Enhancing Value and Uptake for Whole-Population Cohorts of Children and Parents: Methods to Integrate Registries into the Generation Victoria Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. About GenV
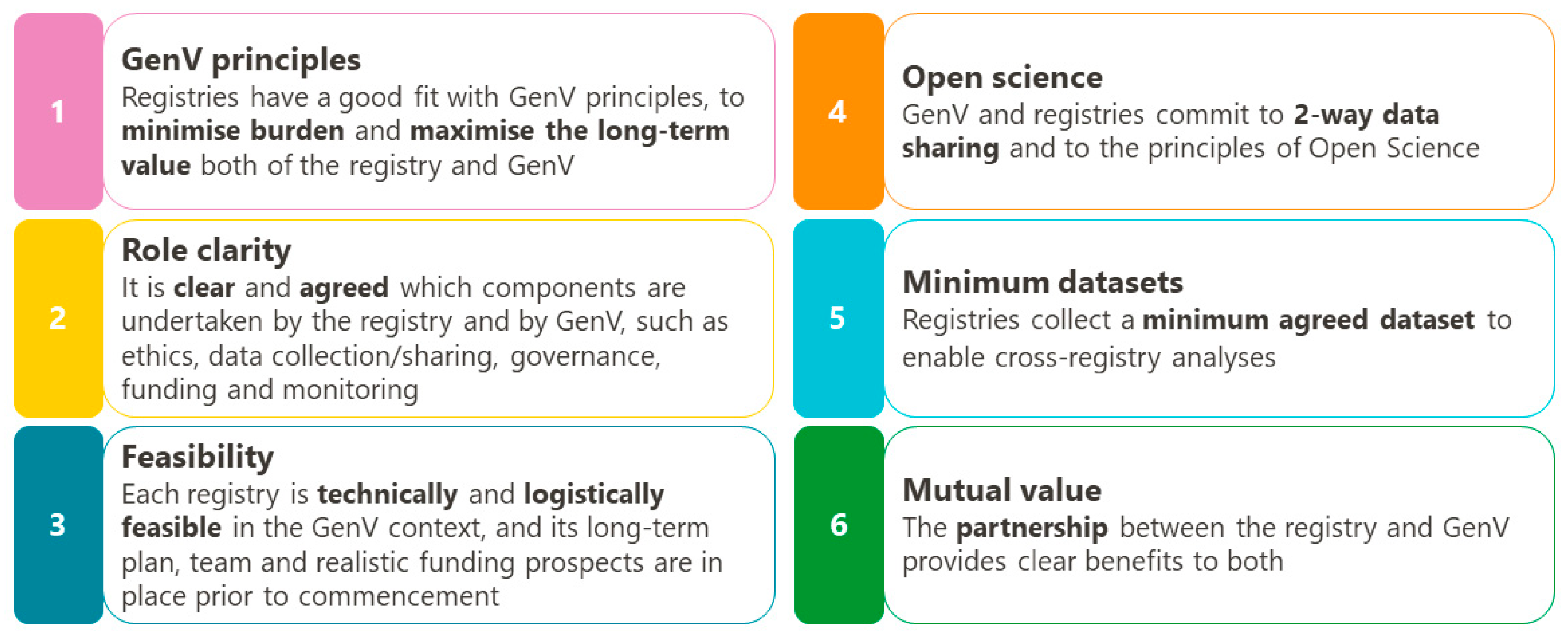
2.2. Principles and Concepts for GenV-Registry Collaboration
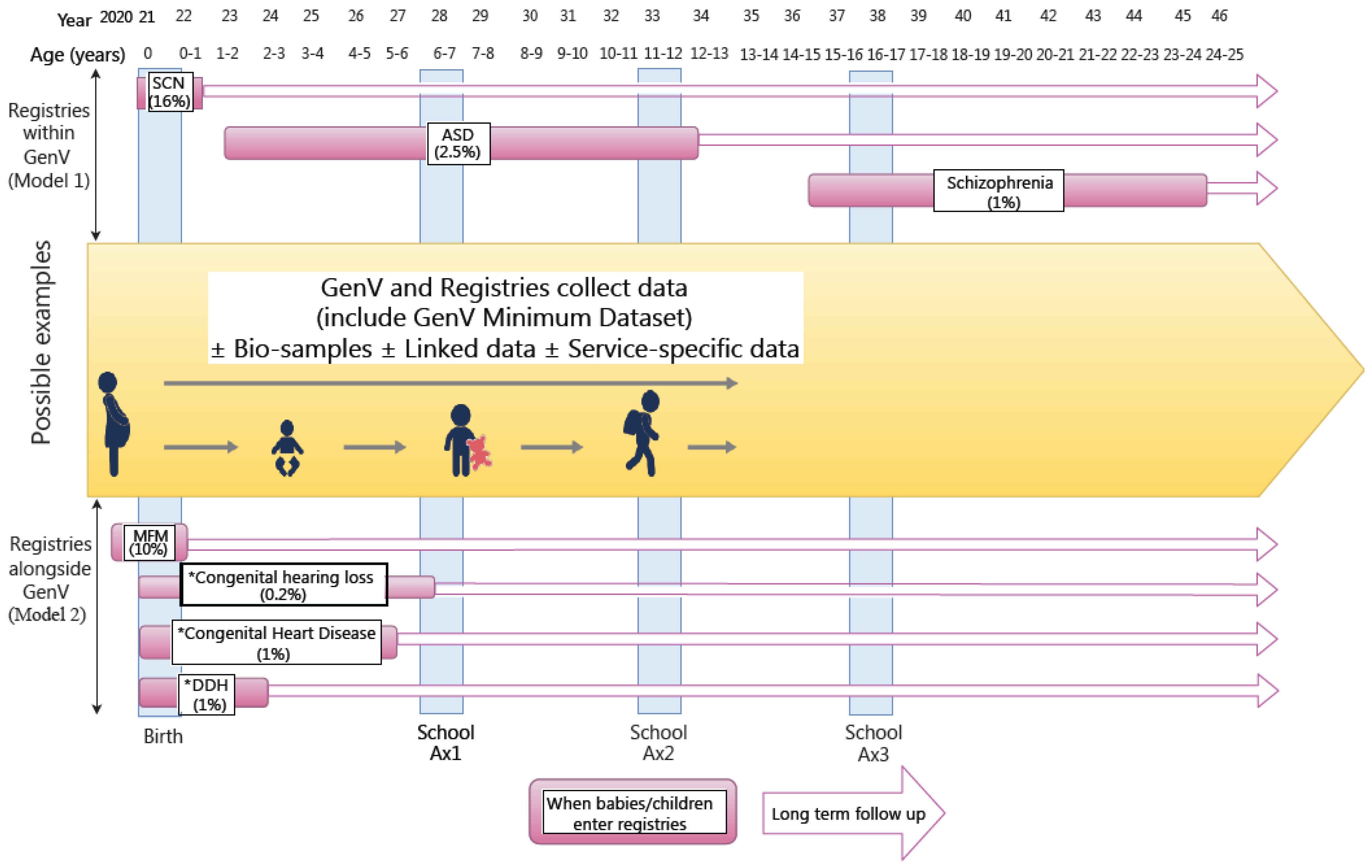
2.3. Preparatory Work
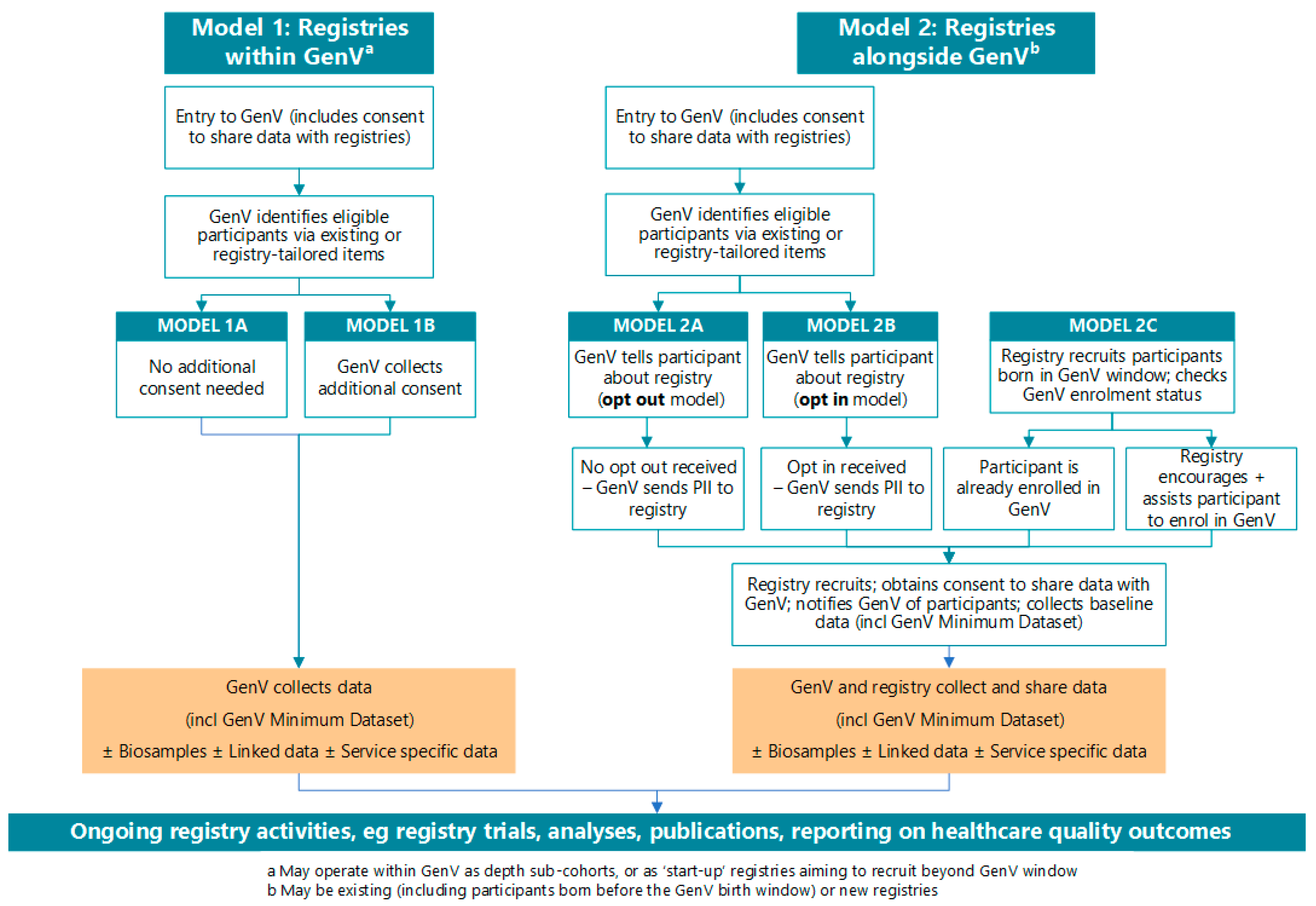
2.4. Consent
- “GenV may offer you the chance to take part in future ethically approved studies working with GenV…..You can always choose whether to take part.”
- “GenV’s data can only be used for ethically approved research to improve health, development, or wellbeing for children and adults. Over time, researchers will use lots of different methods to answer new and important questions. Therefore, the value of your information will keep growing for many years.”
- “GenV participants may also join studies or registries about specific issues such as head injuries or hearing loss.”
- “Trials or studies may ask your consent to share data with GenV, with ethics approval. We support this.”
- “This registry is working with the GenV (Generation Victoria) program. GenV is a research program open to all children living in Victoria and born over two years starting in 2021, and their parents. People in GenV can also be in registries that help prevent, predict and treat problems. This cuts down cost, effort and duplication. It also increases the value of registries. For example, by working with GenV, a registry can access richer data to help solve issues. You can read more about GenV here, and about registries working with GenV here.”
- “We ask that you consent to allow your registry data to be joined up with your GenV data. Then, both studies can answer more questions about health and other outcomes. Under strict conditions and ethical approval, data from this registry can enter GenV’s dataset, and data from GenV can enter this registry’s dataset.”
- [Model 2c only] “It is possible that [you/your child] [are/is] eligible for GenV but not enrolled in it. We encourage you to enroll in GenV. This increases the value of this registry, without adding to your time. You can join GenV by… [registry enrolls participant into GenV; registry passes contact details to GenV; parent contacts GenV directly].
- [Model 2c only—choose relevant wording] You can be in this registry and not in GenV, but we will be missing some information about you/your child or you can only take part in this registry if you are also in GenV.”
2.5. Measures
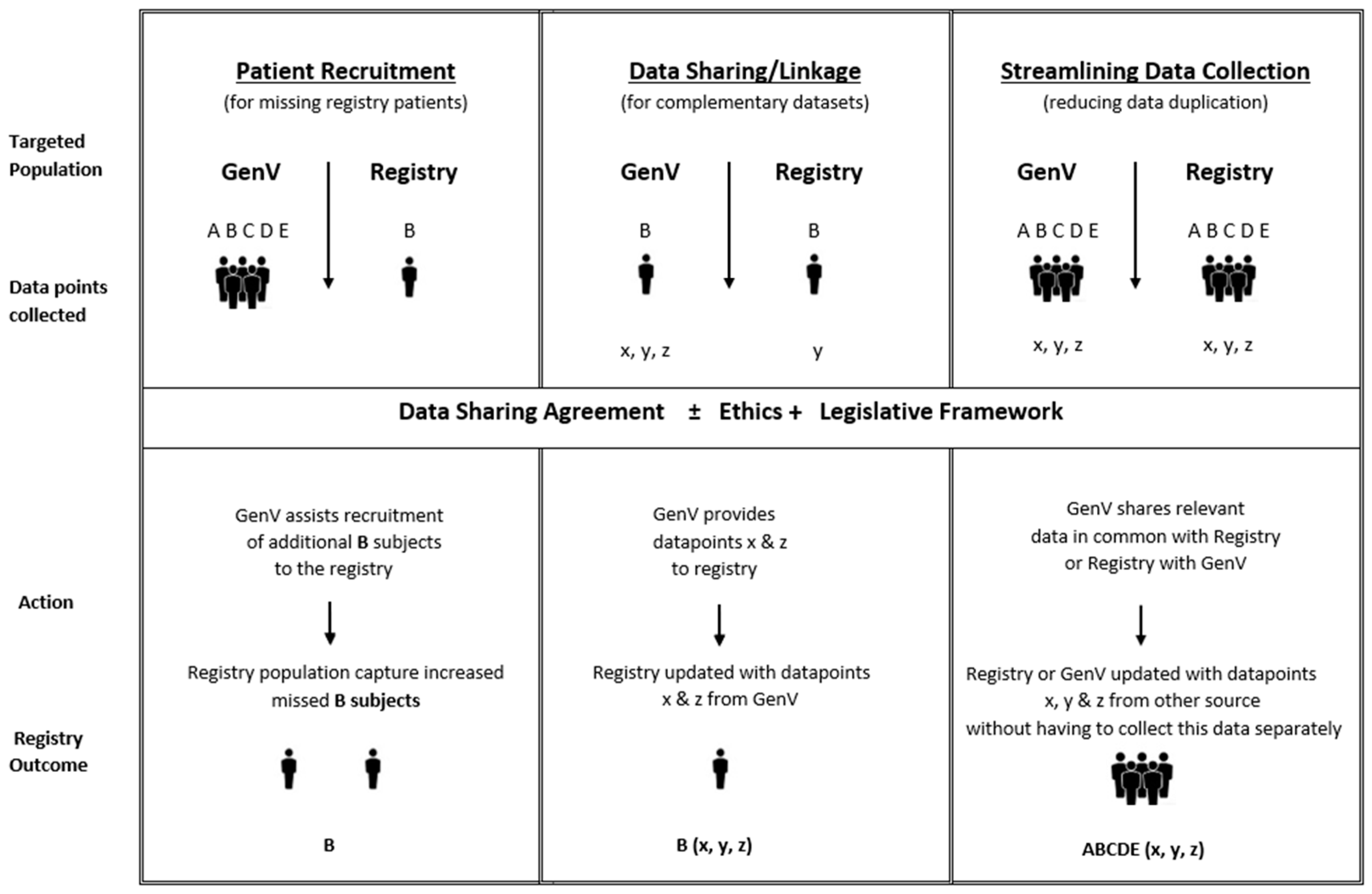
2.6. Data Sharing Considerations
2.7. Governance and Consumer/Stakeholder Considerations
3. Discussion

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McNeil, J.J.; Evans, S.M.; Johnson, N.P.; Cameron, P.A. Clinical-quality registries: Their role in quality improvement. Med. J. Aust. 2010, 192, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, N.; McNeil, J.J. Clinical quality registries have the potential to drive improvements in the appropriateness of care. Med. J. Aust. 2016, 205, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Houben, E.; Broeders, L.; Steegers, E.A.P.; Herings, R.M.C. Cohort profile: The PHARMO Perinatal Research Network (PPRN) in the Netherlands: A population-based mother–child linked cohort. BMJ Open 2020, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Maret-Ouda, J.; Tao, W.; Wahlin, K.; Lagergren, J. Nordic registry-based cohort studies: Possibilities and pitfalls when combining Nordic registry data. Scan. J. Public Health 2017, 45, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H. Massive UK baby study cancelled. Nature 2015, 526, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.; Fearon, P.; Christodoulou, J.; Rotheram-Borus, M.J. Editorial Perspective: Stop describing and start fixing—The promise of longitudinal intervention cohorts. J. Child Psychol. Psychiatry 2020, 61, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.T.; Hagemann, E.; Davis, J.A.; Gibson, L.Y.; Srinivasjois, R.; Palmer, D.J.; Colvin, L.; Tan, J.; Prescott, S.L. Introducing the ORIGINS project: A community-based interventional birth cohort. Rev. Environ. Health 2020, 35, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Magnus, P.; Birke, C.; Vejrup, K.; Haugan, A.; Alsaker, E.; Daltveit, A.K.; Handal, M.; Haugen, M.; Høiseth, G.; Knudsen, G.P.; et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int. J. Epidemiol. 2016, 45, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Australian Population. Victoria–Australia Population. Available online: https://australian-population.com/states/victoria-population (accessed on 25 May 2020).
- Liu, T.; Lingam, R.; Lycett, K.; Mensah, F.K.; Muller, J.; Hiscock, H.; Huque, M.H.; Wake, M. Parent-reported prevalence and persistence of 19 common child health conditions. Arch. Dis. Child. 2018, 103, 548–556. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.; Evans, S.; Brennan, A.; Read, C. Registry Science Handbook; Monash University: Melbourne, Australia, 2013. [Google Scholar]
- Wake, M.; Hu, Y.J.; Warren, H.; Danchin, M.; Fahey, M.; Orsini, F.; Pacilli, M.; Perrett, K.P.; Saffery, R.; Davidson, A. Integrating trials into a whole-population cohort of children and parents: Statement of intent (trials) for the Generation Victoria (GenV) cohort. BMC Med. Res. Methodol. 2020, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Gold, J.; Davenport, L.; Perera, P.; Wake, M.; Goldfeld, S. Rapid Evidence Assessment Report: Large Research-Led PARTNERSHIPS; Generation Victoria Working Paper 2020-02; Murdoch Children’s Research Institute: Melbourne, Australia, 2020. [Google Scholar] [CrossRef]
- Stevens, L. Generation Victoria. Available online: https://mcri.figshare.com/projects/Generation_Victoria/35822 (accessed on 25 May 2020).
- Wake, M.; Gasparini, L.; Hourani, D.; Siero, W. The Victorian Child’s Lifecourse Journey in Data; Murdoch Children’s Research Institute: Melbourne, Australia, 2018. [Google Scholar] [CrossRef]
- Hughes, E.K.; Siero, W.; Welsh, S.; Edwards, B.; Gulenc, A.; Wake, M. GenV Parent Consultations Survey 2019; Generation Victoria Working Paper 2020-05; Murdoch Children’s Research Institute: Melbourne, Australia, 2020. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Clifford, S.; Goldfeld, S.; Wake, M. Selecting lifecourse frameworks to guide and communicate large new cohort studies: Generation Victoria (GenV) case study. J. Dev. Orig. Health Dis. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Clifford, S.; Wake, M. GenV Measures Selection Principles; Generation Victoria Working Paper 2020-01; Murdoch Children’s Research Institute: Melbourne, Australia, 2020. [Google Scholar] [CrossRef]
- Donev, D.; Zaletel-Kragelj, L.; Bjegovic, V.; Burazeri, G. Measuring the burden of disease: Disability adjusted life year (daly). In Methods and Tools in Public Health: A Handbook for Teachers, Researchers and Health Professionals; Zaletel-Kragelj, L., Boţikov, J., Eds.; Hans Jacobs Publishing Company: Lage, Germany, 2010. [Google Scholar]
- Reed, G.M. Toward ICD-11: Improving the clinical utility of WHO’s international classification of mental disorders. Prof. Psychol. Res. Pract. 2010, 41, 457–464. [Google Scholar] [CrossRef]
- The World Health Organisation. International Classification of Functioning, Disability and Health (ICF); World Health Organisation: Geneva, Switzerland, 2001. [Google Scholar]
- Williamson, P.R.; Altman, D.G.; Blazeby, J.M.; Clarke, M.; Devane, D.; Gargon, E.; Tugwell, P. Developing core outcome sets for clinical trials: Issues to consider. Trials 2012, 13, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.; Ritchie, F.; Whelpton, R. Five Safes: Designing Data Access for Research; Economics Working Paper Series 1601; Bristol Centre for Economics and Finance: Bristol, UK, 2019. [Google Scholar]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, V.; Williams, K.; Perlow, E.; Hu, Y.J.; Ahern, S.; Said, J.M.; Karanatsios, B.; Hopper, J.L.; McNeil, J.J.; Donnan, L.; et al. Enhancing Value and Uptake for Whole-Population Cohorts of Children and Parents: Methods to Integrate Registries into the Generation Victoria Cohort. Children 2021, 8, 285. https://doi.org/10.3390/children8040285
Sung V, Williams K, Perlow E, Hu YJ, Ahern S, Said JM, Karanatsios B, Hopper JL, McNeil JJ, Donnan L, et al. Enhancing Value and Uptake for Whole-Population Cohorts of Children and Parents: Methods to Integrate Registries into the Generation Victoria Cohort. Children. 2021; 8(4):285. https://doi.org/10.3390/children8040285
Chicago/Turabian StyleSung, Valerie, Katrina Williams, Ella Perlow, Yanhong J. Hu, Susannah Ahern, Joanne M. Said, Bill Karanatsios, John L. Hopper, John J. McNeil, Leo Donnan, and et al. 2021. "Enhancing Value and Uptake for Whole-Population Cohorts of Children and Parents: Methods to Integrate Registries into the Generation Victoria Cohort" Children 8, no. 4: 285. https://doi.org/10.3390/children8040285
APA StyleSung, V., Williams, K., Perlow, E., Hu, Y. J., Ahern, S., Said, J. M., Karanatsios, B., Hopper, J. L., McNeil, J. J., Donnan, L., Goldfeld, S., & Wake, M. (2021). Enhancing Value and Uptake for Whole-Population Cohorts of Children and Parents: Methods to Integrate Registries into the Generation Victoria Cohort. Children, 8(4), 285. https://doi.org/10.3390/children8040285






