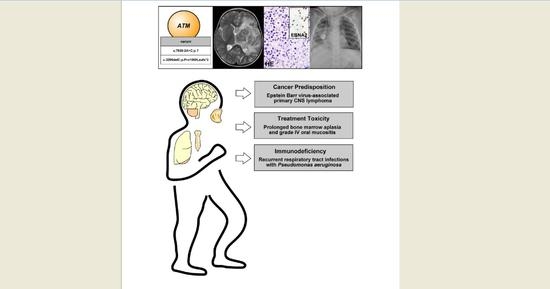
Germline Mutations Including the Rare Pathogenic Variant c.3206delC in the ATM Gene Cause Ataxia Teleangiectasia-Associated Primary Central Nervous System Lymphoma
Abstract
1. Introduction
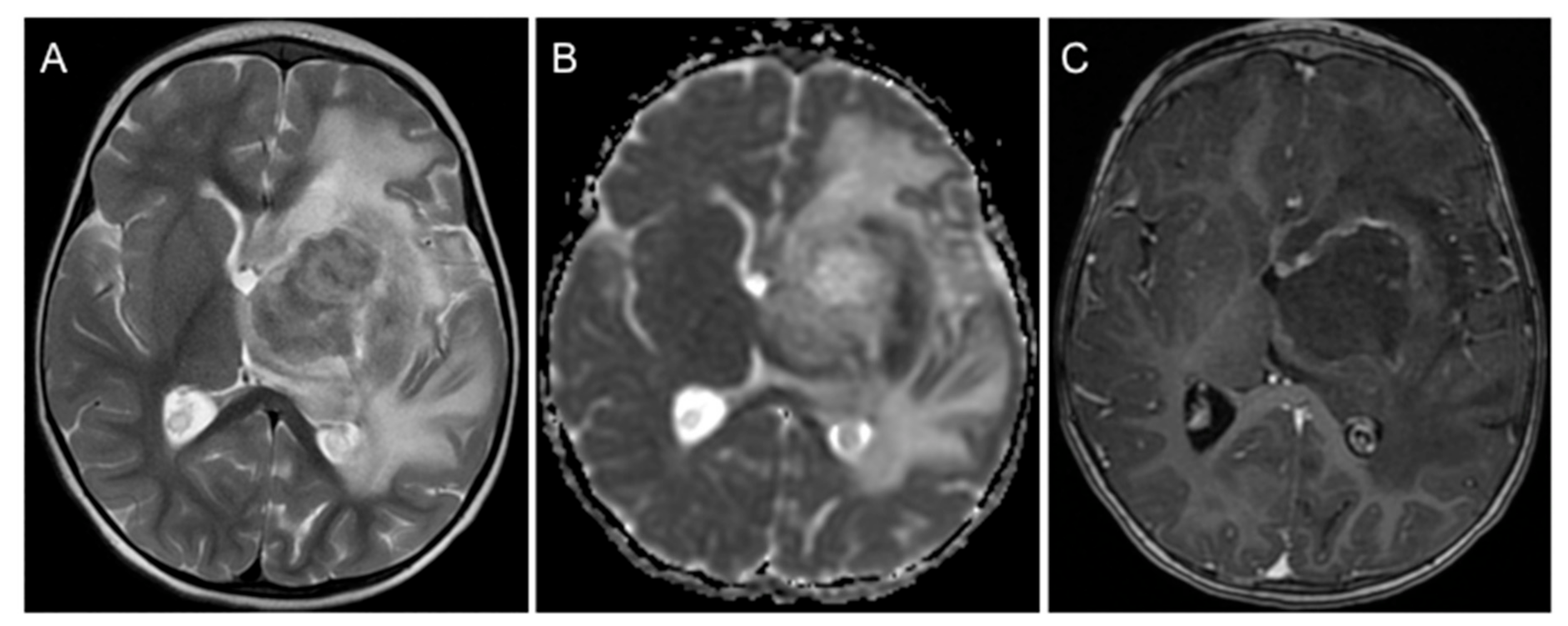
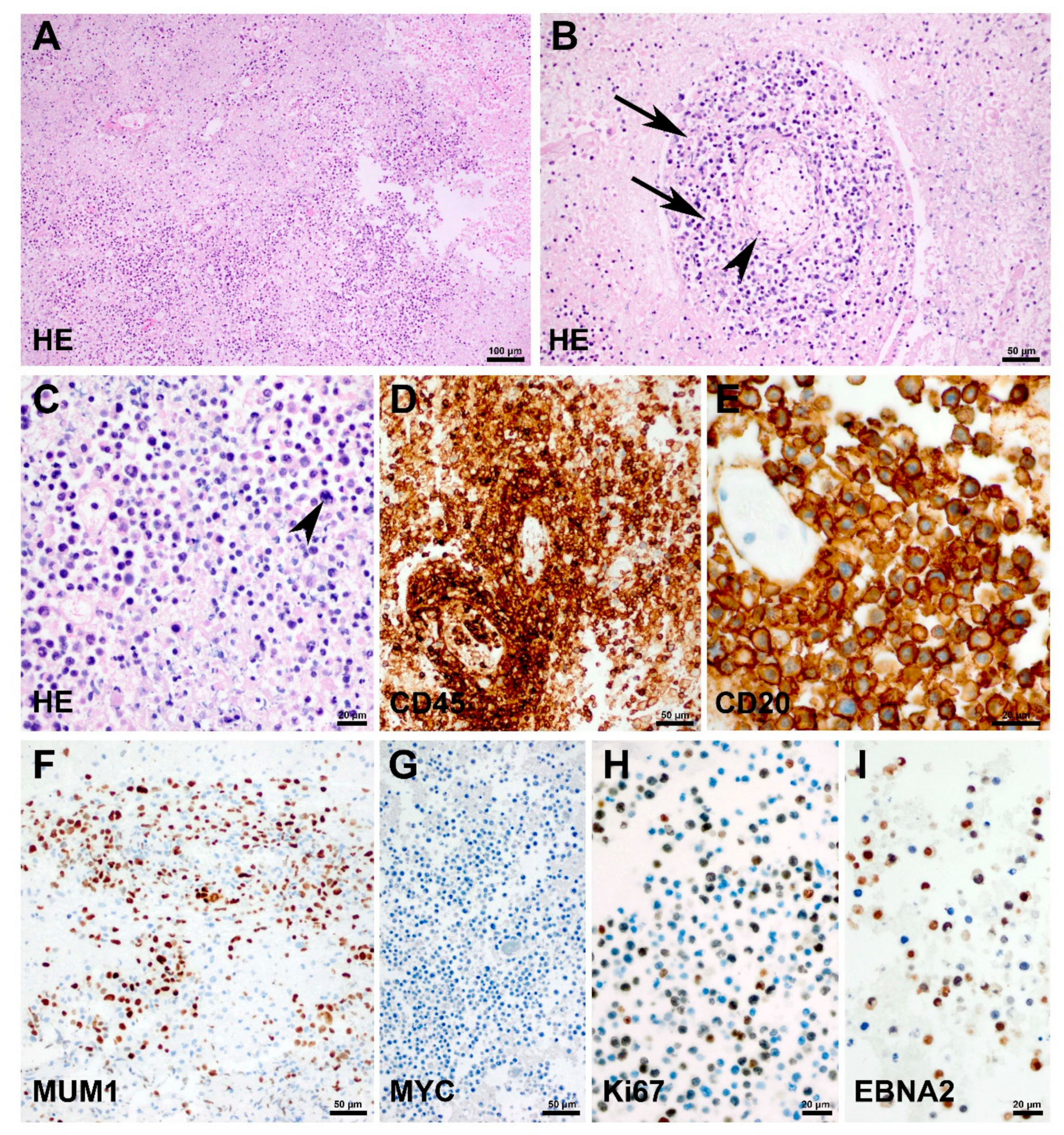
2. Case Presentation
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grommes, C.; De Angelis, L.M. Primary CNS Lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Thorer, H.; Zimmermann, M.; Makarova, O.; Oschlies, I.; Klapper, W.; Lang, P.; Von Stackelberg, A.; Fleischhack, G.; Worch, J.; Juergens, H.; et al. Primary central nervous system lymphoma in children and adolescents: Low relapse rate after treatment according to Non-Hodgkin-Lymphoma Berlin-Frankfurt-Munster protocols for systemic lymphoma. Haematologica 2014, 99, e238–e241. [Google Scholar] [CrossRef]
- Lueth, M.; Stein, H.; Spors, B.; Henze, G.; Driever, P.H. First Case Report of a Peripheral T-cell Lymphoma, not Otherwise Specified, of the Central Nervous System in a Child. J. Pediatr. Hematol. 2012, 34, e66–e68. [Google Scholar] [CrossRef]
- Carnevale, J.; Rubenstein, J.L. The Challenge of Primary Central Nervous System Lymphoma. Hematol. Clin. N. Am. 2016, 30, 1293–1316. [Google Scholar] [CrossRef]
- Cai, Q.; Fang, Y.; Young, K.H. Primary Central Nervous System Lymphoma: Molecular Pathogenesis and Advances in Treatment. Transl. Oncol. 2019, 12, 523–538. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A.B.; Bell, A.I. Epstein–Barr virus-associated lymphomas. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160271. [Google Scholar] [CrossRef]
- Erdag, N.; Bhorade, R.M.; Alberico, R.A.; Yousuf, N.; Patel, M.R. Primary Lymphoma of the Central Nervous System. Am. J. Roentgenol. 2001, 176, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Abla, O.; Sandlund, J.T.; Sung, L.; Brock, P.; Corbett, R.; Kirov, I.; Griffin, T.C.; Blaser, S.; Weitzman, S. A case series of pediatric primary central nervous system lymphoma: Favorable outcome without cranial irradiation. Pediatr. Blood Cancer 2006, 47, 880–885. [Google Scholar] [CrossRef]
- Attarbaschi, A.; Abla, O.; Ronceray, L.; Bansil, S.; Bomken, S.; Burkhardt, B.; Ceppi, F.; Chiang, A.K.S.; Dave, H.; Fedorova, A.; et al. Primary central nervous system lymphoma: Initial features, outcome, and late effects in 75 children and adolescents. Blood Adv. 2019, 3, 4291–4297. [Google Scholar] [CrossRef]
- Zaki-Dizaji, M.; Akrami, S.M.; Abolhassani, H.; Rezaei, N.; Aghamohammadi, A. Ataxia telangiectasia syndrome: Moonlighting ATM. Exp. Rev. Clin. Immunol. 2017, 13, 1155–1172. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, N. Characterization of ATM gene mutations in 66 ataxia telangiectasia families. Hum. Mol. Genet. 1999, 8, 69–79. [Google Scholar] [CrossRef]
- Susswein, L.R.; Marshall, M.L.; Nusbaum, R.; Postula, K.J.V.; Weissman, S.M.; Yackowski, L.; Vaccari, E.M.; Bissonnette, J.; Booker, J.K.; Cremona, M.L.; et al. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet. Med. 2016, 18, 823–832. [Google Scholar] [CrossRef]
- Brand, R.; Borazanci, E.; Speare, V.; Dudley, B.; Karloski, E.; Peters, M.L.B.; Ms, L.S.; Bahary, N.; Zeh, H.; Zureikat, A.; et al. Prospective study of germline genetic testing in incident cases of pancreatic adenocarcinoma. Cancer 2018, 124, 3520–3527. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Parry, E.M.; Gable, D.L.; Stanley, S.E.; Khalil, S.E.; Antonescu, V.; Florea, L.; Armanios, M. Germline mutations in DNA repair genes in lung adenocarcinoma. J. Thorac. Oncol. 2017, 12, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, S.; Woelke, S.; Huenecke, S.; Kieslich, M.; Taylor, A.M.; Schubert, R.; Zielen, S.; Bader, P. Pre-emptive Allogeneic Hematopoietic Stem Cell Transplantation in Ataxia Telangiectasia. Front. Immunol. 2018, 9, 2495. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Duedal, S.; Kirner, J.; McGuffog, L.; Last, J.; Reiman, A.; Byrd, P.; Taylor, M.; Easton, D.F. Cancer risks and mortality in heterozygous ATM mutation carriers. J. Natl. Cancer Inst. 2005, 97, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Abla, O.; Weitzman, S. Primary central nervous system lymphoma in children. Neurosurg. Focus 2006, 21, 1–8. [Google Scholar] [CrossRef]
- Silfen, M.E.; Garvin, J.H.; Hays, A.P.; Starkman, H.S.; Aranoff, G.S.; Levine, L.S.; Feldstein, N.A.; Wong, B.; Oberfield, S.E. Primary Central Nervous System Lymphoma in Childhood Presenting as Progressive Panhypopituitarism. J. Pediatr. Hematol. 2001, 23, 130–133. [Google Scholar] [CrossRef]
- Schulman, H.; Hertzanu, Y.; Maor, E.; Hadar, A. Primary lymphoma of brain in childhood. Pediatr. Radiol. 1991, 21, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovic, I.; Hormigo, A.; Yahalom, J.; DeAngelis, L.M.; Abrey, L.E. Long-Term Follow-Up of High-Dose Methotrexate-Based Therapy With and Without Whole Brain Irradiation for Newly Diagnosed Primary CNS Lymphoma. J. Clin. Oncol. 2006, 24, 4570–4574. [Google Scholar] [CrossRef] [PubMed]
- Balint, M.T.; Jelicic, J.; Mihaljevic, B.; Kostic, J.; Stanic, B.; Balint, B.; Pejanovic, N.; Lucic, B.; Tošić, N.; Marjanović, I.; et al. Gene Mutation Profiles in Primary Diffuse Large B Cell Lymphoma of Central Nervous System: Next Generation Sequencing Analyses. Int. J. Mol. Sci. 2016, 17, 683. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dörr, J.R.; Thorwarth, A.; Mizia-Malarz, A.; Radke, J.; Tietze, A.; Hernáiz-Driever, P.; Horn, D.; Gratopp, A.; Eggert, A.; Deubzer, H.E. Germline Mutations Including the Rare Pathogenic Variant c.3206delC in the ATM Gene Cause Ataxia Teleangiectasia-Associated Primary Central Nervous System Lymphoma. Children 2021, 8, 469. https://doi.org/10.3390/children8060469
Dörr JR, Thorwarth A, Mizia-Malarz A, Radke J, Tietze A, Hernáiz-Driever P, Horn D, Gratopp A, Eggert A, Deubzer HE. Germline Mutations Including the Rare Pathogenic Variant c.3206delC in the ATM Gene Cause Ataxia Teleangiectasia-Associated Primary Central Nervous System Lymphoma. Children. 2021; 8(6):469. https://doi.org/10.3390/children8060469
Chicago/Turabian StyleDörr, Jan R., Anne Thorwarth, Agnieszka Mizia-Malarz, Josefine Radke, Anna Tietze, Pablo Hernáiz-Driever, Denise Horn, Alexander Gratopp, Angelika Eggert, and Hedwig E. Deubzer. 2021. "Germline Mutations Including the Rare Pathogenic Variant c.3206delC in the ATM Gene Cause Ataxia Teleangiectasia-Associated Primary Central Nervous System Lymphoma" Children 8, no. 6: 469. https://doi.org/10.3390/children8060469
APA StyleDörr, J. R., Thorwarth, A., Mizia-Malarz, A., Radke, J., Tietze, A., Hernáiz-Driever, P., Horn, D., Gratopp, A., Eggert, A., & Deubzer, H. E. (2021). Germline Mutations Including the Rare Pathogenic Variant c.3206delC in the ATM Gene Cause Ataxia Teleangiectasia-Associated Primary Central Nervous System Lymphoma. Children, 8(6), 469. https://doi.org/10.3390/children8060469