Characteristics of Giant Cell Tumor of the Bone in Pediatric Patients: Our 18-Year, Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turcotte, R.E. Giant cell tumor of bone. Orthop. Clin. 2006, 37, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Board, W. Soft Tissue and Bone Tumours: International Agency for Research on Cancer; IARC: Lyon, France, 2020. [Google Scholar]
- Schütte, H.E.; Taconis, W.K. Giant cell tumor in children and adolescents. Skelet. Radiol. 1993, 22, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Picci, P.; Manfrini, M.; Zucchi, V.; Gherlinzoni, F.; Rock, M.; Bertoni, F.; Neff, J.R. Giant-cell tumor of bone in skeletally immature patients. J. Bone Jt. Surg. Am. Vol. 1983, 65, 486–490. [Google Scholar] [CrossRef]
- Kransdorf, M.J.; Sweet, D.E.; Buetow, P.C.; A Giudici, M.; Moser, R.P. Giant cell tumor in skeletally immature patients. Radiology 1992, 184, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Agarwal, M.G.; Shah, M.; Jambhekar, N.A.; Anchan, C.; Behle, S. Giant cell tumor of bone in children and adolescents. J. Pediatr. Orthop. 2007, 27, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Hoch, B.; Inwards, C.; Sundaram, M.; Rosenberg, A.E. Multicentric giant cell tumor of bone. Clinicopathologic analysis of thirty cases. J. Bone Jt. Surg. Am. Vol. 2006, 88, 1998–2008. [Google Scholar]
- Campanacci, M.; Baldini, N.; Boriani, S.; Sudanese, A. Giant-cell tumor of bone. J. Bone Jt. Surg. Am. Vol. 1987, 69, 106–114. [Google Scholar] [CrossRef]
- Unni, K.K.; Inwards, C.Y.; Research, M. Dahlin’s Bone Tumors: General Aspects and Data on 10,165 Cases: Wollters Kluwer Health; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Huvos, A.G. Bone Tumors: Diagnosis, Treatment, and Prognosis; Saunders: Philadelphia, PA, USA, 1991. [Google Scholar]
- Bridge, J.A.; Neff, J.R.; Mouron, B.J. Giant cell tumor of bone. Chromosomal analysis of 48 specimens and review of the literature. Cancer Genet. Cytogenet. 1992, 58, 2–13. [Google Scholar] [CrossRef]
- Campanacci, M.; Giunti, A.; Olmi, R. Metaphyseal and diaphyseal localization of giant cell tumors. Chir. Organi Mov. 1975, 62, 29–34. [Google Scholar] [PubMed]
- Goldenberg, R.R.; Campbell, C.J.; Bonfiglio, M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J. Bone Jt. Surg. Am. Vol. 1970, 52, 619–664. [Google Scholar] [CrossRef]
- Boyce, A.M. Denosumab: An Emerging Therapy in Pediatric Bone Disorders. Curr. Osteoporos. Rep. 2017, 15, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, D. Caldwell lecture. Giant Cell Tumor: Highlights of 407 Cases. AJR Am. J. Roentgenol. 1985, 144, 955–960. [Google Scholar] [CrossRef]
- Persson, B.M.; Ekelund, L.; Lövdahl, R.; Gunterberg, B. Favourable results of acrylic cementation for giant cell tumors. Acta Orthop. Scand. 1984, 55, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Gitelis, S.; Mallin, B.A.; Piasecki, P.; Turner, F. Intralesional excision compared with en bloc resection for giant-cell tumors of bone. J. Bone Jt. Surg. Am. Vol. 1993, 75, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.J.; Springfield, D.S.; Motwani, H.K.; Ready, J.E.; Gebhardt, M.C.; Mankin, H.J. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J. Bone Jt. Surg. Am. Vol. 1994, 76, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- von Steyern, F.V.; Bauer, H.C.F.; Trovik, C.; Kivioja, A.; Bergh, P.; Jrgensen, P.H.; Foller̊s, G.; Rydholm, A. Treatment of local recurrences of giant cell tumour in long bones after curettage and cementing. A Scandinavian Sarcoma Group study. J. Bone Jt. Surg. Br. Vol. 2006, 88, 531–535. [Google Scholar] [CrossRef] [Green Version]
- Hutter, R.V.P.; Worcester, J.N.; Francis, K.C.; Foote, F.W., Jr.; Stewart, F.W. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer 1962, 15, 653–690. [Google Scholar] [CrossRef]
- McGrath, P.J. Giant-cell tumour of bone: An analysis of fifty-two cases. J. Bone Jt. Surg. Br. Vol. 1972, 54, 216–229. [Google Scholar] [CrossRef]
- Dahlin, D.C.; Cupps, R.E.; Johnson, E.W. Giant-cell tumor: A study of 195 cases. Cancer 1970, 25, 1061–1070. [Google Scholar] [CrossRef]
- Jaffe, H.L. Tumors and Tumorous Conditions of the Bones and Joints; Lea & Febiger: Philadelphia, PA, USA, 1958. [Google Scholar]
- Conti, A.; Rodriguez, G.C.; Chiechi, A.; Blazquez, R.M.D.; Barbado, V.; Krènacs, T.; Novello, C.; Pazzaglia, L.; Quattrini, I.; Zanella, L.; et al. Identification of potential biomarkers for giant cell tumor of bone using comparative proteomics analysis. Am. J. Pathol. 2011, 178, 88–97. [Google Scholar] [CrossRef]
- Ambrosi, F.; Righi, A.; Benini, S.; Magagnoli, G.; Chiaramonte, I.; Manfrini, M.; Gasbarrini, A.; Frisoni, T.; Gambarotti, M. Giant Cell Tumor of Bone in Patients under 16 Years Old: A Single-Institution Case Series. Cancers 2021, 13, 2585. [Google Scholar] [CrossRef]
- Kim, Y.; Niazami, S.; Goto, H.; Lee, F.Y. Modern interpretation of giant cell tumor of bone: Predominantly osteoclastogenic stromal tumor. Clin. Orthop. Surg. 2012, 4, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
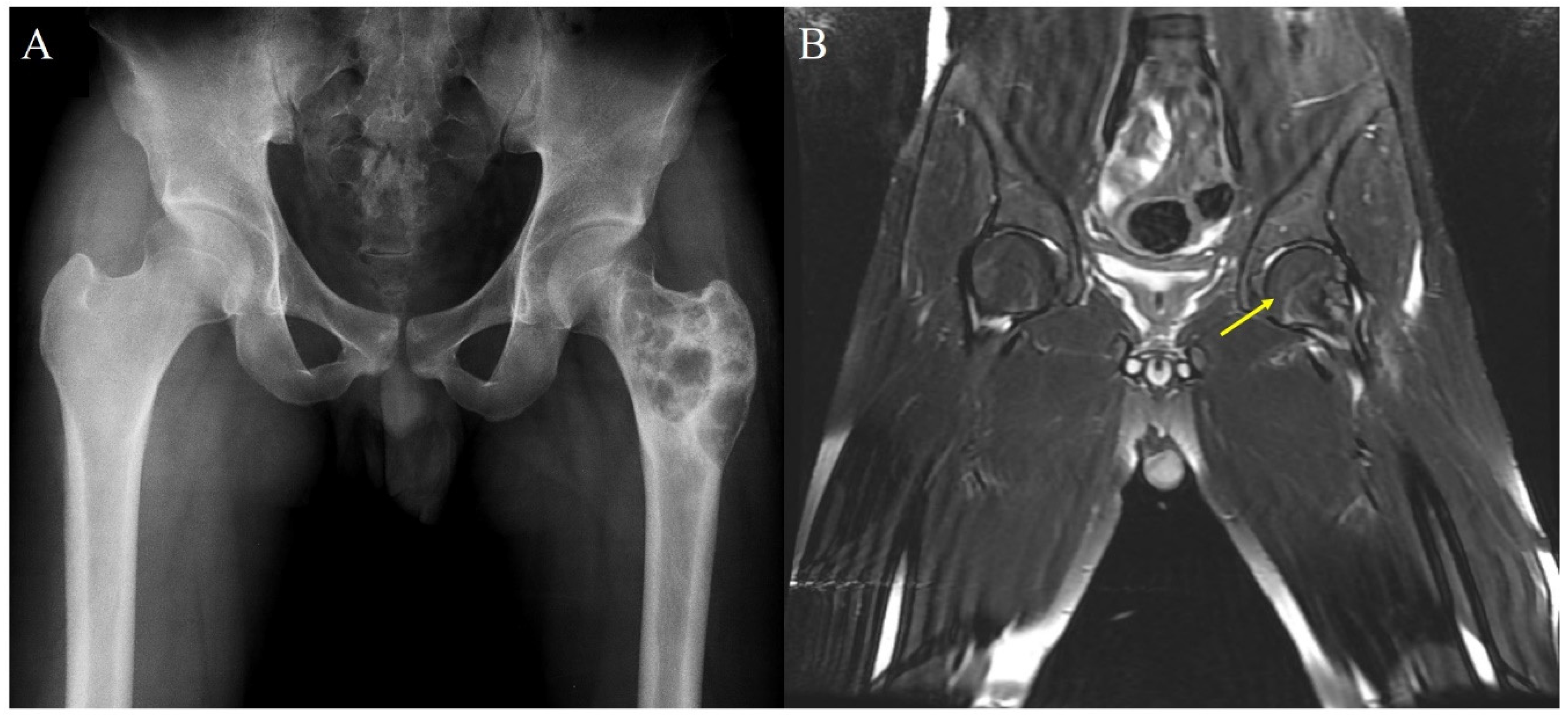
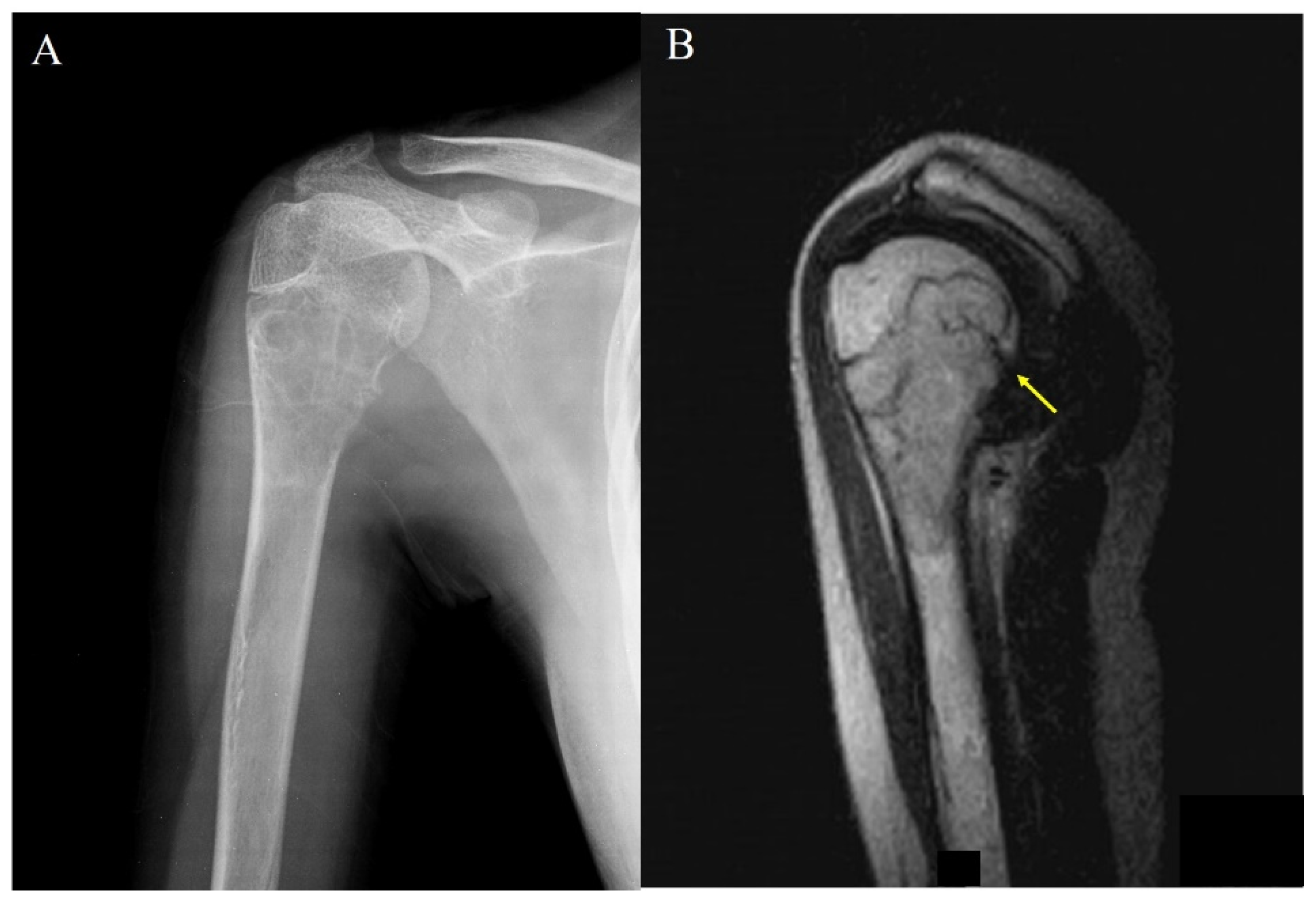
| Case | Age (y) | Sex | Bone | Region | P/R | Grade | Surgery | Follow-Up (mo) | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | F | Prox Humerus | M | P | 3 | Curettage + auto B/G | 49 | Incidentally detect, fracture history, LR 14 mo |
| 1 | 17 | F | Distal femur + Prox Tibia | EM (Femur) + M (Femur, Tibia) | P | 2 | Curettage + Excision (LR) + Prosthesis (LR) | 239 | LR 11 mo |
| 2 | 18 | M | Prox Humerus | EM | P | 2 | Curettage | 101 | 5 months pain |
| 3 | 19 | M | Distal Ulnar | EM | P | 2 | Curettage + Excision + allo B/G | 48 | 1 month pain, LR 2 times (6, 8 mo) |
| 4 | 19 | M | Intermediate Cuneiform | - | P | 2 | Curettage + allo B/G | 31 | 1 year pain |
| 5 | 15 | F | 4,5 Metatarsal | - | P | 2 | Curettage | 256 | 3 months pain |
| 6 | 14 | M | Distal femur + Prox Tibia (Rt) Tibia + Prox Fibular (Lt) | EM (Femur, Tibia, Fibular) | P | 1 | Curettage + Excision (LR) + Prosthesis (LR) | 57 | 1 week pain, LR 28 mo |
| 7 | 15 | F | Prox Humerus | EM | P | 3 | Curettage | 141 | Incidentally detect, pathologic fracture |
| 8 | 18 | M | Prox Femur | M | P | 3 | Curettage | 114 | Incidentally detect, 2 lung metastasis |
| 9 | 16 | F | Dist Radius | EM | P | 3 | Curettage | 85 | 2 weeks pain, pathologic fracture, LR 23 mo |
| 10 | 12 | M | Prox Tibia | M | P | 2 | Curettage | 69 | 10 months pain |
| 11 | 16 | F | Dist Femur | EM | P | 2 | Curettage | 41 | 1 month pain |
| 12 | 19 | M | Dist Fibular | EM | P | 3 | Curettage | 53 | 6 months pain |
| 13 | 18 | M | Prox Humerus | M | P | 1 | Curettage | 36 | 1 year pain |
| 14 | 8 | M | Finger Phalanx | - | P | 1 | Curettage + allo B/G | 24 | 1 month pain |
| Total of 14 Patients, 21 Lesions | |
|---|---|
| Anatomical site (Lesions) | |
| Adjacent to knee joint | 8 (38.0%) |
| Distal femur | 4 (19.0%) |
| Proximal tibia | 4 (19.0%) |
| Proximal fibula | 1 (4.7%) |
| Proximal humerus | 4 (19.0%) |
| Metatarsal bone | 2 (9.5%) |
| Proximal femur | 1 (4.7%) |
| Distal fibula | 1 (4.7%) |
| Distal ulna | 1 (4.7%) |
| Intermediate cuneiform | 2 (9.5%) |
| Phalanx (finger) | 1 (4.7%) |
| Physeal closure (Patients) | |
| Open physis | 11 (78.5%) |
| Closed physis | 1 (7.1%) |
| N/A (d/t short bone) | 2 (14.2%) |
| Location (Lesions) | |
| Physeal involvement | |
| Metaphysis | 6 (28.5%) |
| Epi-metaphysis | 11 (52.3%) |
| Epiphysis | 4 (19.0%) |
| N/A (d/t short bone) | 2 (14.2%) |
| Incidence | Primary Patient Number | Most Common Site | Most Common Location | Recurrence | |
|---|---|---|---|---|---|
| Picci et al. [3] | 1.7% | 6 | Distal femur (4 lesions, 67%) | Epi-metaphysis (5 lesions, 83%) | N/A |
| Puri et al. [5] | 6% | 11 | Distal femur (5 lesions, 29%) | Epi-metaphysis (13 lesions, 76%) | 20% |
| Kransdorf et al. [4] | 5.7% | 50 | Proximal tibia (9 lesions, 18%) | Metaphysis (48 lesions, 96%) | N/A |
| Schutte et al. [2] | 10.6% | 49 | Proximal tibia (10 lesions, 20%) | Epi-metaphysis (27 lesions, 75%) | 8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-J.; Kim, S.; Choi, D.-W.; Lim, G.-H.; Jung, S.-T. Characteristics of Giant Cell Tumor of the Bone in Pediatric Patients: Our 18-Year, Single-Center Experience. Children 2021, 8, 1157. https://doi.org/10.3390/children8121157
Kim W-J, Kim S, Choi D-W, Lim G-H, Jung S-T. Characteristics of Giant Cell Tumor of the Bone in Pediatric Patients: Our 18-Year, Single-Center Experience. Children. 2021; 8(12):1157. https://doi.org/10.3390/children8121157
Chicago/Turabian StyleKim, Woo-Jong, Sungmin Kim, Dae-Woong Choi, Gil-Hwan Lim, and Sung-Taek Jung. 2021. "Characteristics of Giant Cell Tumor of the Bone in Pediatric Patients: Our 18-Year, Single-Center Experience" Children 8, no. 12: 1157. https://doi.org/10.3390/children8121157
APA StyleKim, W.-J., Kim, S., Choi, D.-W., Lim, G.-H., & Jung, S.-T. (2021). Characteristics of Giant Cell Tumor of the Bone in Pediatric Patients: Our 18-Year, Single-Center Experience. Children, 8(12), 1157. https://doi.org/10.3390/children8121157






