Maternal Blood Group and Routine Direct Antiglobulin Testing in Neonates: Is There a Role for Selective Neonatal Testing?
Abstract
1. Introduction
2. Materials and Methods
3. Results
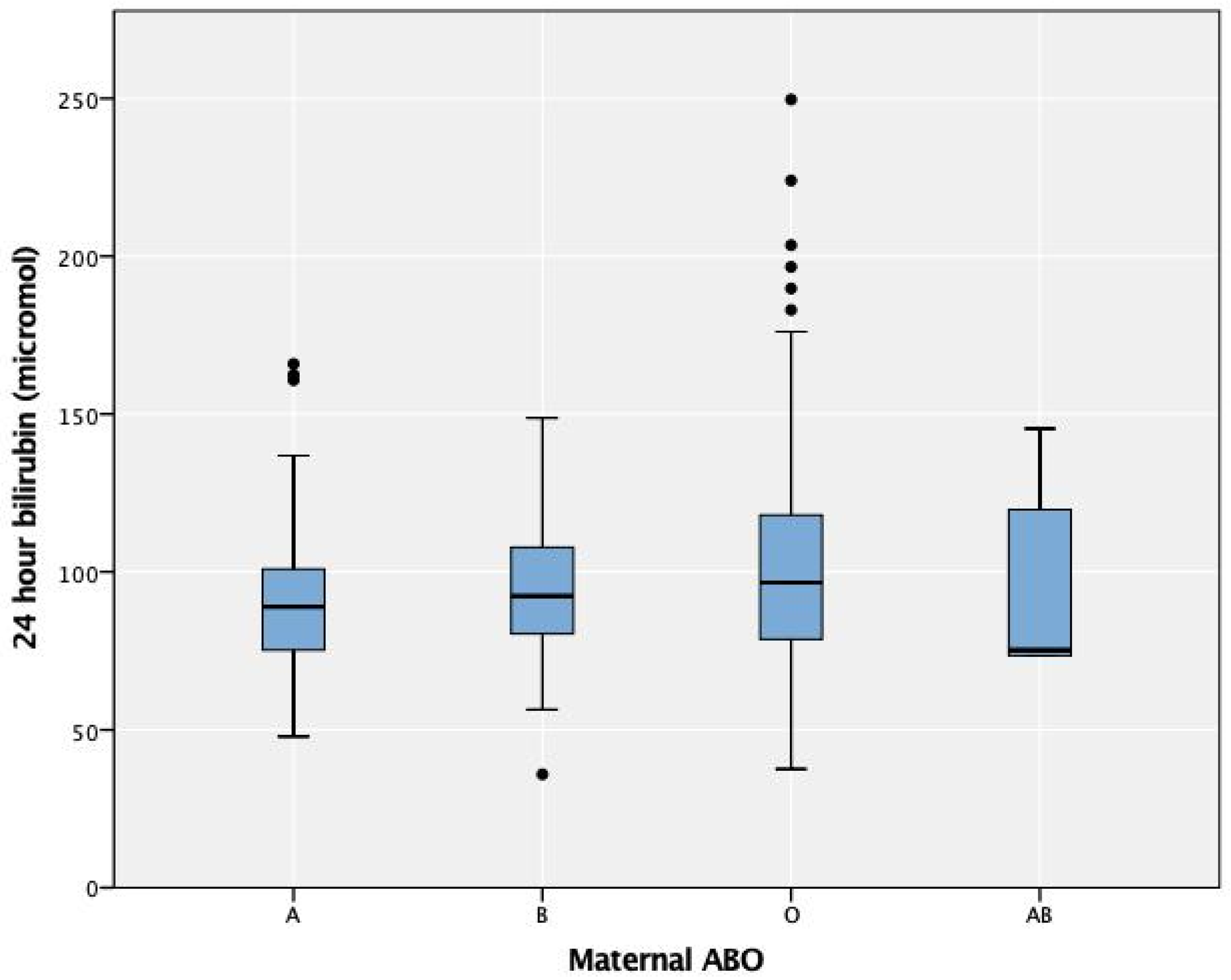
3.1. DAT and Maternal ABO Groups
3.2. DAT and Maternal Group O
3.3. DAT and Maternal RhD Groups
3.4. High-Risk Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Patel, M.K.; Chavda, B.; Ranjan, A.; Ahmad, F. Hemolytic Disease of the Newborn: A study of 50 cases. Int. J. Sci. Study 2013, 1, 13. [Google Scholar]
- Akanmu, A.S.; Oyedeji, O.A.; Adeyemo, T.A.; Ogbenna, A.A. Estimating the Risk of ABO Hemolytic Disease of the Newborn in Lagos. J. Blood Transfus. 2015, 2015, 560738. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.G.; Anstee, D.J. Mollison’s Blood Transfusion in Clinical Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Hendrickson, J.E.; Delaney, M. Hemolytic disease of the fetus and newborn: Modern practice and future investigations. Transfus. Med. Rev. 2016, 30, 159–164. [Google Scholar] [CrossRef]
- Cariani, L.; Romano, E.L.; Martínez, N.; Montaño, R.; Suarez, G.; Ruiz, I.; Soyano, A. ABO-haemolytic disease of the newborn (ABO-HDN): Factors influencing its severity and incidence in Venezuela. J. Trop. Pediatr. 1995, 41, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.A.; Roberts, I.A. Haemolytic disease of the newborn. Arch. Dis. Child. Fetal Neonatal. Ed. 2007, 92, F83–F88. [Google Scholar] [CrossRef]
- Dufour, D.R.; Monoghan, W.P. ABO hemolytic disease of the newborn. A retrospective analysis of 254 cases. Am. J. Clin. Pathol. 1980, 73, 369–373. [Google Scholar] [CrossRef]
- Kaplan, M.; Hammerman, C.; Vreman, H.J.; Wong, R.J.; Stevenson, D.K. Hemolysis and hyperbilirubinemia in antiglobulin positive, direct ABO blood group heterospecific neonates. J. Pediatr. 2010, 157, 772–777. [Google Scholar] [CrossRef][Green Version]
- Bel Hadj, I.; Boukhris, R.; Khalsi, F.; Namouchi, M.; Bougmiza, I.; Tinsa, F.; Hamouda, S.; Boussetta, K. ABO hemolytic disease of newborn: Does newborn’s blood group a risk factor? Tunis. Med. 2019, 97, 455–460. [Google Scholar]
- Keir, A.; Agpalo, M.; Lieberman, L.; Callum, J. How to use: The direct antiglobulin test in newborns. Arch. Dis. Child. Educ. Pract. 2015, 100, 198–203. [Google Scholar] [CrossRef]
- Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004, 114, 297–316. [Google Scholar] [CrossRef]
- Keren, R.; Bhutani, V.K.; Luan, X.; Nihtianova, S.; Cnaan, A.; Schwartz, J.S. Identifying newborns at risk of significant hyperbilirubinaemia: A comparison of two recommended approaches. Arch. Dis. Child. 2005, 90, 415–421. [Google Scholar] [CrossRef] [PubMed]
- BioRad-ABO/Rh for Newborns. Available online: http://www.diamed.com/product_detail.aspx?id=99&navvis=yes (accessed on 9 May 2021).
- Parker, V.; Tormey, C.A. The direct antiglobulin test: Indications, interpretation, and pitfalls. Arch. Pathol. Lab. Med. 2017, 141, 305–310. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Jaundice in Newborn Babies under 28 Days; National Institute for Health and Care Excellence (NICE): London, UK, 2010. [Google Scholar]
- AlKhater, S.A.; Albalwi, R.A.; Alomar, S.A.; Alsultan, A.A.; Almuhaidib, H.R.; Almousa, R.A.; Alanezi, S.M.; Alghamdi, R.K.; Shash, H.A. Value of the Direct Antiglobulin Test in Predicting the Need for Phototherapy in Newborns. J. Blood Med. 2021, 12, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.A. The changing face of haemolytic disease of the newborn. Early Hum. Dev. 2008, 84, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, D. Review of positive direct antiglobulin tests found on cord blood sampling. J. Paediatr. Child Health 2005, 41, 504–507. [Google Scholar] [CrossRef]
- Bucher, K.A.; Patterson, A.M.; Elston, R.C.; Jones, C.A.; Kirkman, H.N. Racial difference in incidence of ABO hemolytic disease. Am. J. Public Health 1976, 66, 854–858. [Google Scholar] [CrossRef][Green Version]
- Huntley, C.C.; Lyerly, A.D.; Littlejohn, M.P.; Rodriguez-Trias, H.; Bowers, G.W. ABO hemolytic disease in Puerto Rico and North Carolina. Pediatrics 1976, 57, 875–883. [Google Scholar] [PubMed]
- Al Jawad, S.; Keenan, P.; Kholeif, S. Incidence of ABO haemolytic disease in a mixed Arab population. Saudi Med. J. 1986, 7, 41–45. [Google Scholar]
- Bashwari, L.; Al-Mulhim, A.A.; Ahmad, M.S.; Ahmed, M.A. Frequency of ABO blood groups in the Eastern region of Saudi Arabia. Saudi Med. J. 2001, 22, 1008–1012. [Google Scholar]
- Akgül, S.; Korkmaz, A.; Yiğit, S.; Yurdakök, M. Neonatal hyperbilirubinemia due to ABO incompatibility: Does blood group matter? Turk. J. Pediatr. 2013, 55, 506–509. [Google Scholar]
- Bhat, Y.R.; Kumar, C.G. Morbidity of ABO haemolytic disease in the newborn. Paediatr. Int. Child Health 2012, 32, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.; Chaudhari, T.; Crispin, P.; Shadbolt, B.; Kent, A. Has anti-D prophylaxis increased the rate of positive direct antiglobulin test results and can the direct antiglobulin test predict need for phototherapy in Rh/ABO incompatibility? J. Paediatr. Child Health 2011, 47, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Maayan-Metzger, A.; Leibovitch, L.; Schushan-Eisen, I.; Morag, I.; Strauss, T. Maternal anti-D prophylaxis during pregnancy and risk of hemolysis among preterm infants. J. Perinatol. 2014, 34, 906–908. [Google Scholar] [CrossRef]
- Chown, B.; Bowman, J.M.; Pollock, J.; Lowen, B.; Pettett, A. The effect of anti-D IgG on D-positive recipients. Can. Med. Assoc. J. 1970, 102, 1161–1164. [Google Scholar] [PubMed]
- Moyer, V.A.; Ahn, C.; Sneed, S. Accuracy of clinical judgment in neonatal jaundice. Arch. Pediatr. Adolesc. Med. 2000, 154, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Riskin, A.; Kugelman, A.; Kuglman, A.; Abend-Weinger, M.; Green, M.; Hemo, M.; Bader, D. In the eye of the beholder: How accurate is clinical estimation of jaundice in newborns? Acta Paediatr. 2003, 92, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Riskin, A.; Tamir, A.; Kugelman, A.; Hemo, M.; Bader, D. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J. Pediatr. 2008, 152, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Hulzebos, C.V.; Vitek, L.; Zabetta, C.D.C.; Dvořák, A.; Schenk, P.; van der Hagen, E.A.; Cobbaert, C.; Tiribelli, C. Screening methods for neonatal hyperbilirubinemia: Benefits, limitations, requirements, and novel developments. Pediatr. Res. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Slusher, T.M.; Imosemi, D.O.; Emokpae, A.A. Maternal detection of neonatal jaundice during birth hospitalization using a novel two-color icterometer. PLoS ONE 2017, 12, e0183882. [Google Scholar] [CrossRef]
- Lee, A.C.; Folger, L.V.; Rahman, M.; Ahmed, S.; Bably, N.N.; Schaeffer, L.; Whelan, R.; Panchal, P.; Rahman, S.; Roy, A.D. A novel Icterometer for hyperbilirubinemia screening in low-resource settings. Pediatrics 2019, 143, e20182039. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Stout, J.W.; de Greef, L.; Goel, M.; Patel, S.; Chung, E.K.; Koduri, A.; McMahon, S.; Dickerson, J.; Simpson, E.A. Use of a smartphone app to assess neonatal jaundice. Pediatrics 2017, 140, e20170312. [Google Scholar] [CrossRef] [PubMed]

| Negative (n = 1398) | Positive (n = 65) | p-Value | Overall (n = 1463) | |
|---|---|---|---|---|
| Maternal Age (y) | ||||
| Median (25th, 75th percentile) | 30.0 (26, 35) | 31.0 (27, 35) | 0.556 | 30.0 (26.0, 35.0) |
| Missing | 1 (0.1%) | 0 | 1 (0.1%) | |
| Parity | ||||
| Median (25th, 75th percentile) | 2.00 (2.00, 4.00) | 3.00 (1.00, 4.0) | 0.549 | 2.00 (1.00, 12.0) |
| Missing | 54 (3.9%) | 1 (1.5%) | 55 (3.8%) | |
| Gestational Age (Categorical) | ||||
| ≤36–38 + 6 | 520 (37.2%) | 23 (35.4%) | 0.444 | 543 (37.1%) |
| 39–40 + 6 | 654 (46.8%) | 26 (40.0%) | 680 (46.5%) | |
| >41 | 119 (8.5%) | 8 (12.3%) | 127 (8.7%) | |
| Missing | 113 | |||
| Neonate Gender | ||||
| Male | 713 (51.0%) | 37 (56.9%) | 0.420 | 750 (51.3%) |
| Female | 685 (49.0%) | 28 (43.1%) | 713 (48.7%) | |
| Birthweight (kg) | ||||
| Median | 3.00 (2.7, 3.3) | 3.2 (2.9, 3.6) | 0.002 | 3.03 (0.470) |
| Missing | 157 (11.2%) | 10 (15.4%) | 167 (11.4%) | |
| Ethnicity | ||||
| Saudi | 1155 (82.6%) | 46 (70.8%) | 0.023 | 1201 (82.1%) |
| Non-Saudi | 243 (17.4%) | 19 (29.2%) | 262 (17.9%) | |
| DAT-Negative (n = 1398) | DAT-Positive (n = 65) | p-Value | Overall (n = 1463) | |
|---|---|---|---|---|
| Maternal ABO | ||||
| Blood group O | 663 (47.4%) | 59 (90.8%) | <0.001 | 722 (49.4%) |
| Blood group A | 396 (28.3%) | 0 (0%) | 396 (27.1%) | |
| Blood group B | 268 (19.2%) | 5 (7.7%) | 273 (18.7%) | |
| Blood group AB | 71 (5.1%) | 1 (1.5%) | 72 (4.9%) | |
| Maternal RhD | ||||
| Positive | 1315 (94.1%) | 56 (86.2%) | 0.021 | 1371 (93.7%) |
| Negative | 83 (5.9%) | 9 (13.8%) | 92 (6.3%) | |
| Received Anti-D (n = 92) | ||||
| Yes | 56 (67.5%) | 5 (55.6%) | 0.013 | 61 (66.3%) |
| No | 18 (21.7%) | 0 (0%) | 18 (19.6%) | |
| Data not available | 9 (10.8%) | 4 (44.4%) | 13 (14.1%) | |
| Maternal/Neonate ABO Compatibility | ||||
| ABO-compatible | 912 (65.2%) | 10 (15.4%) | <0.001 | 922 (63.0%) |
| ABO-incompatible | 486 (34.8%) | 55 (84.6%) | 541 (37.0%) | |
| Type of Maternal/Neonate Incompatibility (n = 541) | ||||
| Maternal A/Baby O | 125 (25.7%) | 0 (0%) | <0.001 | 125 (23.1%) |
| Maternal A/Baby B | 26 (5.3%) | 0 (0%) | 26 (4.8%) | |
| Maternal A/Baby AB | 24 (4.9%) | 0 (0%) | 24 (4.4%) | |
| Maternal B/Baby O | 66 (13.6%) | 2 (3.6%) | 68 (12.6%) | |
| Maternal B/Baby A | 24 (4.9%) | 0 (0%) | 24 (4.4%) | |
| Maternal B/Baby AB | 20 (4.1%) | 1 (1.8%) | 21 (3.9%) | |
| Maternal O/Baby A | 89 (18.3%) | 22 (40%) | 111 (20.5%) | |
| Maternal O/Baby B | 57 (11.7%) | 29 (52.7%) | 86 (15.9%) | |
| Maternal AB/Baby A | 25 (5.1%) | 0 (0%) | 25 (4.6%) | |
| Maternal AB/Baby B | 30 (6.2%) | 1 (1.8%) | 31 (5.7%) | |
| Maternal/Neonatal RhD Compatibility | ||||
| RhD-compatible | 1284 (91.8%) | 57 (87.7%) | 0.340 | 1341 (91.7%) |
| RhD-incompatible | 114 (8.2%) | 8 (12.3%) | 122 (8.3%) | |
| DAT-Negative (n = 663) | DAT-Positive (n = 59) | p-Value | Overall (n = 722) | |
|---|---|---|---|---|
| Maternal/Neonatal Grouping | ||||
| Group O-A | 89 (13.4%) | 22 (37.3%) | <0.001 | 111 (15.4%) |
| Group O-B | 57 (8.6%) | 29 (49.2%) | 86 (11.9%) | |
| Group O-O | 517 (78%) | 8 (13.6%) | 525 (72.7%) | |
| 24-h Bilirubin Level | ||||
| Median [25th, 75th percentile] | 92.34 (77, 107.22) | 106 (82.1, 134.2) | 0.014 | |
| Peak Bilirubin Level | ||||
| Median [25th, 75th percentile] | 111.2 (88.9, 175.3) | 126.5 (89.4, 163.3) | 0.402 | |
| Age at Peak Bilirubin | ||||
| Median [25th, 75th percentile] | 25.00 (24.00, 52.00) | 36.00 (24, 50) | 0.569 | |
| Phototherapy (n = 143) | ||||
| Yes | 11 (13.1%) | 11 (18.6%) | 0.503 | 22 (15.4%) |
| No | 73 (86.9%) | 48 (81.4%) | 212 (84.6%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shash, H.A.; Alkhater, S.A. Maternal Blood Group and Routine Direct Antiglobulin Testing in Neonates: Is There a Role for Selective Neonatal Testing? Children 2021, 8, 426. https://doi.org/10.3390/children8050426
Shash HA, Alkhater SA. Maternal Blood Group and Routine Direct Antiglobulin Testing in Neonates: Is There a Role for Selective Neonatal Testing? Children. 2021; 8(5):426. https://doi.org/10.3390/children8050426
Chicago/Turabian StyleShash, Hwazen A., and Suzan A. Alkhater. 2021. "Maternal Blood Group and Routine Direct Antiglobulin Testing in Neonates: Is There a Role for Selective Neonatal Testing?" Children 8, no. 5: 426. https://doi.org/10.3390/children8050426
APA StyleShash, H. A., & Alkhater, S. A. (2021). Maternal Blood Group and Routine Direct Antiglobulin Testing in Neonates: Is There a Role for Selective Neonatal Testing? Children, 8(5), 426. https://doi.org/10.3390/children8050426






