A Randomised Controlled Study of Low-Dose High-Frequency In-Situ Simulation Training to Improve Newborn Resuscitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. The Neonatal Simulator
2.3. The Study
2.4. Data Collection
2.5. Data Analysis
3. Results
3.1. Participants and CONSORT Flow Chart
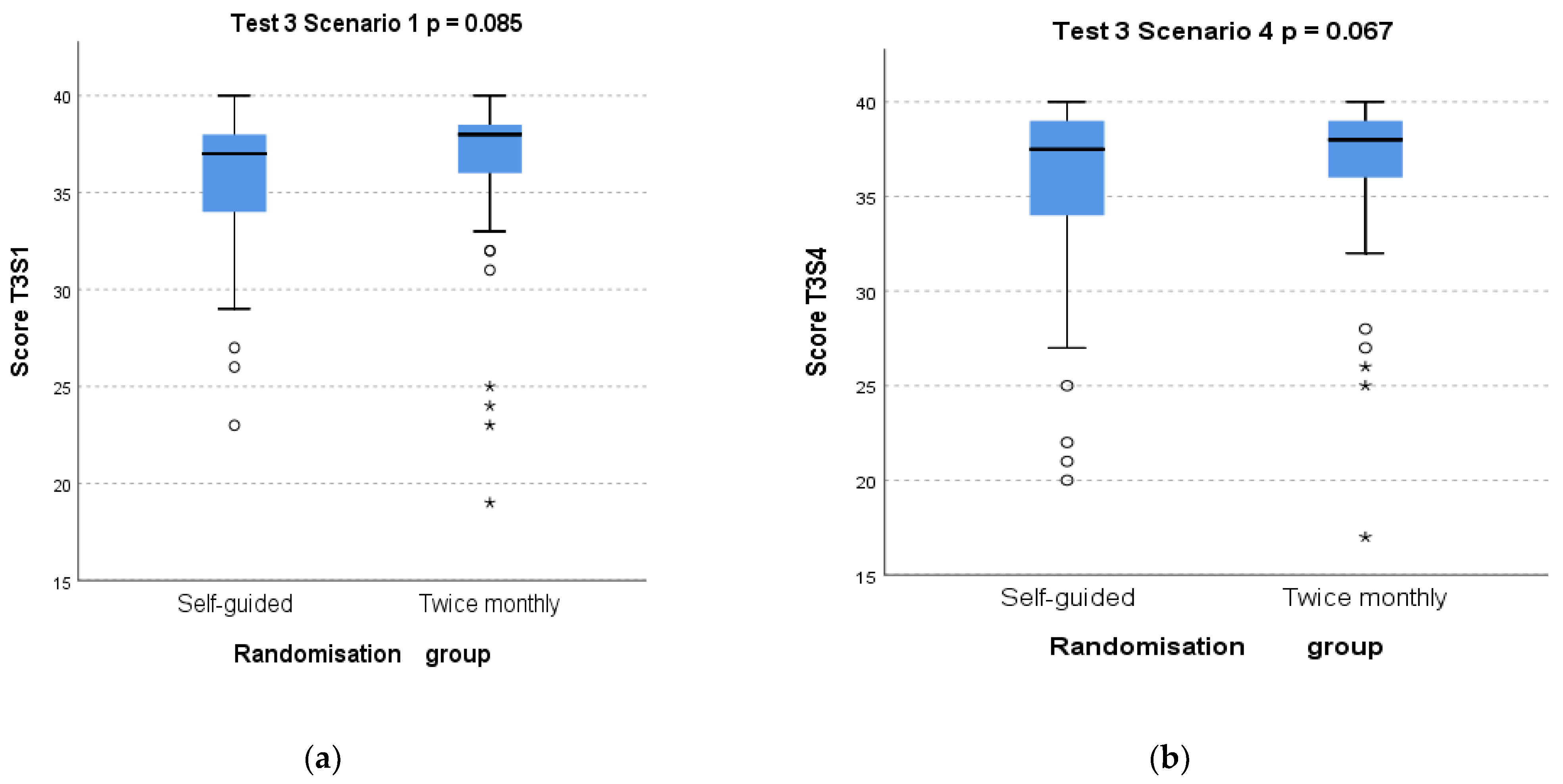
3.2. Primary Outcome: Effect of Randomisation Group on Test 3 Scores
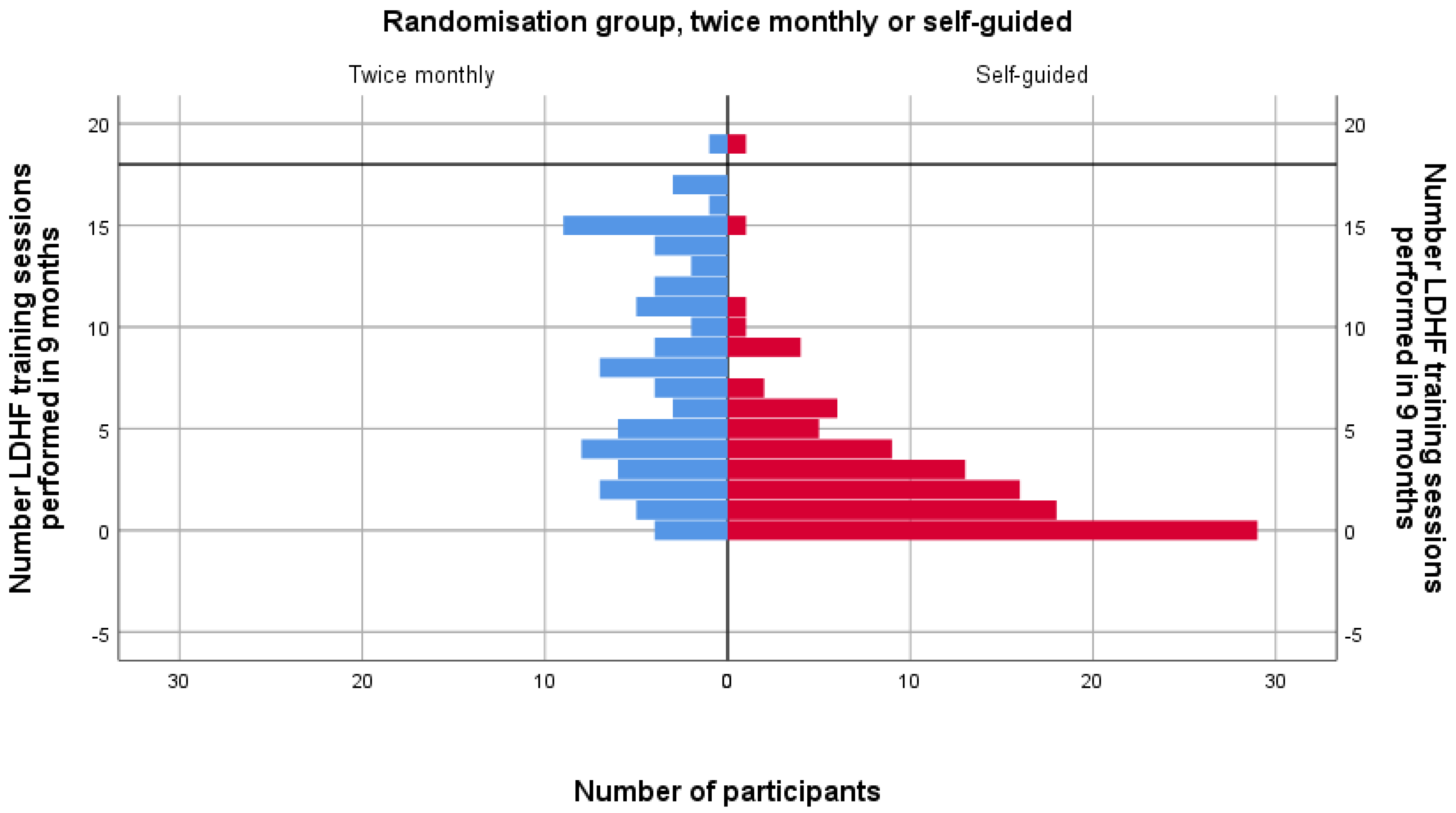
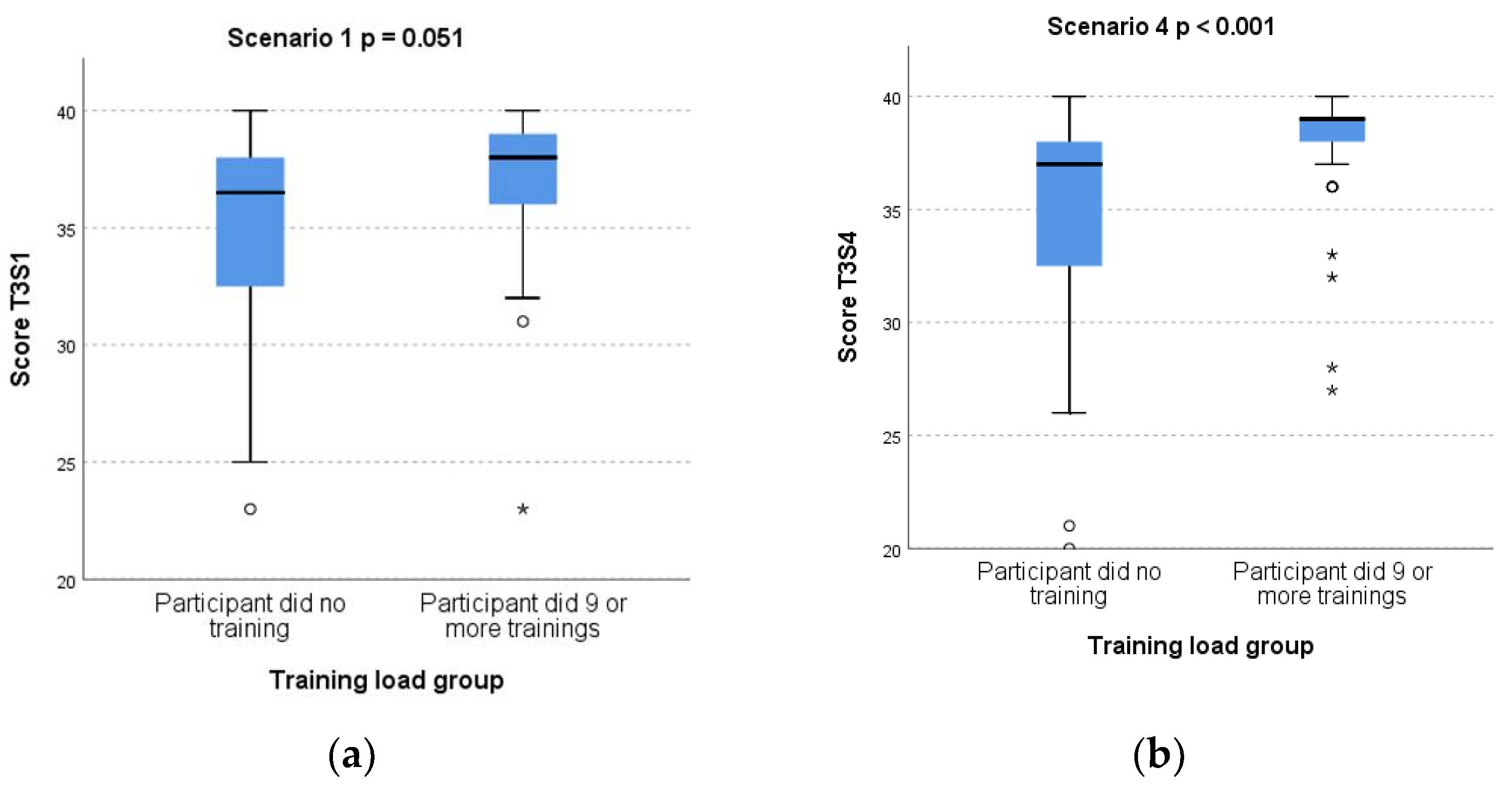
3.3. Subgroup Analysis of Test 3 Scores by Training Load
3.4. Secondary Outcomes: Effect of Study Participation on Test Scores and Comparison across Professional Groups
3.5. Knowledge and Skills Points Lost at Test 3 by Randomisation and by Training Load
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, N.K.; Yaeger, K.A.; Halamek, L.P. Analysis and classification of errors made by teams during neonatal resuscitation. Resuscitation 2015, 96, 109–113. [Google Scholar] [CrossRef]
- Cavicchiolo, M.E.; Cavallin, F.; Bertuola, F.; Pizzol, D.; Segafredo, G.; Manzungu Wingi, O.; Da Dalt, L.; Putoto, G.; Trevisanuto, D. Effect of a Low-Dose/High-Frequency Training on Real-Life Neonatal Resuscitation in a Low-Resource Setting. Neonatology 2018, 114, 294–302. [Google Scholar] [CrossRef]
- Johnson, L.; Mu, T.; Sawyer, T. Use of medical simulation in neonatal-perinatal fellowship training programs. J. Neonatal Perinat. Med. 2012, 5, 339–345. [Google Scholar] [CrossRef]
- Sawyer, T.; Ades, A.; Ernst, K.; Colby, C. Simulation and the Neonatal Resuscitation Program 7th Edition Curriculum. NeoReviews 2016, 17, e447. [Google Scholar] [CrossRef]
- Levitt, C.; Kaczorowski, J.; Outerbridge, E.; Connolly, B.; Jimenez, V.; Slapcoff, B. Knowledge gained during Neonatal Resuscitation Program courses. Fam. Med. 1996, 28, 403–406. [Google Scholar] [PubMed]
- Kaczorowski, J.; Levitt, C.; Hammond, M.; Outerbridge, E.; Grad, R.; Rothman, A.; Graves, L. Retention of Neonatal Resuscitation Skills and Knowledge: A Randomised Controlled Trial. Fam. Med. 1998, 30, 705–711. [Google Scholar]
- Bender, J.; Kennally, K.; Shields, R.; Overly, F. Does simulation booster impact retention of resuscitation procedural skills and teamwork? J. Perinatol. 2014, 34, 664–668. [Google Scholar] [CrossRef]
- Mileder, L.; Urlesberger, B.; Szyld, E.G.; Roehr, C.C.; Schmölzer, G.M. Simulation-Based Neonatal and Infant Resuscitation Teaching: A Systematic Review of Randomised Controlled Trials. Klin. Padiatr. 2014, 226, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.; Gurung, A.; Sunny, A.K.; Basnet, O.; Shrestha, S.K.; Gomo, Ø.H.; Myklebust, H.; Girnary, S.; Ashish, K.C. Effect of skill drills on neonatal ventilation performance in a simulated setting—Observation study in Nepal. BMC Pediatrics 2019, 19, 387. [Google Scholar] [CrossRef] [PubMed]
- Kamath-Rayne, B.D.; Tabangin, M.E.; Taylor, R.G.; Geis, G.L. Retention of Basic Neonatal Resuscitation Skills and Bag-Mask Ventilation in Pediatric Residents Using Just-in-Place Simulation of Varying Frequency and Intensity: A Pilot Randomized Controlled Study. Hosp. Pediatr. 2019, 9, 681–689. [Google Scholar] [CrossRef]
- Mduma, E.; Ersdal, H.; Svensen, E.; Kidanto, H.L.; Auestad, B.; Perlman, J. Frequent brief on-site simulation training and reduction in 24-h neonatal mortality—An educational intervention study. Resuscitation 2015, 93, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Størdal, K.; Eilevstjønn, J.; Mduma, E.; Holte, K.; Thallinger, M.; Linde, J.; Mdoe, P.; Kidanto, H.; Ersdal, H. Increased perinatal survival and improved ventilation skills over a five-year period: An observational study. PLoS ONE 2020, 15, e0240520. [Google Scholar] [CrossRef] [PubMed]
- Bjorland, P.A.; Øymar, K.; Ersdal, H.L.; Rettedal, S.I. Incidence of newborn resuscitative interventions at birth and short-term outcomes: A regional population-based study. BMJ Paediatr. Open 2019, 3, e000592. [Google Scholar] [CrossRef] [Green Version]
- Bjorland, P.A.; Ersdal, H.L.; Eilevstjønn, J.; Øymar, K.; Davis, P.G.; Rettedal, S.I. Changes in heart rate from 5 s to 5 min after birth in vaginally delivered term newborns with delayed cord clamping. Arch. Dis. Child Fetal Neonatal Ed. 2021, 106, F311–F315. [Google Scholar] [CrossRef]
- Linde, J.E.; Eilevstjønn, J.; Øymar, K.; Ersdal, H.L. Feasibility of a prototype newborn resuscitation monitor to study transition at birth, measuring heart rate and ventilator parameters, an animal experimental study. BMC Res. Notes 2017, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Haynes, J.; Bjorland, P.; Gomo, Ø.; Ushakova, A.; Rettedal, S.; Perlman, J.; Ersdal, H. Novel Neonatal Simulator Provides High-Fidelity Ventilation Training Comparable to Real-Life Newborn Ventilation. Children 2021, 8, 940. [Google Scholar] [CrossRef]
- Eilevstjønn, J.; Linde, J.E.; Blacy, L.; Kidanto, H.; Ersdal, H.L. Distribution of heart rate and responses to resuscitation among 1237 apnoeic newborns at birth. Resuscitation 2020, 152, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://nrr.org/images/nedlasting/pdf/NRR_Guidelines_2021_Stabilisering_og_resuscitering_av_nyfodte.pdf (accessed on 10 October 2021).
- Rubio-Gurung, S.; Putet, G.; Touzet, S.; Gauthier-Moulinier, H.; Jordan, I.; Beissel, A.; Labaune, J.M.; Blanc, S.; Amamra, N.; Balandras, C.; et al. In Situ Simulation Training for Neonatal Resuscitation: An RCT. Pediatrics 2014, 134, e790. [Google Scholar] [CrossRef] [Green Version]
- O’Currain, E.; Davis, P.G.; Thio, M. Educational Perspectives: Toward More Effective Neonatal Resuscitation: Assessing and Improving Clinical Skills. NeoReviews 2019, 20, e248. [Google Scholar] [CrossRef] [PubMed]
- Budhathoki, S.S.; Gurung, R.; Ewald, U.; Thapa, J.; Ashish, K.C. Does the Helping Babies Breathe Programme impact on neonatal resuscitation care practices? Results from systematic review and meta-analysis. Acta Pædiatr. 2019, 108, 806–813. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.O.; Brown, L.L.; Bender, J.; Machan, J.T.; Overly, F.L. A Medical Simulation-based Educational Intervention for Emergency Medicine Residents in Neonatal Resuscitation. Acad. Emerg. Med. 2012, 19, 577–585. [Google Scholar] [CrossRef]
- Mileder, L.P.; Gressl, J.; Urlesberger, B.; Raith, W. Paramedics’ Newborn Life Support Knowledge and Skills before and after a Targeted Simulation-Based Educational Intervention. Front. Pediatr. 2019, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Skidmore, M.B.; Urquhart, H. Retention of skills in neonatal resuscitation. Paediatr. Child Health 2001, 6, 31–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, K.M.; Miller, M.P.; Schmidt, K.; Sagy, M. Pediatric residents experience a significant decline in their response capabilities to simulated life-threatening events as their training frequency in cardiopulmonary resuscitation decreases. Pediatr. Crit. Care Med. 2011, 12, e141–e144. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Posencheg, M.; Ades, A. Proficiency and Retention of Neonatal Resuscitation Skills by Pediatric Residents. Pediatrics 2012, 130, 515–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosley, C.M.J.; Shaw, B.N.J. A longitudinal cohort study to investigate the retention of knowledge and skills following attendance on the Newborn Life support course. Arch. Dis. Child. 2013, 98, 582–586. [Google Scholar] [CrossRef]
- Matterson, H.H.; Szyld, D.; Green, B.R.; Howell, H.B.; Pusic, M.V.; Mally, P.V.; Bailey, S.M. Neonatal resuscitation experience curves: Simulation based mastery learning booster sessions and skill decay patterns among pediatric residents. J. Perinat. Med. 2018, 46, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Djarv, T.; Hsieh, M.J.; Sawyer, T.; Lockey, A.; Finn, J.; Greif, R.; on behalf of the Education, Implementation and Team Task Force and Neonatal Life Support Task Force of the International Liaison Committee on Resuscitation (ILCOR). Spaced learning versus massed learning in resuscitation—A systematic review. Resuscitation 2020, 156, 61–71. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Aziz, K.; Escobedo, M.B.; Kapadia, V.S.; Kattwinkel, J.; Perlman, J.M.; Simon, W.M.; Weiner, G.M.; Zaichkin, J.G. Part 13: Neonatal Resuscitation 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132, S543–S560. [Google Scholar] [CrossRef] [PubMed]
- McGaghie, W.C.; Issenberg, S.B.; Petrusa, E.R.; Scalese, R.J. Effect of practice on standardized learning outcomes in simulation-based medical education. Med. Educ. 2006, 40, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, K.A. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad. Med. 2008, 15, 988–994. [Google Scholar] [CrossRef]
- Ashish, K.C.; Wrammert, J.; Nelin, V.; Clark, R.B.; Ewald, U.; Peterson, S.; Målqvist, M. Evaluation of Helping Babies Breathe Quality Improvement Cycle (HBB-QIC) on retention of neonatal resuscitation skills six months after training in Nepal. BMC Pediatrics 2017, 17, 103. [Google Scholar] [CrossRef]
- Tabangin, M.E.; Josyula, S.; Taylor, K.K.; Vasquez, J.C.; Kamath-Rayne, B.D. Resuscitation skills after Helping Babies Breathe training: A comparison of varying practice frequency and impact on retention of skills in different types of providers. Int. Health 2018, 10, 163–171. [Google Scholar] [CrossRef]
- Mduma, E.; Kvaløy, J.T.; Søreide, E.; Svensen, E.; Mdoe, P.; Perlman, J.; Johnson, C.; Kidanto, H.L.; Ersdal, H.L. Frequent refresher training on newborn resuscitation and potential impact on perinatal outcome over time in a rural Tanzanian hospital: An observational study. BMJ Open 2019, 9, e030572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nourkami-Tutdibi, N.; Hilleke, A.B.; Zemlin, M.; Wagenpfeil, G.; Tutdibi, E. Novel modified Peyton’s approach for knowledge retention on newborn life support training in medical students. Acta Paediatr. 2020, 109, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.; Niday, P.; Watters, N.E.; McGrath, P.; Alcock, D. The provision and evaluation of a neonatal resuscitation program. J. Contin. Educ. Nurs. 1992, 23, 118–126. [Google Scholar] [CrossRef]
- van der Heide, P.A.; van Toledo-Eppinga, L.; van der Heide, M.; van der Lee, J.H. Assessment of neonatal resuscitation skills: A reliable and valid scoring system. Resuscitation 2006, 71, 212–221. [Google Scholar] [CrossRef]
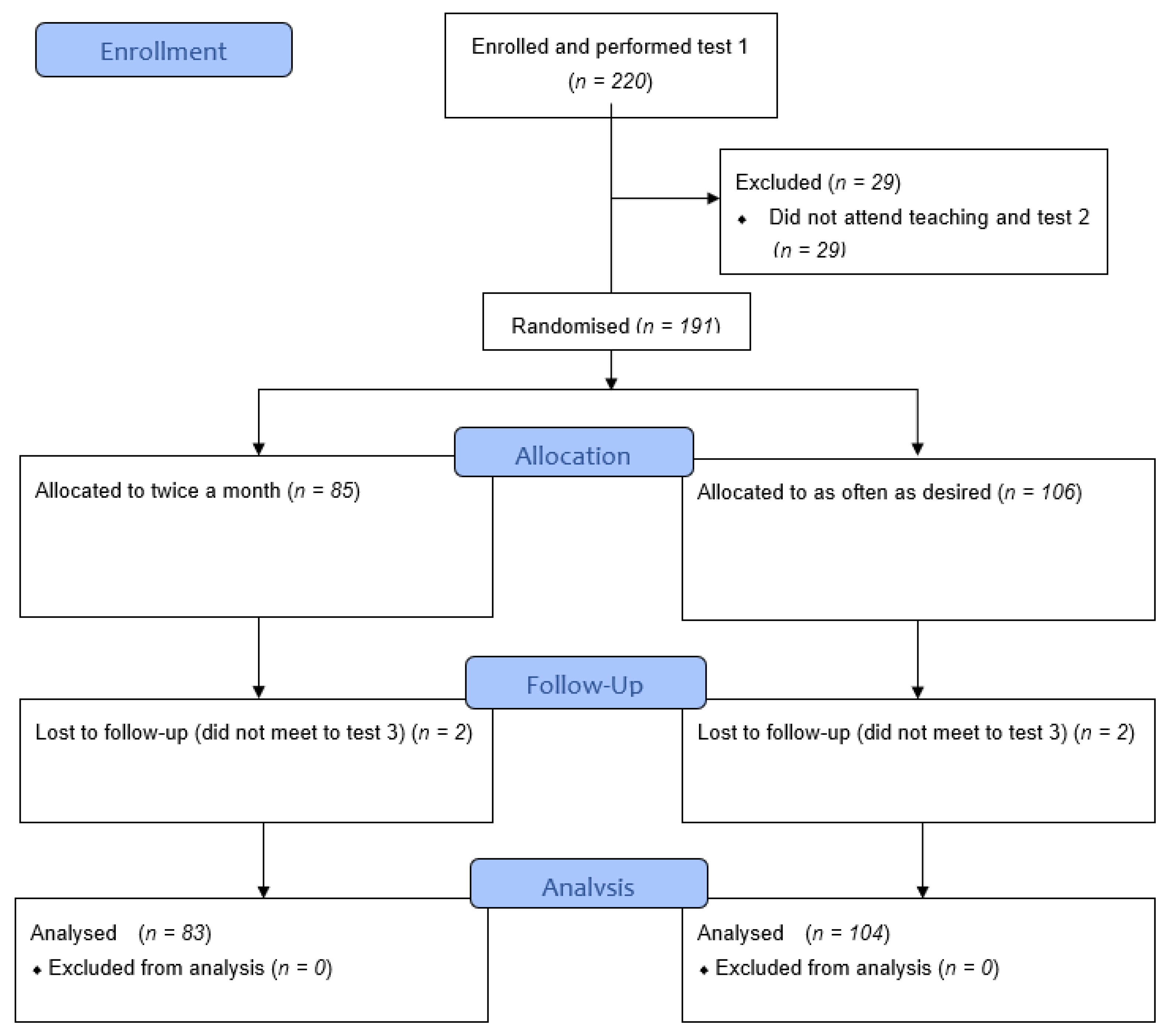


| Total Recruited and Completed Test 1 | Educated and Completed Test 2 | Randomised to Twice a Month (of Which x Did not Complete Test 3) | Randomised to as often as Desired (of Which x did not Complete Test 3) | Final Total Completing Study and Analysed after Test 3 | ||
|---|---|---|---|---|---|---|
| Profession | Anaesthesia nurse | 54 | 46 | 20 (0) | 26 (0) | 46 |
| Anaesthetist | 38 | 34 | 19 (0) | 15 (0) | 34 | |
| Midwife | 72 | 62 | 28 (0) | 34 (2) | 60 | |
| Paediatric nurse assistant | 17 | 17 | 6 (0) | 11 (0) | 17 | |
| Paediatrician | 18 | 18 | 7 (1) | 11 (0) | 17 | |
| Obstetrician | 21 | 14 | 5 (1) | 9 (0) | 13 | |
| Total | 220 | 191 | 85 (2) | 106 (2) | 187 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haynes, J.; Rettedal, S.; Perlman, J.; Ersdal, H. A Randomised Controlled Study of Low-Dose High-Frequency In-Situ Simulation Training to Improve Newborn Resuscitation. Children 2021, 8, 1115. https://doi.org/10.3390/children8121115
Haynes J, Rettedal S, Perlman J, Ersdal H. A Randomised Controlled Study of Low-Dose High-Frequency In-Situ Simulation Training to Improve Newborn Resuscitation. Children. 2021; 8(12):1115. https://doi.org/10.3390/children8121115
Chicago/Turabian StyleHaynes, Joanna, Siren Rettedal, Jeffrey Perlman, and Hege Ersdal. 2021. "A Randomised Controlled Study of Low-Dose High-Frequency In-Situ Simulation Training to Improve Newborn Resuscitation" Children 8, no. 12: 1115. https://doi.org/10.3390/children8121115
APA StyleHaynes, J., Rettedal, S., Perlman, J., & Ersdal, H. (2021). A Randomised Controlled Study of Low-Dose High-Frequency In-Situ Simulation Training to Improve Newborn Resuscitation. Children, 8(12), 1115. https://doi.org/10.3390/children8121115







