Antenatal Corticosteroids: Extending the Practice for Late-Preterm and Scheduled Early-Term Deliveries?
Abstract
1. Introduction
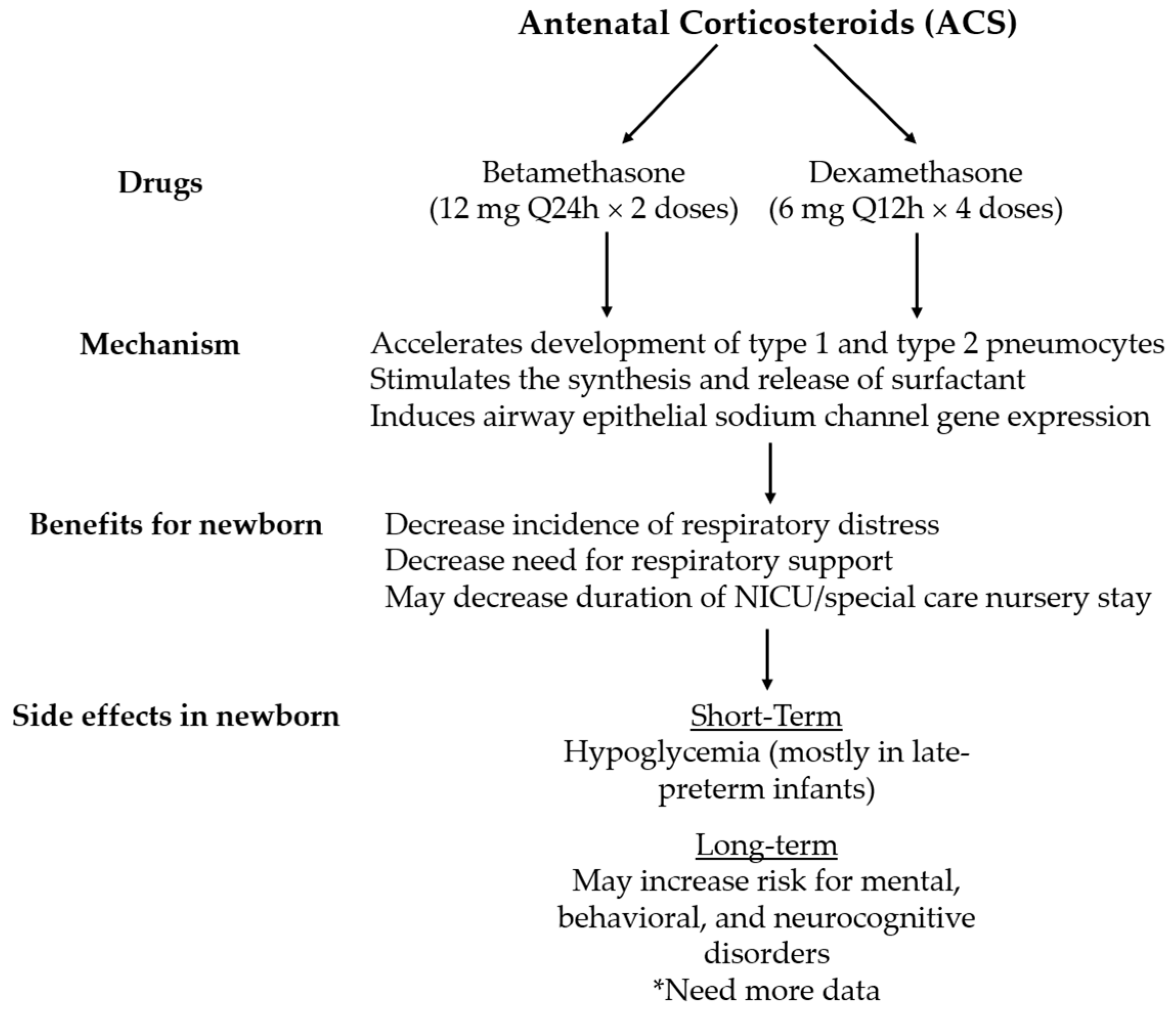
2. Antenatal Corticosteroid Agents and Current Recommendations
3. Antenatal Corticosteroids for Late Preterm Delivery
4. Antenatal Corticosteroids for Early Term Elective Caesarean Delivery
5. Short- and Long-Term Effects
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACOG | American College of Obstetricians and Gynecologists |
| ALPS | Antenatal Betamethasone for Women at Risk for Late Preterm Delivery |
| ACS | Antenatal corticosteroids |
| ASTECS | Antenatal Steroids for Term Elective Caesarean Section |
| CI | Confidence interval |
| CPAP | Continuous positive airway pressure |
| HR | Hazard ratio |
| IUGR | Intrauterine growth restriction |
| IVH | Intraventricular hemorrhage |
| NC | Nasal cannula |
| NICU | Neonatal Intensive Care Unit |
| NNH | Number needed to harm |
| RR | Relative risk |
| RDS | Respiratory distress |
| SMFM | Society of Maternal Fetal Medicine |
| SDQ | Strengths and difficulties questionnaire |
| TTN | Transient tachypnea of the newborn |
References
- Warren, J.B.; Anderson, J.M. Core Concepts: Respiratory Distress Syndrome. NeoReviews 2009, 10, e351–e361. [Google Scholar] [CrossRef]
- Anadkat, J.S.; Kuzniewicz, M.W.; Chaudhari, B.P.; Cole, F.S.; Hamvas, A. Increased risk for respiratory distress among white, male, late preterm and term infants. J. Perinatol. 2012, 32, 780–785. [Google Scholar] [CrossRef]
- McPherson, C.; Wambach, J.A. Prevention and Treatment of Respiratory Distress Syndrome in Preterm Neonates. Neonatal Netw. 2018, 37, 169–177. [Google Scholar] [CrossRef]
- Hibbard, J.U.; Wilkins, I.; Sun, L.; Gregory, K.; Haberman, S.; Hoffman, M.; Kominiarek, M.A.; Reddy, U.; Bailit, J. Respiratory Morbidity in Late Preterm Births. JAMA 2010, 304, 419–425. [Google Scholar] [CrossRef][Green Version]
- Shah, P.S.; Shah, V.; Ye, X.Y.; Lee, S.K.; Jefferies, A.L.; Bassil, K.L.; Network, A.T.C.N. Impact of Late Preterm and Early Term Infants on Canadian Neonatal Intensive Care Units. Am. J. Perinatol. 2013, 31, 269–278. [Google Scholar] [CrossRef]
- Riyami, N.; Hadhrami, A.; Lawati, T.; Pillai, S.; Abdellatif, M.; Jaju, S. Respiratory Distress Syndrome in Neonates Delivered at Term-gestation by Elective Cesarean Section at Tertiary Care Hospital in Oman. Oman Med. J. 2020, 35, e133. [Google Scholar] [CrossRef]
- Engle, W.A.; Tomashek, K.M.; Wallman, C.; the Committee on Fetus and Newborn. “Late-Preterm” Infants: A Population at Risk. Pediatrics 2007, 120, 1390–1401. [Google Scholar] [CrossRef]
- Guglani, L.; Lakshminrusimha, S.; Ryan, R.M. Transient Tachypnea of the Newborn. Pediatr. Rev. 2008, 29, e59–e65. [Google Scholar] [CrossRef]
- Mitanchez, D. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J. Diabetes 2015, 6, 734–743. [Google Scholar] [CrossRef]
- Bourbon, J.R.; Farrell, P.M. Fetal Lung Development in the Diabetic Pregnancy. Pediatr. Res. 1985, 19, 253–267. [Google Scholar] [CrossRef][Green Version]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2020, 12, CD004454. [Google Scholar] [CrossRef] [PubMed]
- O’Brodovich, H.M. Immature epithelial Na+ channel expression is one of the pathogenetic mechanisms leading to human neonatal respiratory distress syndrome. Proc. Assoc. Am. Physicians 1996, 108, 345–355. [Google Scholar] [PubMed]
- Null, N. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am. J. Obstet. Gynecol. 2016, 215, B13–B15. [Google Scholar] [CrossRef]
- Ballard, P.L.; Ballard, R.A. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am. J. Obstet. Gynecol. 1995, 173, 254–262. [Google Scholar] [CrossRef]
- Grier, D.G.; Halliday, H.L. Effects of Glucocorticoids on Fetal and Neonatal Lung Development. Treat. Respir. Med. 2004, 3, 295–306. [Google Scholar] [CrossRef]
- WHO. Recommendations on Interventions to Improve Preterm Birth Outcomes. 2015. Available online: https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/preterm-birth-guideline/en/ (accessed on 26 January 2021).
- Committee Opinion No. 713 Summary: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet. Gynecol. 2017, 130, 493–494. [CrossRef]
- Crane, J.; Armson, A.; Brunner, M. Antenatal Corticosteroid Therapy for Fetal Maturation. Pediatrics 2017, 140, e20172082. [Google Scholar] [CrossRef]
- Information NCfB. PubChem Compound Summary for CID 9782, Betamethasone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Betamethasone (accessed on 15 August 2020).
- Information NCfB. PubChem Compound Summary for CID 5743, Dexamethasone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dexamethasone (accessed on 15 August 2020).
- Roberts, D.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2006, 2006, CD004454. [Google Scholar] [CrossRef]
- Sotiriadis, A.; Tsiami, A.; Papatheodorou, S.; Baschat, A.A.; Sarafidis, K.; Makrydimas, G. Neurodevelopmental Outcome After a Single Course of Antenatal Steroids in Children Born Preterm. Obstet. Gynecol. 2015, 125, 1385–1396. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Gagliardi, D.; Bain, E.; Middleton, P.; Crowther, C. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2013, 2013, CD006764. [Google Scholar] [CrossRef]
- Lee, B.H.; Stoll, B.J.; McDonald, S.A.; Higgins, R.D. Neurodevelopmental Outcomes of Extremely Low Birth Weight Infants Exposed Prenatally to Dexamethasone Versus Betamethasone. Pediatrics 2008, 121, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Antenatal Corticosteroid Clinical Practice Guidelines Panel. In Antenatal Corticosteroids Given to Women Prior to Birth to Improve Fetal, Infant, Child and Adult Health: Clinical Practice Guidelines; Liggins Institute, The University of Auckland: Auckland, New Zealand, 2015.
- Skoll, A.; Boutin, A.; Bujold, E.; Burrows, J.; Crane, J.; Geary, M.; Jain, V.; Lacaze-Masmonteil, T.; Liauw, J.; Mundle, W.; et al. No. 364-Antenatal Corticosteroid Therapy for Improving Neonatal Outcomes. J. Obstet. Gynaecol. Can. 2018, 40, 1219–1239. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi-Bannerman, C.; Thom, E.A.; Blackwell, S.C.; Tita, A.T.; Reddy, U.M.; Saade, G.R.; Rouse, D.J.; McKenna, D.S.; Clark, E.A.; Thorp, J.M.; et al. Antenatal Betamethasone for Women at Risk for Late Preterm Delivery. N. Engl. J. Med. 2016, 374, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Martin, J.; Osterman, M.J.K. Describing the Increase in Preterm Births in the United States, 2014–2016. NCHS Data Brief 2018, 312, 1–8. [Google Scholar]
- Harris, D.L.; Weston, P.J.; Harding, J.E. Incidence of Neonatal Hypoglycemia in Babies Identified as at Risk. J. Pediatr. 2012, 161, 787–791. [Google Scholar] [CrossRef]
- Morrison, J.J.; Rennie, J.M.; Milton, P.J. Neonatal respiratory morbidity and mode of delivery at term: Influence of timing of elective caesarean section. BJOG Int. J. Obstet. Gynaecol. 1995, 102, 101–106. [Google Scholar] [CrossRef]
- Zanardo, V.; Simbi, A.K.; Franzoi, M.; Soldá, G.; Salvadori, A.; Trevisanuto, D. Neonatal respiratory morbidity risk and mode of delivery at term: Influence of timing of elective caesarean delivery. Acta Paediatr. 2004, 93, 643–647. [Google Scholar] [CrossRef]
- Hansen, A.K.; Wisborg, K.; Uldbjerg, N.; Henriksen, T.B. Elective caesarean section and respiratory morbidity in the term and near-term neonate. Acta Obstet. Et Gynecol. Scand. 2007, 86, 389–394. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.; Driscoll, A.K. Births: Final Data for 2018. Natl. Vital Stat. Rep. 2018, 68, 13. [Google Scholar]
- Stutchfield, P.; Whitaker, R.; Russell, I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: Pragmatic randomised trial. BMJ 2005, 331, 662. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.R.; Ahmed, W.A.S.; Mohammed, T.Y. Antenatal steroids at 37 weeks, does it reduce neonatal respiratory morbidity? A randomized trial. J. Matern. Neonatal Med. 2014, 28, 1486–1490. [Google Scholar] [CrossRef]
- Nada, A.; Shafeek, M.; El Maraghy, M.; Nageeb, A.; El Din, A.S.; Awad, M. Antenatal corticosteroid administration before elective caesarean section at term to prevent neonatal respiratory morbidity: A randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 88–91. [Google Scholar] [CrossRef]
- Gyamfi-Bannerman, C.; Zupancic, J.A.F.; Sandoval, G.; Grobman, W.A.; Blackwell, S.C.; Tita, A.T.N.; Reddy, U.M.; Jain, L.; Saade, G.R.; Rouse, D.J.; et al. Cost-effectiveness of Antenatal Corticosteroid Therapy vs No Therapy in Women at Risk of Late Preterm Delivery. JAMA Pediatr. 2019, 173, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Yellanthoor, R.B.; Ramdas, V. Frequency and Intensive Care Related Risk Factors of Pneumothorax in Ventilated Neonates. Pulm. Med. 2014, 2014, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Räikkönen, K.; Gissler, M.; Kajantie, E. Associations Between Maternal Antenatal Corticosteroid Treatment and Mental and Behavioral Disorders in Children. JAMA 2020, 323, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Asztalos, E.; Murphy, K.; Zaltz, A.; Redelmeier, D.; Shah, B.R.; Barrett, J. Neurodevelopmental disorders among term infants exposed to antenatal corticosteroids during pregnancy: A population-based study. BMJ Open 2019, 9, e031197. [Google Scholar] [CrossRef] [PubMed]
- Stutchfield, P.R.; Whitaker, R.; Gliddon, A.; Hobson, L.; Kotecha, S.; Doull, I.J.M. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F195–F200. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.; Alberti, C.; Ursino, M.; Baud, O.; Aupiais, C.; for the BETADOSE study group and the GROG (Groupe de Recherche en Gynécologie Obstétrique). Full versus half dose of antenatal betamethasone to prevent severe neonatal respiratory distress syndrome associated with preterm birth: Study protocol for a randomised, multicenter, double blind, placebo-controlled, non-inferiority trial (BETADOSE). BMC Pregnancy Childbirth 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Gilbert, W.M.; Danielsen, B. Pregnancy outcomes associated with intrauterine growth restriction. Am. J. Obstet. Gynecol. 2003, 188, 1596–1601. [Google Scholar] [CrossRef]
| Population | Antenatal steroid | Indication (s) | Research Gaps |
|---|---|---|---|
| Late-Preterm (34 0/7–36/7 weeks) | Betamethasone or dexamethasone (IM, 24 mg total) |
|
|
| Early Term (37 0/7–38 6/7 weeks) |
| ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Htun, Z.T.; Hairston, J.C.; Gyamfi-Bannerman, C.; Marasch, J.; Duarte Ribeiro, A.P. Antenatal Corticosteroids: Extending the Practice for Late-Preterm and Scheduled Early-Term Deliveries? Children 2021, 8, 272. https://doi.org/10.3390/children8040272
Htun ZT, Hairston JC, Gyamfi-Bannerman C, Marasch J, Duarte Ribeiro AP. Antenatal Corticosteroids: Extending the Practice for Late-Preterm and Scheduled Early-Term Deliveries? Children. 2021; 8(4):272. https://doi.org/10.3390/children8040272
Chicago/Turabian StyleHtun, Zeyar T., Jacqueline C. Hairston, Cynthia Gyamfi-Bannerman, Jaime Marasch, and Ana Paula Duarte Ribeiro. 2021. "Antenatal Corticosteroids: Extending the Practice for Late-Preterm and Scheduled Early-Term Deliveries?" Children 8, no. 4: 272. https://doi.org/10.3390/children8040272
APA StyleHtun, Z. T., Hairston, J. C., Gyamfi-Bannerman, C., Marasch, J., & Duarte Ribeiro, A. P. (2021). Antenatal Corticosteroids: Extending the Practice for Late-Preterm and Scheduled Early-Term Deliveries? Children, 8(4), 272. https://doi.org/10.3390/children8040272






