Neurologic Sequelae Associated with Hypertensive Disorders of Pregnancy
Abstract
:1. Introduction
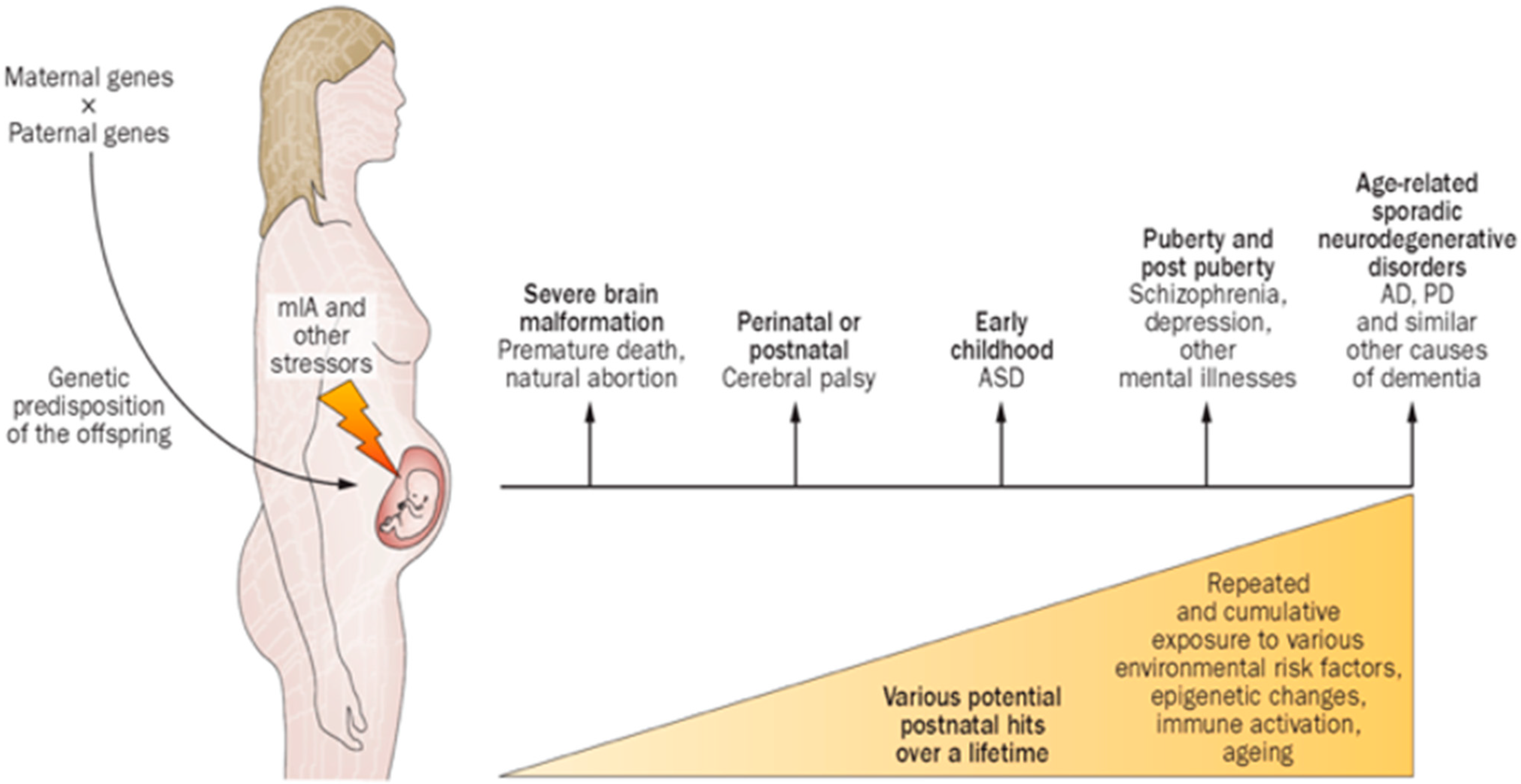
2. Gene/Environment Interactions Influence Clinical Expression
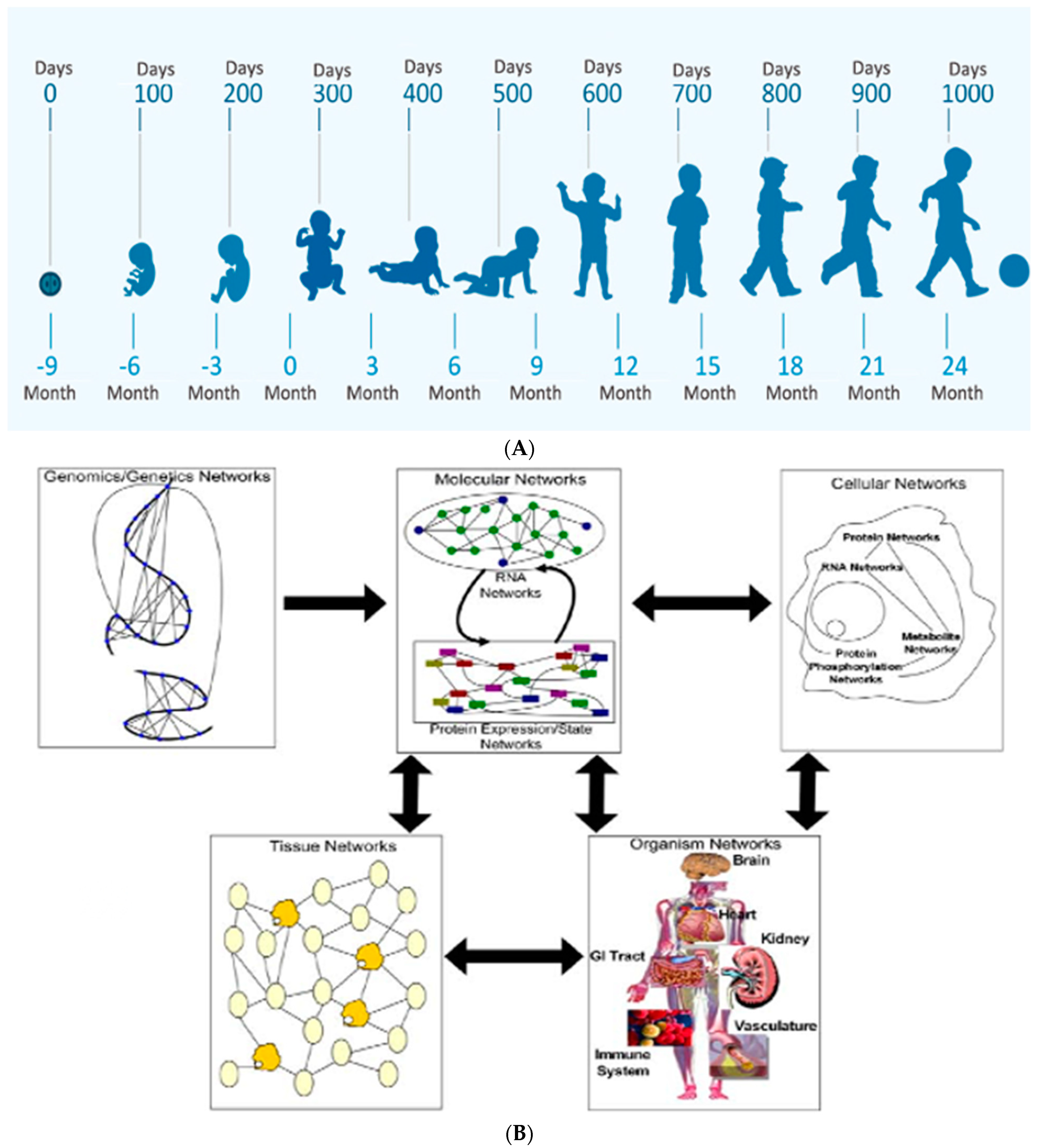
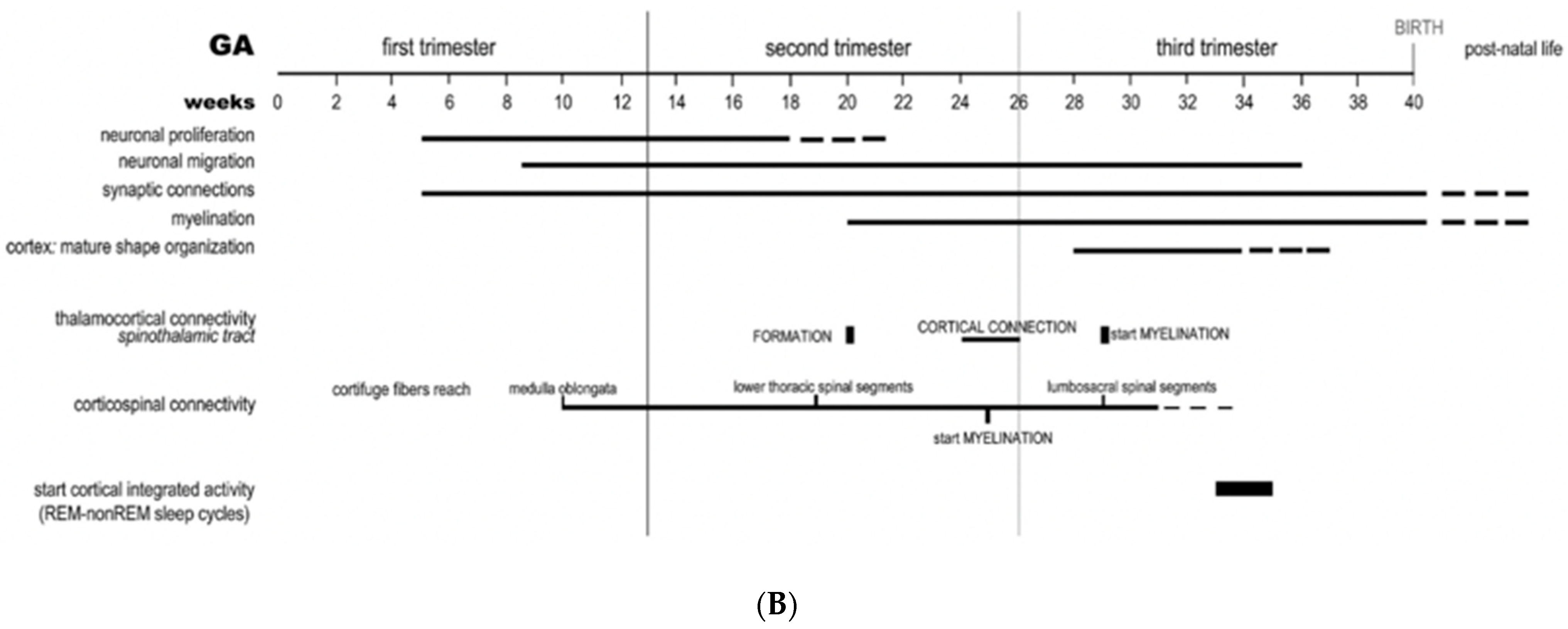
3. Early Pregnancy Effects on Embryonic/Fetal Brain
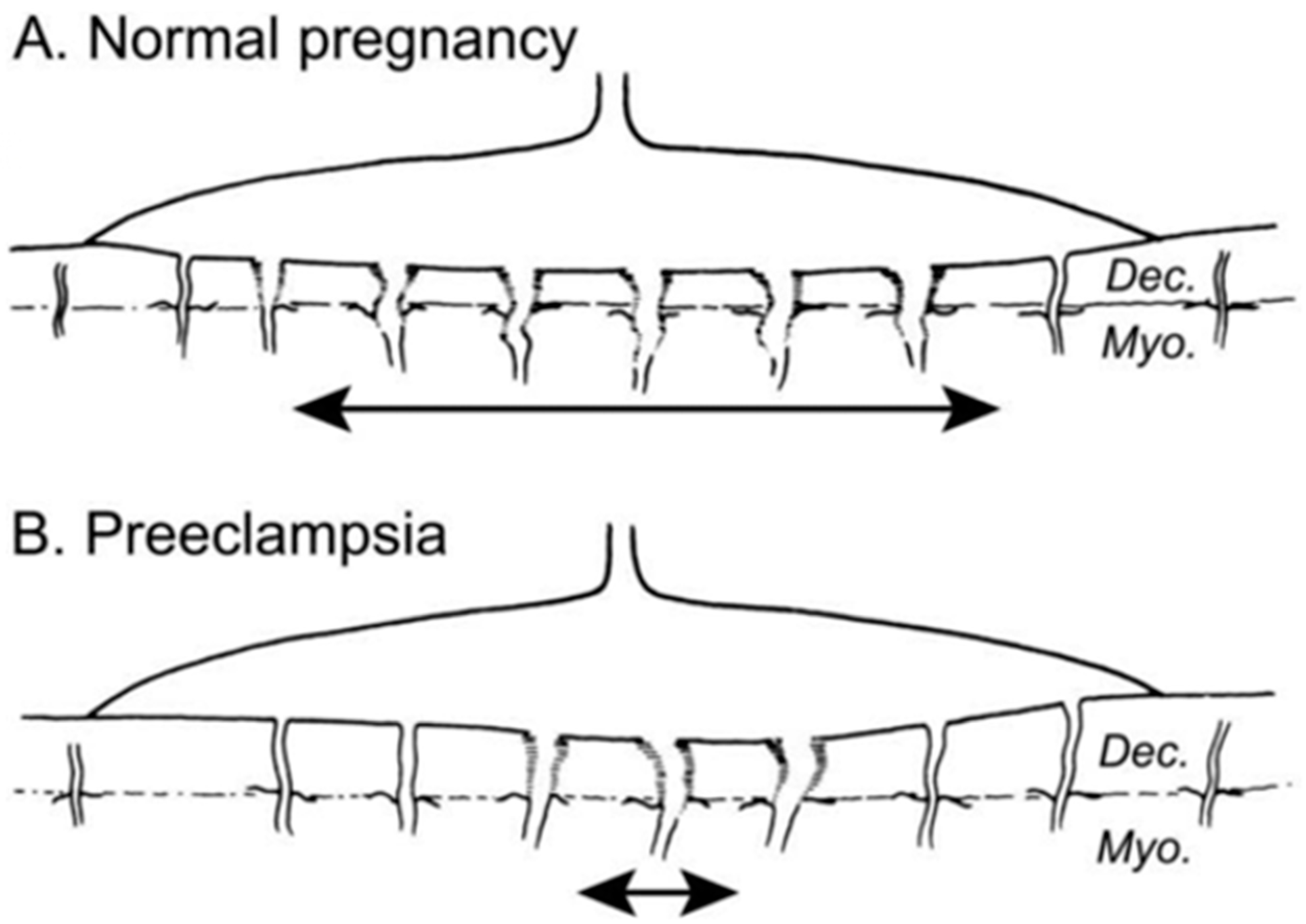
4. Later Pregnancy Effects Associated with the Great Obstetrical Syndromes
5. Peripartum and Intrapartum Considerations
6. The Great Neonatal Neurological Syndromes
7. Pediatric Neurology Evaluations after HDP
8. Childhood, Adolescent and Adult Sequelae
9. Current Practice Guidelines and Therapeutic Research Advances
10. Reduction of the Global Burden of Disease
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HDP | hypertensive disorders of pregnancy |
| G × E | gene/environment interactions |
| PE | pre-eclampsia |
| MPF | maternal/placental/fetal |
| FNN | fetal neonatal neurology |
| MIA | maternal immune activation |
| IPS | ischemic placental syndrome |
| GOS | great obstetrical syndromes |
| GNNS | great neonatal neurological syndromes |
| NE | neonatal encephalopathy |
| NS | neonatal seizures |
| NSK | neonatal stroke |
| EP | encephalopathy of prematurity |
References
- Scher, M.S. The First Thousand Days Define a Fetal/Neonatal Neurology Program. Front Pediatr. 2021, 9. [Google Scholar] [CrossRef]
- Sieberts, S.K.; Schadt, E.E. Moving toward a system genetics view of disease. Mamm. Genome 2007, 18, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Scher, M.S. Chapter 1—Fetal neurology: Principles and practice with a life-course perspective. Handb. Clin. Neurol. 2019, 162, 1–29. [Google Scholar] [CrossRef]
- Garovic, V.D.; White, W.M.; Vaughan, L.; Saiki, M.; Parashuram, S.; Garcia-Valencia, O.; Weissgerber, T.L.; Milic, N.; Weaver, A.; Mielke, M.M. Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J. Am. Coll. Cardiol. 2020, 75, 2323–2334. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Barron, A.; McCarthy, C.M.; O’Keeffe, G.W. Preeclampsia and Neurodevelopmental Outcomes: Potential Pathogenic Roles for Inflammation and Oxidative Stress? Mol. Neurobiol. 2021, 58, 2734–2756. [Google Scholar] [CrossRef]
- Brand, J.S.; Lawlor, D.A.; Larsson, H.; Montgomery, S. Association Between Hypertensive Disorders of Pregnancy and Neurodevelopmental Outcomes Among Offspring. JAMA Pediatr. 2021, 175, 577. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.K.; Bouhouch, R.R.; Walson, J.L.; Daelmans, B.; Bahl, R.; Darmstadt, G.L.; Dua, T. Enhancing the child survival agenda to promote, protect, and support early child development. Semin. Perinatol. 2015, 39, 373–386. [Google Scholar] [CrossRef]
- De Bernis, L.; Kinney, M.V.; Stones, W.; Hoope-Bender, P.T.; Vivio, D.; Leisher, S.H.; Bhutta, Z.A.; Gülmezoglu, M.; Mathai, M.; Belizán, J.M.; et al. Stillbirths: Ending preventable deaths by 2030. Lancet 2016, 387, 703–716. [Google Scholar] [CrossRef] [Green Version]
- Tolu, L.B.; Yigezu, E.; Urgie, T.; Feyissa, G. Maternal and perinatal outcome of preeclampsia without severe feature among pregnant women managed at a tertiary referral hospital in urban Ethiopia. PLoS ONE 2020, 15, e0230638. [Google Scholar] [CrossRef]
- Pinheiro, T.V.; Brunetto, S.; Ramos, J.G.L.; Bernardi, J.; Goldani, M.Z. Hypertensive disorders during pregnancy and health outcomes in the offspring: A systematic review. J. Dev. Orig. Health Dis. 2016, 7, 391–407. [Google Scholar] [CrossRef]
- Benny, P.A.; Al-Akwaa, F.M.; Schlueter, R.J.; Lassiter, C.B.; Garmire, L.X. A review of omics approaches to study preeclampsia. Placenta 2020, 92, 17–27. [Google Scholar] [CrossRef]
- Mohamad, M.A.; Manzor, N.F.M.; Zulkifli, N.F.; Zainal, N.; Hayati, A.R.; Asnawi, A.W.A. A Review of Candidate Genes and Pathways in Preeclampsia-An Integrated Bioinformatical Analysis. Biology 2020, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Than, N.G.; Romero, R.; Tarca, A.L.; Kekesi, K.A.; Xu, Y.; Xu, Z.; Juhasz, K.; Bhatti, G.; Leavitt, R.J.; Gelencser, Z.; et al. Integrated Systems Biology Approach Identifies Novel Maternal and Placental Pathways of Preeclampsia. Front. Immunol. 2018, 9, 1661. [Google Scholar] [CrossRef]
- Gray, K.J.; Kovacheva, V.P.; Mirzakhani, H.; Bjonnes, A.C.; Almoguera, B.; DeWan, A.T.; Triche, E.W.; Saftlas, A.F.; Hoh, J.; Bodian, D.L.; et al. Gene-Centric Analysis of Preeclampsia Identifies Maternal Association at PLEKHG1. Hypertension 2018, 72, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, C.; Liu, Y.; Wang, N.; Gao, S.; Qiu, S.; Wang, Z.; Ding, J.; Zhang, L.; Wang, H.; et al. Identifying key genes and drug screening for preeclampsia based on gene expression profiles. Oncol. Lett. 2020, 20, 1585–1596. [Google Scholar] [CrossRef]
- Hanson, M.; Skinner, M.K. Developmental origins of epigenetic transgenerational inheritance. Environ. Epigenetics 2016, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scorza, P.; Duarte, C.S.; Hipwell, A.E.; Posner, J.; Ortin, A.; Canino, G.; Monk, C. Program Collaborators for Environmental influences on Child Health Outcomes Research Review: Intergenerational transmission of disadvantage: Epigenetics and parents’ childhoods as the first exposure. J. Child. Psychol. Psychiatry 2018, 60, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U. Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends Neurosci. 2019, 42, 793–806. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Kwiatkowska, E.; Rzepka, R.; Torbe, A.; Dolegowska, B. Ischemic placental syndrome—Prediction and new disease monitoring. J. Matern. Neonatal. Med. 2015, 29, 2033–2039. [Google Scholar] [CrossRef]
- Borsani, E.; Della Vedova, A.M.; Rezzani, R.; Rodella, L.F.; Cristini, C. Correlation between human nervous system development and acquisition of fetal skills: An overview. Brain Dev. 2019, 41, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Vasung, L.; Turk, E.A.; Ferradal, S.; Sutin, J.; Stout, J.N.; Ahtam, B.; Lin, P.-Y.; Grant, P.E. Exploring early human brain development with structural and physiological neuroimaging. NeuroImage 2019, 187, 226–254. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.A.; Mayo, J.A.; Carmichael, S.L.; Stevenson, D.K.; Winn, V.D.; Shaw, G.M. Occurrence of Selected Structural Birth Defects Among Women with Preeclampsia and Other Hypertensive Disorders. Am. J. Epidemiology 2017, 187, 668–676. [Google Scholar] [CrossRef]
- Li, Y.; Thompson, W.K.; Reuter, C.; Nillo, R.; Jernigan, T.; Dale, A.; Sugrue, L.P.; Brown, J.; Dougherty, R.F.; Rauschecker, A.; et al. Rates of Incidental Findings in Brain Magnetic Resonance Imaging in Children. JAMA Neurol. 2021, 78, 578. [Google Scholar] [CrossRef] [PubMed]
- Adnani, L.; Han, S.; Li, S.; Mattar, P.; Schuurmans, C. Mechanisms of Cortical Differentiation. Int. Rev. Cell Mol. Biol. 2018, 336, 223–320. [Google Scholar] [CrossRef]
- Freese, J.L.; Pino, D.; Pleasure, S.J. Wnt signaling in development and disease. Neurobiol. Dis. 2010, 38, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Takei, N.; Nawa, H. mTOR signaling and its roles in normal and abnormal brain development. Front. Mol. Neurosci. 2014, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Menassa, D.; Gomez-Nicola, D. Microglial Dynamics during Human Brain Development. Front. Immunol. 2018, 9, 1014. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.G. The Origins of Cortical Interneurons: Mouse versus Monkey and Human. Cereb. Cortex 2009, 19, 1953–1956. [Google Scholar] [CrossRef] [Green Version]
- Huntley, M.A.; Srinivasan, K.; Friedman, B.A.; Wang, T.-M.; Yee, A.X.; Wang, Y.; Kaminker, J.S.; Sheng, M.; Hansen, D.V.; Hanson, J.E. Genome-Wide Analysis of Differential Gene Expression and Splicing in Excitatory Neurons and Interneuron Subtypes. J. Neurosci. 2020, 40, 958–973. [Google Scholar] [CrossRef]
- Katsarou, A.; Moshé, S.L.; Galanopoulou, A.S. Interneuronopathies and their role in early life epilepsies and neurodevelopmental disorders. Epilepsia Open 2017, 2, 284–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prins, J.R.; Eskandar, S.; Eggen, B.J.; Scherjon, S.A. Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment? J. Reprod. Immunol. 2018, 126, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Lara, E.; Acurio, J.; Leon, J.; Penny, J.; Torres-Vergara, P.; Escudero, C. Are the Cognitive Alterations Present in Children Born From Preeclamptic Pregnancies the Result of Impaired Angiogenesis? Focus on the Potential Role of the VEGF Family. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Muralimanoharan, S.; Maloyan, A.; Myatt, L. Evidence of sexual dimorphism in the placental function with severe preeclampsia. Placenta 2013, 34, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Barnes, M.D.; Heaton, T.L.; Goates, M.C.; Packer, J.M. Intersystem Implications of the Developmental Origins of Health and Disease: Advancing Health Promotion in the 21st Century. Healthcare 2016, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Z.; Clowry, G.J.; Šestan, N.; Alzu’Bi, A.; Bakken, T.; Hevner, R.F.; Hüppi, P.; Kostović, I.; Rakic, P.; Anton, E.S.; et al. New insights into the development of the human cerebral cortex. J. Anat. 2019, 235, 432–451. [Google Scholar] [CrossRef] [Green Version]
- Truttmann, A.C.; Ginet, V.; Puyal, J. Current Evidence on Cell Death in Preterm Brain Injury in Human and Preclinical Models. Front. Cell Dev. Biol. 2020, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Di Renzo, G.C. The Great Obstetrical Syndromes. J. Matern. Neonatal Med. 2009, 22, 633–635. [Google Scholar] [CrossRef]
- Brosens, I.; Puttemans, P.; Benagiano, G. Placental bed research: I. The placental bed: From spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019, 221, 437–456. [Google Scholar] [CrossRef]
- Harris, L.; Benagiano, M.; D’Elios, M.M.; Brosens, I.; Benagiano, G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am. J. Obstet. Gynecol. 2019, 221, 457–469. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raguema, N.; Moustadraf, S.; Bertagnolli, M. Immune and Apoptosis Mechanisms Regulating Placental Development and Vascularization in Preeclampsia. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Roescher, A.M.; Timmer, A.; Erwich, J.J.H.M.; Bos, A.F. Placental Pathology, Perinatal Death, Neonatal Outcome, and Neurological Development: A Systematic Review. PLoS ONE 2014, 9, e89419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwertbroek, E.F.; Franssen, M.T.; Broekhuijsen, K.; Langenveld, J.; Bremer, H.; Ganzevoort, W.; van Loon, A.J.; van Pampus, M.; Rijnders, R.J.; Sikkema, M.J.; et al. Neonatal developmental and behavioral outcomes of immediate delivery versus expectant monitoring in mild hypertensive disorders of pregnancy: 2-year outcomes of the HYPITAT-II trial. Am. J. Obstet. Gynecol. 2019, 221, 154. [Google Scholar] [CrossRef]
- Scher, M.S. Peripartum Consultations Expand the Role of the Fetal/Neonatal Neurologist. Pediatr. Neurol. 2012, 47, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Lear, C.A.; Wassink, G.; Westgate, J.A.; Nijhuis, J.G.; Ugwumadu, A.; Galinsky, R.; Bennet, L.; Gunn, A.J. The peripheral chemoreflex: Indefatigable guardian of fetal physiological adaptation to labour. J. Physiol. 2018, 596, 5611–5623. [Google Scholar] [CrossRef]
- Okereafor, A.; Allsop, J.; Counsell, S.; Fitzpatrick, J.; Azzopardi, D.; Rutherford, M.; Cowan, F.M. Patterns of Brain Injury in Neonates Exposed to Perinatal Sentinel Events. Pediatrics 2008, 121, 906–914. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. American Academy of Pediatrics. Neonatal Encephalopathy and Neurologic Outcome; American College of Obstetricians and Gynecologists: Washington, DC, USA, 2019. [Google Scholar]
- Thomason, M.E.; Grove, L.E.; Lozon, T.A.; Vila, A.M.; Ye, Y.; Nye, M.J.; Manning, J.H.; Pappas, A.; Hernandez-Andrade, E.; Yeo, L.; et al. Age-related increases in long-range connectivity in fetal functional neural connectivity networks in utero. Dev. Cogn. Neurosci. 2015, 11, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.M.; Mitchell, M.; Kumar, S.S. The physiology of intrapartum fetal compromise at term. Am. J. Obstet. Gynecol. 2020, 222, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Wang, L.; Tian, T.; Liu, L.; Jin, L.; Liu, J.; Ren, A. Maternal hypertensive disorders in pregnancy and risk of hypoxic-ischemia encephalopathy. J. Matern. Neonatal Med. 2019, 34, 1754–1762. [Google Scholar] [CrossRef]
- Nakamura, N.; Ushida, T.; Nakatochi, M.; Kobayashi, Y.; Moriyama, Y.; Imai, K.; Nakano-Kobayashi, T.; Hayakawa, M.; Kajiyama, H.; Kikkawa, F.; et al. Mortality and neurological outcomes in extremely and very preterm infants born to mothers with hypertensive disorders of pregnancy. Sci. Rep. 2021, 11, 1729. [Google Scholar] [CrossRef]
- Marins, L.R.; Anizelli, L.B.; Romanowski, M.D.; Sarquis, A.L. How does preeclampsia affect neonates? Highlights in the disease’s immunity. J. Matern. Neonatal Med. 2019, 32, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.K.; Krakowiak, P.; Baker, A.; Hansen, R.L.; Ozonoff, S.; Hertz-Picciotto, I. Preeclampsia, Placental Insufficiency, and Autism Spectrum Disorder or Developmental Delay. JAMA Pediatr. 2015, 169, 154–162. [Google Scholar] [CrossRef]
- Chen, K.; Yu, T.; Kang, L.; Lien, Y.; Kuo, P. Childhood neurodevelopmental disorders and maternal hypertensive disorder of pregnancy. Dev. Med. Child. Neurol. 2021, 63, 1107–1113. [Google Scholar] [CrossRef]
- Scher, M.S. An Interdisciplinary Fetal/Neonatal Neurology Program. J. Child. Neurol. 2012, 27, 496–502. [Google Scholar] [CrossRef]
- Biban, P.; Spaggiari, S. “Cohabitation” between NICU and PICU. J. Matern. Neonatal Med. 2011, 24, 91–93. [Google Scholar] [CrossRef]
- Crow, S.S.; Undavalli, C.; Warner, D.O.; Katusic, S.K.; Kandel, P.; Murphy, S.L.; Schroeder, D.R.; Watson, R.S. Epidemiology of Pediatric Critical Illness in a Population-Based Birth Cohort in Olmsted County, MN. Pediatr. Crit. Care Med. 2017, 18, e137–e145. [Google Scholar] [CrossRef] [Green Version]
- Kanata, M.; Liazou, E.; Chainoglou, A.; Kotsis, V.; Stabouli, S. Clinical outcomes of hypertensive disorders in pregnancy in the offspring during perinatal period, childhood, and adolescence. J. Hum. Hypertens. 2021, 1–11. [Google Scholar] [CrossRef]
- Lahti-Pulkkinen, M.; Girchenko, P.; Tuovinen, S.; Sammallahti, S.; Reynolds, R.M.; Lahti, J.; Heinonen, K.; Lipsanen, J.; Hämäläinen, E.; Villa, P.M.; et al. Maternal Hypertensive Pregnancy Disorders and Mental Disorders in Children. Hypertension 2020, 75, 1429–1438. [Google Scholar] [CrossRef]
- Wang, H.; László, K.D.; Gissler, M.; Li, F.; Zhang, J.; Yu, Y.; Li, J. Maternal hypertensive disorders and neurodevelopmental disorders in offspring: A population-based cohort in two Nordic countries. Eur. J. Epidemiology 2021, 36, 519–530. [Google Scholar] [CrossRef]
- Tuovinen, S.; Räikkönen, K.; Pesonen, A.-K.; Lahti-Pulkkinen, M.; Heinonen, K.; Wahlbeck, K.; Kajantie, E.; Osmond, C.; Barker, D.J.; Eriksson, J.G. Hypertensive disorders in pregnancy and risk of severe mental disorders in the offspring in adulthood: The Helsinki Birth Cohort Study. J. Psychiatr. Res. 2012, 46, 303–310. [Google Scholar] [CrossRef]
- O’Leary, L.; Cooper, S.; Hughes-McCormack, L. Early death and causes of death of people with intellectual disabilities: A systematic review. J. Appl. Res. Intellect. Disabil. 2018, 31, 325–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawalt, L.S.; Hong, J.; Greenberg, J.S.; Mailick, M.R. Mortality in individuals with autism spectrum disorder: Predictors over a 20-year period. Autism 2019, 23, 1732–1739. [Google Scholar] [CrossRef]
- Maric-Bilkan, C.; Abrahams, V.M.; Arteaga, S.S.; Bourjeily, G.; Conrad, K.P.; Catov, J.M.; Costantine, M.M.; Cox, B.; Garovic, V.; George, E.; et al. Research Recommendations from the National Institutes of Health Workshop on Predicting, Preventing, and Treating Preeclampsia. Hypertension 2019, 73, 757–766. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.; Zhang, J.; Lye, S.J.; Parker, J.D.; Kingdom, J.C. Phenotypes of Pregnant Women Who Subsequently Develop Hypertension in Pregnancy. J. Am. Hear. Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, S.; Bombay, K.; Lanes, A.; Walker, M.; Corsi, D.J. Potential biological therapies for severe preeclampsia: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2019, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Ma’Ayeh, M.; Costantine, M.M. Prevention of preeclampsia. Semin. Fetal Neonatal Med. 2020, 25, 101123. [Google Scholar] [CrossRef] [PubMed]
- Hitzerd, E.; Broekhuizen, M.; Neuman, R.I.; Colafella, K.M.M.; Merkus, D.; Schoenmakers, S.; Simons, S.; Reiss, I.K.; Danser, A.J. Human Placental Vascular Reactivity in Health and Disease: Implications for the Treatment of Pre-eclampsia. Curr. Pharm. Des. 2019, 25, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Turanov, A.A.; Lo, A.; Hassler, M.R.; Makris, A.; Ashar-Patel, A.; Alterman, J.F.; Coles, A.H.; Haraszti, R.A.; Roux, L.; Godinho, B.M.D.C.; et al. RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat. Biotechnol. 2018, 36, 1164–1173. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Correia-Branco, A.; Adhikari, N.; Mercan, E.; Mallidi, S.; Wallingford, M.C. New Frontiers in Placenta Tissue Imaging. EMJ Radiol. 2020, 1, 54–62. [Google Scholar]
- Andescavage, N.; du Plessis, A.; Limperopoulos, C. Advanced MR imaging of the placenta: Exploring the in utero placenta–brain connection. Semin. Perinatol. 2015, 39, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girardi, G. MRI-based methods to detect placental and fetal brain abnormalities in utero. J. Reprod. Immunol. 2016, 114, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Lew, S.; Hämäläinen, M.; Okada, Y. Toward noninvasive monitoring of ongoing electrical activity of human uterus and fetal heart and brain. Clin. Neurophysiol. 2017, 128, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.R. Global Burden of Pediatric Neurological Disorders. Semin. Pediatr. Neurol. 2018, 27, 10–15. [Google Scholar] [CrossRef]
- Aagaard-Hansen, J.; Norris, S.A.; Maindal, H.T.; Hanson, M.; Fall, C. What are the public health implications of the life course perspective? Glob. Health Action 2019, 12, 1603491. [Google Scholar] [CrossRef] [Green Version]
- Hanson, M.; Gluckman, P. Developmental origins of health and disease—Global public health implications. Best Pr. Res. Clin. Obstet. Gynaecol. 2015, 29, 24–31. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scher, M.S. Neurologic Sequelae Associated with Hypertensive Disorders of Pregnancy. Children 2021, 8, 945. https://doi.org/10.3390/children8110945
Scher MS. Neurologic Sequelae Associated with Hypertensive Disorders of Pregnancy. Children. 2021; 8(11):945. https://doi.org/10.3390/children8110945
Chicago/Turabian StyleScher, Mark S. 2021. "Neurologic Sequelae Associated with Hypertensive Disorders of Pregnancy" Children 8, no. 11: 945. https://doi.org/10.3390/children8110945
APA StyleScher, M. S. (2021). Neurologic Sequelae Associated with Hypertensive Disorders of Pregnancy. Children, 8(11), 945. https://doi.org/10.3390/children8110945




