Physical Activity Promotion Programmes in Childhood Cancer Patients and Their Impact on Fatigue and Pain: A Systematic Review
Abstract
:1. Introduction
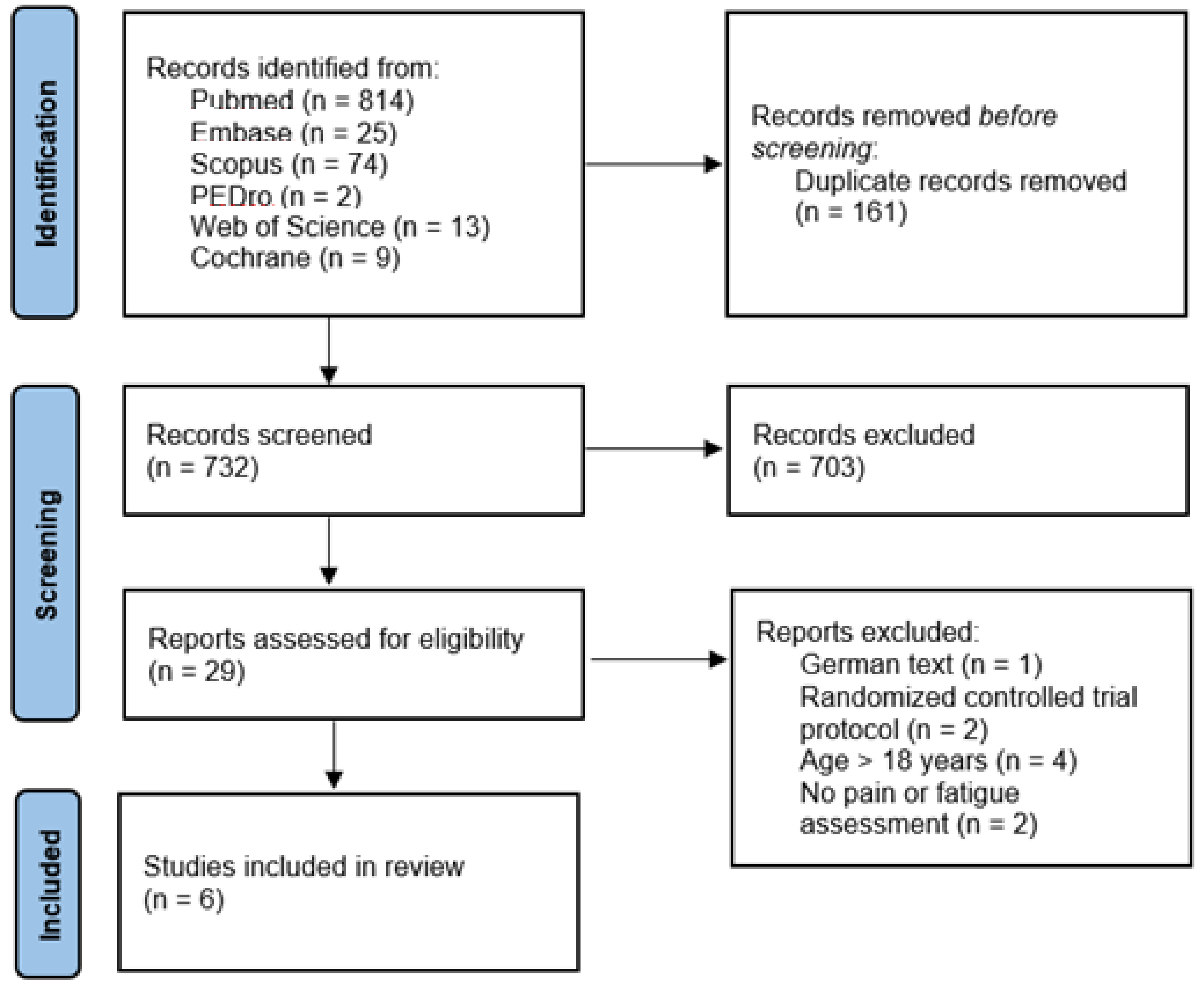
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Procedure
2.4. Methodological Quality Assessment
3. Results
4. Discussion
4.1. Effects on Pain Symptoms
4.2. Effects on Fatigue
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO World Health Organization. Available online: https://www.who.int/cancer/media/news/Childhood_cancer_day/en/ (accessed on 14 March 2021).
- Filbin, M.; Monje, M. Developmental Origins and Emerging Therapeutic Opportunities for Childhood Cancer. Nat. Med. 2019, 25, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Bertruccio, P.; Alicandro, G.; Malvezzi, M.; Carioli, G.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. Childhood Cancer Mortality Trends in Europe, 1990–2017, with Focus on Geographic Differences. Cancer Epidemiol. 2020, 67, 101768. [Google Scholar] [CrossRef]
- Berlanga, P.; Vicente, M.L.; Cañete, A.; Alberich, C.; Castel, V. Cancer in Children and Adolescents in Spain: Incidence, Treatment Setting and Provider Specialty. Clin. Transl. Oncol. 2016, 18, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Schüz, J.; Roman, E. Childhood Cancer: A Global Perspective. Cancer Epidemiol. 2021, 7, 101878. [Google Scholar] [CrossRef]
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Falck Winther, J. Childhood Cancer: Survival, Treatment Modalities, Late Effects and Improvements over Time. Cancer Epidemiol. 2021, 71, 101733. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-W.; Xu, S.-M.; An, Q.; Wang, P. A Review of Risk Factors for Childhood Leukemia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3760–3764. [Google Scholar]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and Adolescent Cancer Statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef]
- Platschek, A.-M.; Kehe, L.; Abeln, V.; Berthold, F.; Simon, T.; Strüder, H.K. Computer-Based Exercise Program: Effects of a 12-Week Intervention on Mood and Fatigue in Pediatric Patients with Cancer. Clin. J. Oncol. Nurs. 2017, 21, E280–E286. [Google Scholar] [CrossRef]
- Atkinson, M.; Murnane, A.; Goddard, T.; Pendergrast, C.; Rogers, P.; Manudhane, R.; Osborn, M. A Randomized Controlled Trial of a Structured Exercise Interventon after the Completion of Acute Cancer Treatment in Adolescents and Young Adults. Pediatr. Blood Cancer 2020, 68, e28751. [Google Scholar] [CrossRef] [PubMed]
- Landier, W.; Armenian, S.; Bhatia, S. Late Effects of Childhood Cancer and Its Treatment. Pediatr. Clin. N. Am. 2015, 62, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.S.; Santana-Sosa, E.; Santos-Lozano, A.; Baño-Rodrigo, A.; Valenzuela, P.L.; Rincón-Castañedo, C.; Fernández-Moreno, D.; González Vicent, M.; Pérez-Somarriba, M.; Madero, L.; et al. Intrahospital Exercise Benefits in Childhood Cancer: A Prospective Cohort Study. Scand. J. Med. Sports 2020, 30, 126–134. [Google Scholar] [CrossRef]
- Scott, J.M.; Li, N.; Liu, Q.; Yasui, Y.; Leisenring, W.; Nathan, P.C.; Gibson, T.; Armenian, S.H.; Nilsen, T.S.; Oeffinger, K.C.; et al. Association of Exercise with Mortality in Adult Survivors of Childhood Cancer. JAMA Oncol. 2018, 4, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Baime, J.; Swisher, A.K.; Winters-Stone, K.M.; Welsh, J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005–2017). PM & R 2017, 9, S347–S384. [Google Scholar] [CrossRef]
- Braam, K.I.; van der Torre, P.; Takken, T.; Veening, M.M.; van Dulmen-den Broeder, E.; Kaspers, G.J.L. Physical Exercise Training Interventions for Children and Young Adults during and after Treatment for Childhood Cancer. Cochrane Database Syst. Rev. 2016, 3, 1–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diorio, C.; Celis Ekstrand, A.; Hesser, T.; O’Sullivan, C.; Lee, M.; Schechter, T.; Sung, L. Development of an Individualized Yoga Intervention to Adress Fatigue in Hospitalized Children Undergoing Intensive Chemotherapy. Integr. Cancer Ther. 2016, 15, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearney, J.A.; Salley, C.G.; Muriel, A.C. Standards of Psychosocial Care for Parents of Children with Cancer. Pediatr. Blood Cancer 2015, 62, S632–S683. [Google Scholar] [CrossRef] [Green Version]
- Darcy, L.; Enskär, K.; Granlund, M.; Simeonsson, R.J.; Peterson, C.; Björk, M. Health and Functioning in the Everyday Lives of Young Children with Cancer: Documenting with the International Classification of Functioning, Disability and Health—Children and Youth (ICF-CY). Child. Care Health Dev. 2015, 41, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kauhanen, L.; Järvelä, L.; Lähteenmäki, P.M.; Arola, M.; Heinonen, O.J.; Axelin, A.; Lilius, J.; Vahlberg, T.; Salanterä, S. Active Video Games to Promote Physical Activity in Children with Cancer: A Randomized Clinical Trial with Follow-Up. BMC Pediatr. 2014, 14, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, R.R.; Gupta, A.; Mayer, E.A.; Zeltzer, L.K. Chronic Pain in Children: Structural and Resting-State Functional Brain Imaging within a Developmental Perspective. Pediatr. Res. 2020, 88, 840–849. [Google Scholar] [CrossRef]
- Conn, S.; Curtain, S. Health Coaching as a Lifestyle Medicine Process in Primary Care. Aust. J. Gen. Pract. 2019, 48, 677–680. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, C.G.; Sherrington, C.; Elkins, M.; Herbert, R.D.; Moseley, A.M. Challenges for Evidence-Based Physical Therapy: Accessing and Interpreting High-Quality Evidence on Therapy. Phys. Ther. 2004, 84, 644–654. [Google Scholar] [CrossRef] [Green Version]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.C.; Ho, K.Y.; Lam, K.K.W.; Lam, H.S.; Chui, S.Y.; Chan, G.C.F.; Cheung, A.T.; Ho, L.L.K.; Chung, O.K. Adventure-Based Training to Promote Physical Activity and Reduce Fatigue among Childhood Cancer Survivors: A Randomized Controlled Trial. Int. J. Nurs. Stud. 2018, 83, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Baker, K.; Moreno, M.; Whitlock, K.; Abbey-Lambertz, M.; Waite, A.; Colburn, T.; Chow, E. A Fitbit and Facebook MHealth Intervention for Promoting Physical Activity among Adolescent and Young Adult Childhood Cancer Survivors: A Pilot Study. Pediatr. Blood Cancer 2017, 64, e26660. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Zhu, L.; Kaste, S.; Srivastava, K.; Barnes, L.; Nathan, P.; Wells, R.; Ness, K. Modifying Bone Mineral Density, Physical Function, and Quality of Life in Children with Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2018, 65, e26929. [Google Scholar] [CrossRef]
- Lam, K.; Li, W.; Chung, O.; Ho, K.; Chiu, S.; Lam, H.; Chan, G. An Integrated Experiential Training Programme with Coaching to Promote Physical Activity and Reduce Fatigue among Childhood Cancer Survivors: A Randomized Controlled Trial. Patient Educ. Couns. 2018, 101, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Hooke, M.; Hoelscher, A.; Tanner, L.; Langevin, M.; Bronas, U.; Maciej, A.; Mathiason, M. Kids Are Moving: A Physical Activity Program for Children with Cancer. J. Pediatr. Oncol. Nurs. 2019, 36, 379–389. [Google Scholar] [CrossRef]
- Ovans, J.; Hooke, M.; Bendel, A.; Tanner, L. Physical Therapist Coaching to Improve Physical Activity in Children with Brain Tumors: A Pilot Study. Pediatr. Phys. Ther. 2018, 30, 310–317. [Google Scholar] [CrossRef]
- McWilliam, R.A. Working with Families of Young Children with Special Needs, 1st ed.; The Guilford Press: New York, NY, USA, 2010; ISBN 1-282-49016-8. [Google Scholar]
- Van Dijk-Lokkart, E.M.; Braam, K.I.; van Dulmen-den Broeder, E.; Kaspers, G.J.L.; Takken, T.; Grootenhuis, M.A.; Streng, I.C.; Bierings, M.; Merks, J.H.; van den Heuvel-Eibrink, M.M.; et al. Effects of a Combined Physical and Psychosocial Intervention Program for Childhood Cancer Patients on Quality of Life and Psychosocial Functioning: Results of the QLIM Randomized Clinical Trial. Psycho Oncol. 2016, 25, 815–822. [Google Scholar] [CrossRef]
| Author and Year | Sample Size | Study Design | Cancer Type | Age | Intervention | PEDro Scale |
|---|---|---|---|---|---|---|
| Cox et al. [27] (2018) | n = 77: - E.G.: 36 - C.G.: 41 | Randomized controlled trial (RCT) | Acute lymphoblastic leukaemia | 4–18 years | E.G.: - 30 min personalised PA routine 5 days/week aimed at strengthening, improvement of ROM, of gross motor function and endurance, together with a PA education programme for patients and their family members through 37 home visits or phone calls by physical therapists. - 43 home visits or phone calls by nurses with strategies aimed at supporting motivation and long-term behavioural changes for patients and their family members. C.G.: - Attention-control home visits or phone calls by physical therapists at the same time interval as the E.G., with recommendations of passive ankle stretching to slow down the limitation of ankle ROM due to the pharmacological treatment with vincristine. | 6/10 |
| Hooke et al. [29] (2019) | n = 30 | Quasi-experimental study with a nonrandomized comparative group design | - Acute lymphoblastic leukaemia (n = 7). - Solid tumours (n = 12). - Lymphoma (n = 11). | 6–18 years | “Kids Are Moving” programme starting the second month of cancer treatment. This programme is based on typical PA for children, such as playing hide and seek or riding a bike, and was implemented in combination with PA coaching in five steps: determining the patients’ current PA level, assessing possible health barriers to PA, determining the stage of change the patients and their caretakers are in, writing the personalised PA prescription with recommendations on frequency and intensity, and providing information on resources. | N/A |
| Lam et al. [28] (2018) | n = 70 - E.G.: 37 - C.G.: 33 | Randomized controlled trial (RCT) | Not specified | 9–18 years | E.G.: - An integrated experiential training programme guided in 28 1-h home visits by student nurses, combined with a coaching programme aimed at increasing the patients’ PA and self-efficacy levels through the setting of new challenging goals and a series of difficult physical activities. PA focused mainly on aerobic, resistance, stretching and relaxation exercise of mild-moderate intensity. C.G.: - Home visits at similar time intervals as the E.G., with placebo interventions which do not have any specific effects on the outcome measures: chess and card games, providing health advice on the importance of a healthy diet, etc. Both groups: - 15 min health education talk during hospitalisation. - 30 min individual English tutorials during the home visits to promote treatment adherence. | 7/10 |
| Li et al. [25] (2018) | n = 222 - E.G.: 117 - C.G.: 105 | Randomized controlled trial (RCT) | Child cancer survivors (type not specified) | 9–16 years | E.G.: - 4 sessions of an adventure-based training programme (2 weeks–2 months–4 months–6 months after randomisation) with a previous 45 min briefing session including health education components. Each exercise in the training programme was designed with a different objective in mind: improving muscle strength and vital capacity, promoting self-esteem, empowering the patient, … C.G.: - Placebo intervention at the same time intervals as the E.G., without any specific effects on the outcome measures: board games, movies, museum visits, … | 7/10 |
| Mendoza et al. [26] (2017) | n = 60 - E.G.: 30 - C.G.: 30 | Pilot randomized controlled trial (RCT) | Child cancer survivors (type not specified) | 14–18 years | E.G.: - A 10-week PA promotion programme through the use of a FitBit Flex®. The research staff set new daily step goals every week based on the mean daily step number of the previous week, and gradually increased their step goal in the following weeks until reaching or maintaining the general step recommendations for adolescents. - Participation in a Facebook group created specifically for this RCT, composed by study participants and moderated by research staff members. This group was dedicated to motivating and reminding patients of their PA target, as well as sharing their personal experiences. Participation in both interventions was voluntary in order to obtain real-world feasibility. C.G.: - Advice on PA for childhood cancer survivors and its importance to their health. The C.G. did not receive an active intervention. | 5/10 |
| Ovans et al. [30] (2018) | n = 15 | Quasi-experimental study | Brain tumours | 7–18 years | A 12-week intervention combining: - The use of a FitBit Flex® with a personalised step goal based on the average steps of the previous days and the daily step recommendations for healthy children. - Coaching by physical therapists every 2–3 weeks (a total number of 5 coaching sessions): count of the average number of steps of the previous days, patients’ progress, identifying strategies to increase patients’ PA level in the coming weeks, possible barriers to PA and how to overcome them. After each coaching session, a new step goal was suggested and programmed into the FitBit Flex®. | N/A |
| Author and Year | Time of Assessment | Outcome Variables | Assessment Tools | Outcomes: Mean (SD) | Conclusions | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | T0 | T1 | T2 | T3 | ||||||
| Cox et al. [27] (2018) | T0: initial assessment (n = 107). T1: 8 weeks (n = 97). T2: 15 weeks (n = 92). T3:135 weeks (n = 77). | Health-related quality of life (includes pain): patients and parents. | Patients: Child Health Questionnaire a (bodily pain) | E.G. | 59.58 (28.36) | 55.58 (25.19) | 60.73 (25.53) | 71.58 (23.31) | Although there were improvements in outcomes over time, there were no statistically significant differences between the two groups | |
| C.G. | 56.38 (26.82) | 65.23 (26.37) | 66.67 (25.05) | 76.34 (22.78) | ||||||
| Parents: Child Health Questionnaire (bodily pain) | E.G. | 46.79 (24.24) | 46.6 (19.14) | 58.37 (19.02) | 71.08 (23.78) | |||||
| C.G. | 49.81 (23) | 50.42 (16.01) | 60.21 (22.55) | 72.38 (25.26) | ||||||
| PA patterns (hours/day) | SenseWear Pro III accelerometer | E.G. | - | 8.45 (9.94) | 5.81 (5.91) | 11.86 (10.06) | ||||
| C.G. | - | 9.32 (12.26) | 7.84 (10.17) | 12.84 (12.80) | ||||||
| Hooke et al. [29] (2019) | T1: 2 months (n = 28). T2: 4 months b (n = 21). T3: 6 months (n = 12). | PA level | Self-report: leisure score index of the GLTEQ | E.G. | 54.9 (10.8) | 60.4 (7.8) | 51.1 (10.2) | No statistically significant differences have been found in the outcomes between both groups of over time. | ||
| Historical C.G b. | - | 49.3 (63.1) | - | |||||||
| Actigraphy c: Actigraph GT3X accelerometer. | E.G. | Steps/day | 4000 | 3500 | 4300 | |||||
| PA minutes/day | 95 | 70 | 70 | |||||||
| % sedentarism/day | 80 | 85 | 85 | |||||||
| Fatigued | FS-Cb and FS-A b | E.G. | Combined group | 53.9 (2.0) | 51.0 (2.0) | 48.7 (2.6) | ||||
| ALL | 59.6 (5.2) | 54.6 (4.3) | - | |||||||
| Lymphoma | 54.3 (2.2) | 47.6 (5.2) | - | |||||||
| Solid tumours | 50.8 (3.8) | 55.0 (2.9) | 58.7 (2.1) | |||||||
| Historical C.Gb. | - | 51.1 (9.1) | - | |||||||
| Lam et al. [28] (2018) | T0: initial assessment (n = 70). T1: 6 months (n = 70). T2: 9 months (n = 70). | Fatigue | Chinese version of the FS-C | E.G. | 49.2 (7.5) | 48.2 (7.2) | 47.6 (7.5) | - | Statistically significant improvement in all outcome variables of the E.G. from T0 to T2. | |
| C.G. | 49.7 (6.9) | 53.7 (7.0) | 54.7 (6.7) | - | ||||||
| PA level | CUHK-PARCY questionnaire | E.G. | 2 (1.2) | - | 4 (2.0) | - | ||||
| C.G. | 2 (1.3) | - | 1.9 (1.3) | - | ||||||
| PA self-efficacy | PA-SE scale | E.G. | 7.8 (2.3) | 8.4 (1.8) | 8.6 (2.0) | - | ||||
| C.G. | 7.7 (2.7) | 6.4 (2.0) | 6.3 (2.2) | - | ||||||
| QOL | Chinese version of the PedsQL questionnaire | E.G. | 63.0 (7.1) | 64.0 (6.0) | 64.7 (6.0) | - | ||||
| C.G. | 62.3 (9.2) | 60.4 (9.0) | 58.0 (8.5) | - | ||||||
| Li et al. [25] (2018) | T0: initial assessment (n = 222). T1: 6 months (n = 222). T2: 12 months (n = 222). | Fatigue | Chinese version of the FS-C | E.G. | 29.4 (4.2) | 26.6 (4.9) | 22.3 (4.2) | - | Statistically significant improvement in all outcome variables of the E.G. from T0 to T2. | |
| C.G. | 29.2 (4.1) | 28.5 (4.2) | 28.9 (4.9) | - | ||||||
| PA level | CUKH-PARCY questionnaire | E.G. | 3.0 (1.2) | 5.2 (1.6) | 6.0 (1.8) | - | ||||
| C.G: | 3.2 (1.1) | 3.3 (1.2) | 3.5 (1.6) | - | ||||||
| PA self-efficacy | PA-SE scale | E.G. | 9.1 (3.4) | 10.5 (3.0) | 11.9 (3.0) | - | ||||
| C.G. | 9.0 (3.1) | 9.1 (3.0) | 9.0 (3.1) | - | ||||||
| QOL | Chinese version of the PedsQL scale | E.G. | 68.6 (11.6) | 70.3 (11.9) | 79.8 (13.2) | - | ||||
| C.G. | 68.4 (11.5) | 68.4 (12.0) | 70.1 (12.8) | - | ||||||
| Mendoza et al. [26] (2017) | T0: initial assessment (n = 59). T1: 8–10 weeks (n = 59). | PA level | Actigraph GT3X+ e accelerometer | PA minutes/day | E.G | - | +4.4 | - | - | No significant differences were found for any of the outcome variables in either of the two study groups. |
| C.G | - | +5 | - | - | ||||||
| Sedentarism | E.G | - | −4.5 | - | - | |||||
| C.G | - | +1 | - | - | ||||||
| Health-related QOL (includes pain) | PedsQL 4.0 Generic Core f scale | E.G. | 79.7 | 79.1 | - | - | ||||
| C.G. | 77.5 | 79.4 | - | - | ||||||
| PedsQL Cancer Module Scores f: bodily pain | E.G. | 73.2 | 69.6 | - | - | |||||
| C.G. | 73.5 | 70.8 | - | - | ||||||
| Ovans et al. [30] (2018) | T0: initial assessment (n = 15). T1: 12 weeks (n = 15). T2: 24 weeks (n = 11). | PA level | Objective: FitBit Flex® (steps/day) | E.G. | 8956 (4589) | 8944 (3022) | 10,141 (3260) | - | Statistically significant improvements in total, general and sleep/rest-related fatigue between T0 and T1, as well as in total and general fatigue between T0 and T2 of the 11 participants left. | |
| Subjective: leisure score index of the GLTEQ f | E.G. | 45 | 52 | 73 | - | |||||
| QOL | PedsQL Generic Core Scale f | E.G. | 84.38 | 85.50 | 91.75 | - | ||||
| Fatigue | PedsQL Multidimensional Fatigue Scale f | E.G. | Tot.F. | 72.22 | 83.33 | - | - | |||
| Gen.F. | 70.83 | 83.33 | 87.50 | |||||||
| S-R. F. | 62.50 | 75.00 | 79.17 | |||||||
| Cog.F. | 79.17 | 83.33 | 87.50 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malysse, C.; Romero-Galisteo, R.P.; Merchán-Baeza, J.A.; Durán-Millán, J.I.; González-Sánchez, M.; Galan-Mercant, A. Physical Activity Promotion Programmes in Childhood Cancer Patients and Their Impact on Fatigue and Pain: A Systematic Review. Children 2021, 8, 1119. https://doi.org/10.3390/children8121119
Malysse C, Romero-Galisteo RP, Merchán-Baeza JA, Durán-Millán JI, González-Sánchez M, Galan-Mercant A. Physical Activity Promotion Programmes in Childhood Cancer Patients and Their Impact on Fatigue and Pain: A Systematic Review. Children. 2021; 8(12):1119. https://doi.org/10.3390/children8121119
Chicago/Turabian StyleMalysse, Catherine, Rita Pilar Romero-Galisteo, Jose Antonio Merchán-Baeza, J. Ignacio Durán-Millán, Manuel González-Sánchez, and Alejandro Galan-Mercant. 2021. "Physical Activity Promotion Programmes in Childhood Cancer Patients and Their Impact on Fatigue and Pain: A Systematic Review" Children 8, no. 12: 1119. https://doi.org/10.3390/children8121119
APA StyleMalysse, C., Romero-Galisteo, R. P., Merchán-Baeza, J. A., Durán-Millán, J. I., González-Sánchez, M., & Galan-Mercant, A. (2021). Physical Activity Promotion Programmes in Childhood Cancer Patients and Their Impact on Fatigue and Pain: A Systematic Review. Children, 8(12), 1119. https://doi.org/10.3390/children8121119








