Changes in Umbilico–Placental Circulation during Prolonged Intact Cord Resuscitation in a Lamb Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
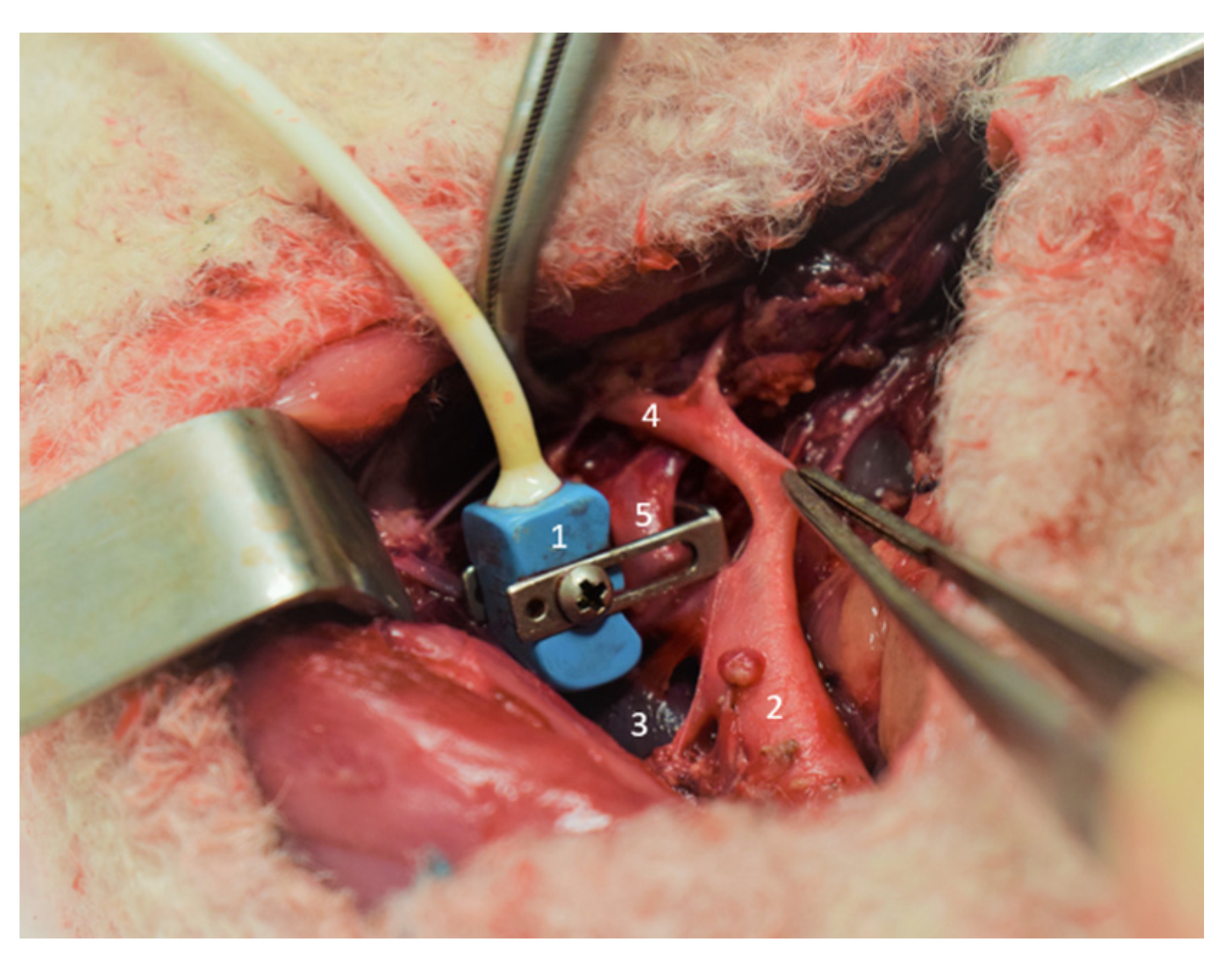
2.2. Surgical Procedure
2.3. Experimental Design
2.4. Hemodynamic and Biological Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crossley, K.J.; Allison, B.J.; Polglase, G.R.; Morley, C.J.; Davis, P.G.; Hooper, S.B. Dynamic Changes in the Direction of Blood Flow through the Ductus Arteriosus at Birth: Blood Flow through the Ductus Arteriosus at Birth. J. Physiol. 2009, 587, 4695–4704. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.J.; Middleton, P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; p. CD004074.pub2. [Google Scholar]
- Pratesi, S.; Montano, S.; Ghirardello, S.; Mosca, F.; Boni, L.; Tofani, L.; Dani, C. Placental Circulation Intact Trial (PCI-T)-Resuscitation With the Placental Circulation Intact vs. Cord Milking for Very Preterm Infants: A Feasibility Study. Front. Pediatr. 2018, 6, 364. [Google Scholar] [CrossRef] [PubMed]
- Farrar, D.; Airey, R.; Law, G.R.; Tuffnell, D.; Cattle, B.; Duley, L. Measuring Placental Transfusion for Term Births: Weighing Babies with Cord Intact. BJOG 2011, 118, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.C.; Wist, A.; Lind, J. The Blood Volume of the Newborn Infant Delivered by Caesarean Section. Acta Paediatr. Scand. 1967, 56, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.C.; Lind, J. Blood Flow in the Umbilical Vessels during the Third Stage of Labor. Biol. Neonate 1974, 25, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Viard, R.; Tourneux, P.; Storme, L.; Girard, J.-M.; Betrouni, N.; Rousseau, J. Magnetic Resonance Imaging Spatial and Time Study of Lung Water Content in Newborn Lamb: Methods and Preliminary Results. Invest. Radiol. 2008, 43, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Boere, I.; Roest, A.A.W.; Wallace, E.; Ten Harkel, A.D.J.; Haak, M.C.; Morley, C.J.; Hooper, S.B.; Te Pas, A.B. Umbilical Blood Flow Patterns Directly after Birth before Delayed Cord Clamping. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F121–F125. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Rakza, T.; Weslinck, N.; Vaast, P.; Houfflin-debarge, V.; Mur, S.; Storme, L. Feasibility and Safety of Intact Cord Resuscitation in Newborn Infants with Congenital Diaphragmatic Hernia (CDH). Resuscitation 2017, 120, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Houeijeh, A.; Aubry, E.; Coridon, H.; Montaigne, K.; Sfeir, R.; Deruelle, P.; Storme, L. Effects of N-3 Polyunsaturated Fatty Acids in the Fetal Pulmonary Circulation. Crit. Care Med. 2011, 39, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Houfflin-Debarge, V.; Sabbah-Briffaut, E.; Aubry, E.; Deruelle, P.; Alexandre, C.; Storme, L. Effects of Environmental Tobacco Smoke on the Pulmonary Circulation in the Ovine Fetus. Am. J. Obstet. Gynecol. 2011, 204, 450.e8–450.e14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Nutrition for Health and Development. In World Health Organization Guideline; World Health Organization: Geneva, Switzerland, 2014; ISBN 978-92-4-150820-9. [Google Scholar]
- Brouwer, E.; Te Pas, A.B.; Polglase, G.R.; McGillick, E.V.; Böhringer, S.; Crossley, K.J.; Rodgers, K.; Blank, D.; Yamaoka, S.; Gill, A.W.; et al. Effect of Spontaneous Breathing on Umbilical Venous Blood Flow and Placental Transfusion during Delayed Cord Clamping in Preterm Lambs. Arch. Dis Child. Fetal Neonatal Ed. 2020, 105, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.J.; Green, T.J. Foetal Placental Blood Flow in the Lamb. J. Physiol. 1972, 223, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Kiserud, T.; Ebbing, C.; Kessler, J.; Rasmussen, S. Fetal Cardiac Output, Distribution to the Placenta and Impact of Placental Compromise. Ultrasound Obstet. Gynecol. 2006, 28, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Adamson, S.L.; Myatt, L.; Byrne, B.M.P. Regulation of Umbilical Blood Flow. In Fetal and Neonatal Physiology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 827–837. ISBN 978-1-4160-3479-7. [Google Scholar]
- McGrath, J.C.; MacLennan, S.J.; Mann, A.C.; Stuart-Smith, K.; Whittle, M.J. Contraction of Human Umbilical Artery, but Not Vein, by Oxygen. J. Physiol. 1986, 380, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Evensen, A.; Anderson, J.M.; Fontaine, P. Postpartum Hemorrhage: Prevention and Treatment. Am. Fam. Physician 2017, 95, 442–449. [Google Scholar] [PubMed]
- Olofsson, P.; Thuring-Jönsson, A.; Marsál, K. Uterine and Umbilical Circulation during the Oxytocin Challenge Test. Ultrasound Obstet. Gynecol. 1996, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Yamakage, M.; Tsujiguchi, N.; Chen, X.; Kamada, Y.; Namiki, A. Sevoflurane Inhibits Contraction of Uterine Smooth Muscle from Pregnant Rats Similarly to Halothane and Isoflurane. Can. J. Anesth. 2002, 49, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Novoa, R.H.; Quintana, W.; Castillo-Urquiaga, W.; Ventura, W. EXIT (Ex Utero Intrapartum Treatment) Surgery for the Management of Fetal Airway Obstruction: A Systematic Review of the Literature. J. Pediatr. Surg. 2020, 55, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, S.; Johnson, M.P.; Flake, A.W.; Howell, L.J.; Myers, L.B.; Adzick, N.S.; Crombleholme, T.M. The EXIT Procedure: Experience and Outcome in 31 Cases. J. Pediatr. Surg. 2002, 37, 418–426. [Google Scholar] [CrossRef] [PubMed]
| Time | M0 | M20 | M40 | M60 |
|---|---|---|---|---|
| pH | 7.1 ± 0.1 | 7.1 ± 0.2 | 7.2 ± 0.2 | 7.3 ± 0.1 * |
| PaO2 (mmHg) | 15 ± 4 | 126 ± 61 * | 70 ± 29 * | 68 ± 12 * |
| PaCO2 (mmHg) | 75 ± 17 | 62 ± 12 | 49 ± 11 * | 44 ± 8 * |
| HCO3 (mEq.L−1) | 23 ± 8 | 20 ± 5 | 22 ± 6 | 23 ± 7 |
| Time | M-5 | M0 | M10 | M20 | M30 | M40 | M50 | M60 |
|---|---|---|---|---|---|---|---|---|
| HR (Beats/min) | 147 ± 40 | 144 ± 35 | 133 ± 10 | 140 ± 20 | 132 ± 14 | 132 ± 13 | 140 ± 14 | 148 ± 14 |
| PAo (mmHg) | 43 ± 4 | 43 ± 8 | 48 ± 4 | 49 ± 4 | 47 ± 5 | 51 ± 7 | 53 ± 8 | 50 ± 6 |
| Pv (mmHg) | 7 ± 3 | 8 ± 2 | 8 ± 2 | 9 ± 2 | 9 ± 2 | 8 ± 2 | 8 ± 2 | 7 ± 2 |
| Pre-ductal Spo2 (%) | 80 ± 20 | 80 ± 20 | 98 ± 3 | 99 ± 2 | 97 ± 4 | 97 ± 3 | 98 ± 3 | 96 ± 2 |
| Post-ductal Spo2 (%) | 38 ± 23 | 38 ± 23 | 50 ± 32 * | 90 ± 15 * | 95 ± 6 * | 93 ± 5 * | 94 ± 3 * | 95 ± 5 * |
| Qup (mL/min) | 232 ± 38 | 234 ± 75 | 252 ± 107 | 214 ± 73 | 204 ± 98 | 186 ± 79 | 148 ± 33 * | 158 ± 12 * |
| Rup (µW) | 158 ± 10 | 151 ± 26 | 179 ± 66 | 205 ± 58 | 224 ± 104 | 262 ± 111 | 280 ± 106 * | 299 ± 82 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Duc, K.; Aubry, E.; Mur, S.; Besengez, C.; Garabedian, C.; De Jonckheere, J.; Storme, L.; Sharma, D. Changes in Umbilico–Placental Circulation during Prolonged Intact Cord Resuscitation in a Lamb Model. Children 2021, 8, 337. https://doi.org/10.3390/children8050337
Le Duc K, Aubry E, Mur S, Besengez C, Garabedian C, De Jonckheere J, Storme L, Sharma D. Changes in Umbilico–Placental Circulation during Prolonged Intact Cord Resuscitation in a Lamb Model. Children. 2021; 8(5):337. https://doi.org/10.3390/children8050337
Chicago/Turabian StyleLe Duc, Kévin, Estelle Aubry, Sébastien Mur, Capucine Besengez, Charles Garabedian, Julien De Jonckheere, Laurent Storme, and Dyuti Sharma. 2021. "Changes in Umbilico–Placental Circulation during Prolonged Intact Cord Resuscitation in a Lamb Model" Children 8, no. 5: 337. https://doi.org/10.3390/children8050337
APA StyleLe Duc, K., Aubry, E., Mur, S., Besengez, C., Garabedian, C., De Jonckheere, J., Storme, L., & Sharma, D. (2021). Changes in Umbilico–Placental Circulation during Prolonged Intact Cord Resuscitation in a Lamb Model. Children, 8(5), 337. https://doi.org/10.3390/children8050337






