Higher versus Lower Oxygen Concentration during Respiratory Support in the Delivery Room in Extremely Preterm Infants: A Pilot Feasibility Study
Abstract
:1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Consent
2.3. Sample Size Calculation
2.4. Blinding
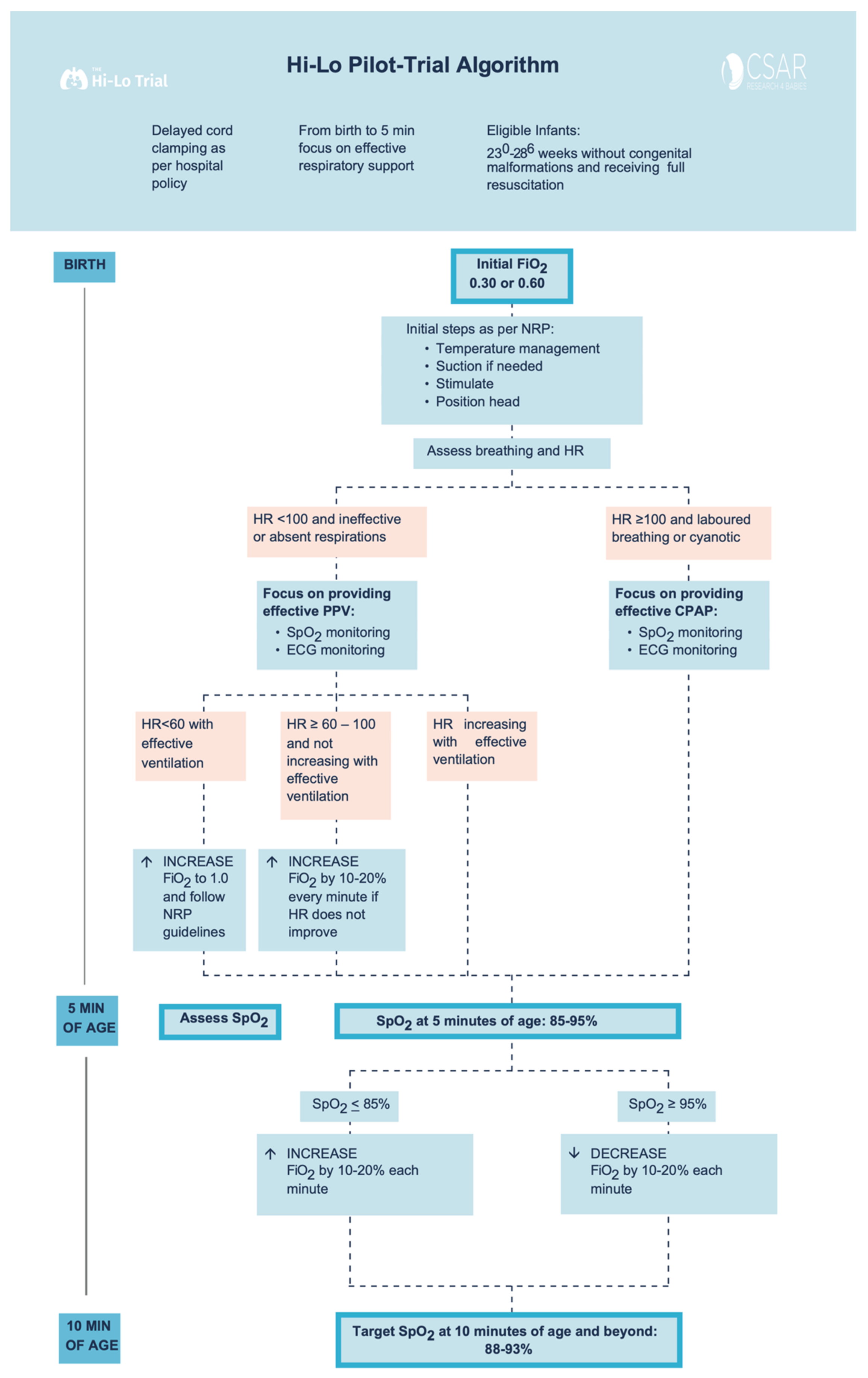
2.5. Study Interventions
2.6. Outcomes
3. Statistical Analysis
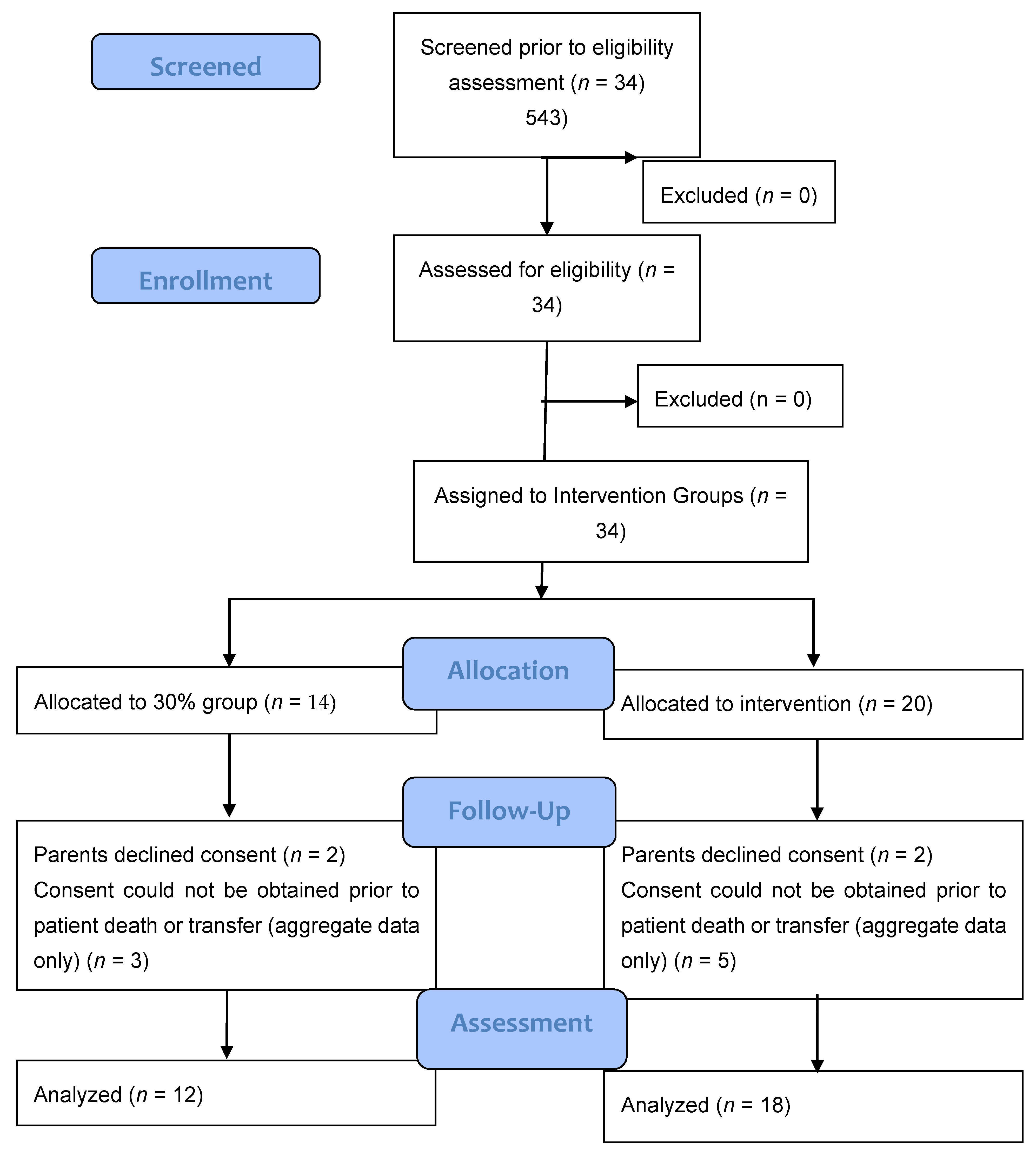
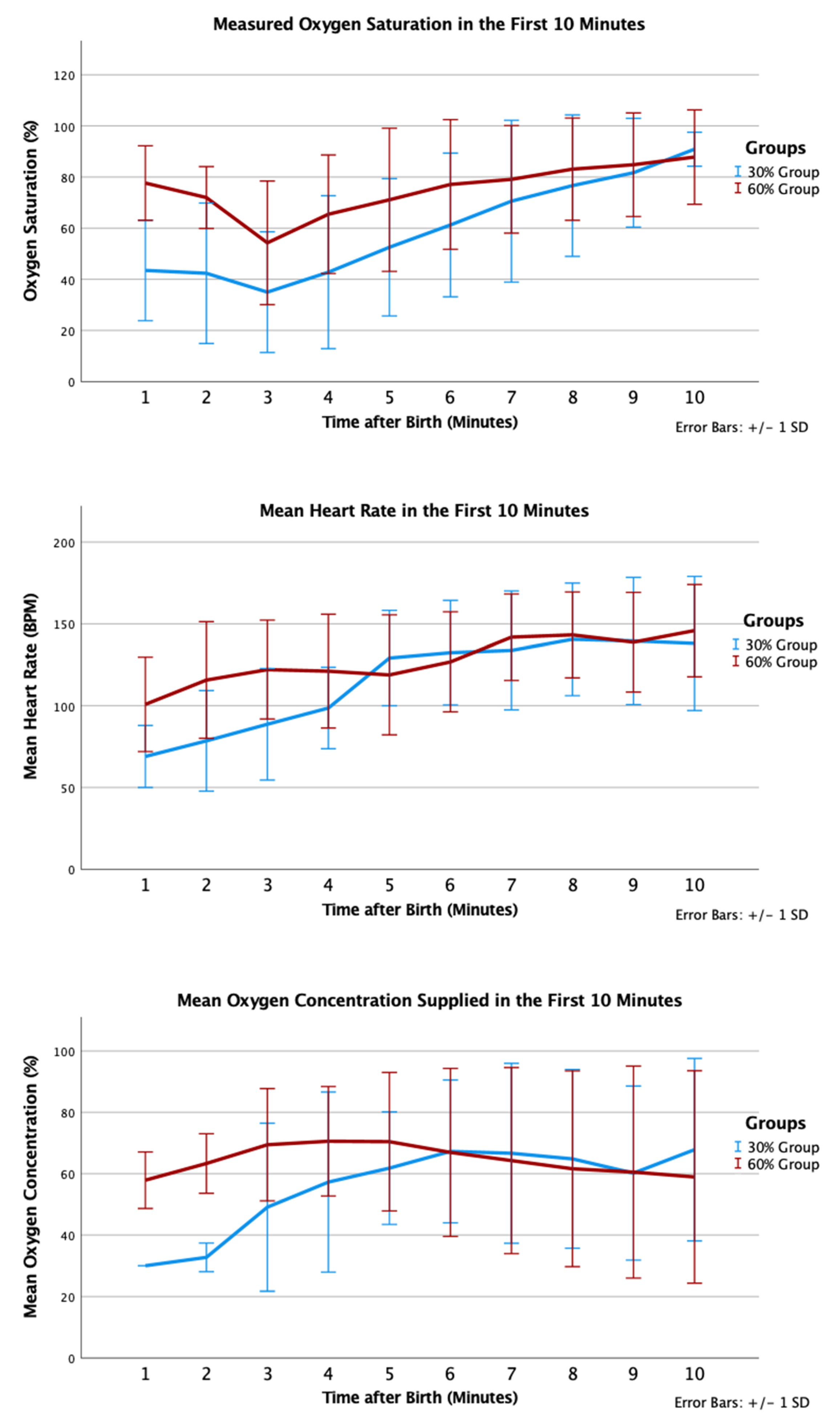
4. Results
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SpO2 | Oxygen saturation |
| HR | Heart rate |
| NICU | Neonatal Intensive Care Unit |
| SD | Standard deviation |
| IQR | Interquartile range |
References
- East, C.E.; Colditz, P.B.; Begg, L.M.; Brennecke, S.P. Update on intrapartum fetal pulse oximetry. Aust. N. Z. J. Obstet. Gynaecol. 2002, 42, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.A.; Kamlin, C.O.F.; Vento, M.; Wong, C.; Cole, T.J.; Donath, S.M.; Davis, P.G.; Morley, C.J. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010, 125, e1340–e1347. [Google Scholar] [CrossRef] [Green Version]
- Kapadia, P.; Hurst, C.; Harley, D.; Flenady, V.; Johnston, T.; Bretz, P.; Liley, H.G. Trends in neonatal resuscitation patterns in Queensland, Australia—A 10-year retrospective cohort study. Resuscitation 2020, 157, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kattwinkel, J.; Perlman, J.M.; Aziz, K.; Colby, C.; Fairchild, K.; Gallagher, J.; Hazinski, M.F.; Halamek, L.P.; Kumar, P.; Little, G.; et al. Part 15: Neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010, 122, S909–S919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oei, J.L.; Ghadge, A.; Coates, E.; Wright, I.M.; Saugstad, O.D.; Vento, M.; Buonocore, G.; Nagashima, T.; Suzuki, K.; Hosono, S.; et al. Clinicians in 25 countries prefer to use lower levels of oxygen to resuscitate preterm infants at birth. Acta Paediatr. Int. J. Paediatr. 2016, 105, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; de Almeida, M.F.; Fabres, J.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2020, 142, S185–S221. [Google Scholar] [CrossRef] [PubMed]
- Welsford, M.; Nishiyama, C.; Shortt, C.; Weiner, G.; Roehr, C.C.; Isayama, T.; Dawson, J.A.; Wyckoff, M.H.; Rabi, Y. Initial oxygen use for preterm newborn resuscitation: A systematic review with meta-analysis. Pediatrics 2019, 143, e20181828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vento, M.; Saugstad, O.D. Oxygen supplementation in the delivery room: Updated information. J. Pediatr. 2011, 158, e5–e7. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D.; Ramji, S.; Vento, M. Oxygen for newborn resuscitation: How much is enough? Pediatrics 2006, 118, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Finer, N.; Saugstad, O.; Vento, M.; Barrington, K.; Davis, P.; Duara, S.; Leone, T.; Lui, K.; Martin, R.; Morley, C.; et al. Use of oxygen for resuscitation of the extremely low birth weight infant. Pediatrics 2010, 125, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Katheria, A.C.; Hassen, K.; Rich, W.; Poeltler, D.; Finer, N. Resuscitation outcomes of infants that do not achieve a 5 min target SpO2 saturation. J. Perinatol. 2019, 39, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D.; Aune, D.; Aguar, M.; Kapadia, V.; Finer, N.; Vento, M. Systematic review and meta-analysis of optimal initial fraction of oxygen levels in the delivery room at ≤32 weeks. Acta Paediatr. Int. J. Paediatr. 2014, 103, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Oei, J.L.; Finer, N.N.; Saugstad, O.D.; Wright, I.M.; Rabi, Y.; Tarnow-Mordi, W.; Rich, W.; Kapadia, V.; Rook, D.; Smyth, J.P.; et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F446–F454. [Google Scholar] [CrossRef] [PubMed]
- Oei, J.L.; Vento, M.; Rabi, Y.; Wright, I.; Finer, N.; Rich, W.; Kapadia, V.; Aune, D.; Rook, D.; Tarnow-Mordi, W.; et al. Higher or lower oxygen for delivery room resuscitation of preterm infants below 28 completed weeks gestation: A meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F24–F30. [Google Scholar] [CrossRef] [PubMed]
- Oei, J.L.; Saugstad, O.D.; Lui, K.; Wright, I.M.; Smyth, J.P.; Craven, P.; Wang, Y.A.; McMullan, R.; Coates, E.; Ward, M.; et al. Targeted Oxygen in the Resuscitation of Preterm Infants, a Randomized Clinical Trial. Pediatrics 2017, 139, e20161452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethesda (MD): National Library of Medicine (US). Identifier NCT01773746, Study of Room Air Versus 60% Oxygen for Resuscitation of Premature Infants (PRESOX). Available online: https://clinicaltrials.gov/ct2/show/NCT01773746 (accessed on 21 July 2021).
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A.; Altman, D.; Bretz, F.; Campbell, M.; et al. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethesda (MD): National Library of Medicine (US). Identifier NCT03706586, The Hilo Trial to Improve Neurodevelopmental Outcomes in Very Low Birthweight Infants—Pilot-Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT03706586 (accessed on 21 July 2021).
- Gale, C.; Hyde, M.J.; Modi, N. Research ethics committee decision-making in relation to an efficient neonatal trial. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F291–F298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Boer, M.C.; Houtlosser, M.; Foglia, E.E.; Davis, P.G.; Van Kaam, A.H.; Kamlin, C.O.F.; Schmölzer, G.M.; De Vries, M.C.; Te Pas, A.B. Deferred consent for the enrolment of neonates in delivery room studies: Strengthening the approach. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F348–F352. [Google Scholar] [CrossRef] [PubMed]
- Canadian Institutes of Health Research; Natural Sciences and Engineering Research Council of Canada; Social Sciences and Humanities Research Council. Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans—TCPS 2. Available online: https://ethics.gc.ca/eng/tcps2-eptc2_2018_introduction.html (accessed on 12 October 2017).
- Songstad, N.T.; Roberts, C.T.; Manley, B.J.; Owen, L.S.; Davis, P.G. Retrospective consent in a neonatal randomized controlled trial. Pediatrics 2018, 141, e20172092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, W.D.; Katheria, A.C. Waiver of consent in a trial intervention occurring at birth-how do parents feel? Front. Pediatr. 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethesda (MD): National Library of Medicine (US). Identifier NCT03825835, 30% or 60% Oxygen at Birth to Improve Neurodevelopmental Outcomes in Very Low Birthweight Infants (HiLo). Available online: https://clinicaltrials.gov/ct2/show/NCT03825835 (accessed on 21 July 2021).

| 30% Oxygen (n = 12) | 60% Oxygen (n = 18) | p-Value | |
|---|---|---|---|
| Birth weight (g) | 847 (265) | 1000 (247) | 0.127 |
| Gestational age (weeks) | 25 (1.8) | 26 (1.6) | 0.131 |
| Male (n) * | 7 (58%) | 6 (35%) | 0.219 |
| Antenatal steroids (n) * | 11 (92%) | 16 (94%) | 1.00 |
| Apgar 1 min # | 2 (1–5) | 5 (2–5) | 0.149 |
| Apgar 5 min # | 6 (6–7) | 7 (4–8) | 0.258 |
| Delayed Cord Clamping (n) * | 5 (42%) | 15 (83%) | 0.045 |
| 30% Oxygen (n = 12) | 60% Oxygen (n = 18) | p-Value | |
|---|---|---|---|
| Surfactant | 11 (92%) | 13 (72%) | 0.521 |
| Death before discharge | 3 (25%) | 4 (22%) | 1.000 |
| Intraventricular hemorrhage all grades | 7 (58%) | 8 (44%) | 0.701 |
| Intraventricular hemorrhage grade ≥ 3 | 4 (33%) | 4 (22%) | 0.677 |
| Patent ductus arteriosus | 6 (50%) | 9 (50%) | 1.000 |
| Necrotizing enterocolitis | 2 (17%) | 3 (17%) | 1.000 |
| Chronic lung disease in survivor | 6 (67%) | 1 (7%) | 0.0049 |
| Retinopathy of prematurity in survivor | 3 (33%) | 3 (27%) | 0.643 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Law, B.H.Y.; Asztalos, E.; Finer, N.N.; Yaskina, M.; Vento, M.; Tarnow-Mordi, W.; Shah, P.S.; Schmölzer, G.M. Higher versus Lower Oxygen Concentration during Respiratory Support in the Delivery Room in Extremely Preterm Infants: A Pilot Feasibility Study. Children 2021, 8, 942. https://doi.org/10.3390/children8110942
Law BHY, Asztalos E, Finer NN, Yaskina M, Vento M, Tarnow-Mordi W, Shah PS, Schmölzer GM. Higher versus Lower Oxygen Concentration during Respiratory Support in the Delivery Room in Extremely Preterm Infants: A Pilot Feasibility Study. Children. 2021; 8(11):942. https://doi.org/10.3390/children8110942
Chicago/Turabian StyleLaw, Brenda Hiu Yan, Elizabeth Asztalos, Neil N. Finer, Maryna Yaskina, Maximo Vento, William Tarnow-Mordi, Prakesh S. Shah, and Georg M. Schmölzer. 2021. "Higher versus Lower Oxygen Concentration during Respiratory Support in the Delivery Room in Extremely Preterm Infants: A Pilot Feasibility Study" Children 8, no. 11: 942. https://doi.org/10.3390/children8110942
APA StyleLaw, B. H. Y., Asztalos, E., Finer, N. N., Yaskina, M., Vento, M., Tarnow-Mordi, W., Shah, P. S., & Schmölzer, G. M. (2021). Higher versus Lower Oxygen Concentration during Respiratory Support in the Delivery Room in Extremely Preterm Infants: A Pilot Feasibility Study. Children, 8(11), 942. https://doi.org/10.3390/children8110942








