Abstract
Background: Extracorporeal membrane oxygenation (ECMO) implantation for neonates with severe cardiorespiratory life-threatening conditions is highly effective. However, since ECMO is a high-risk and complex therapy, this treatment is usually performed in centers with proven expertise. Methods: A retrospective review of neonates, from January 2014 to January 2020, presenting with life-threatening conditions and treated by means of Hub and Spoke (HandS) ECMO in peripheral (spoke) hospitals. Data were retrieved from our internal ECMO registry. Protocols and checklists were revised and shared with all spoke hospitals located in North-Eastern Italy. Results: Eleven neonates receiving maximal respiratory and cardiovascular support at a spoke hospital underwent HandS ECMO management. All but three patients were affected by life-threatening meconium aspiration syndrome (MAS). The median ECMO support duration and hospitalization were four (range 2–32) and 30 days (range 8–50), respectively. All but two patients (with congenital diaphragmatic hernia), were weaned off ECMO and discharged home. At a mean follow up of 33.7 ± 29.2 months, all survivors were alive and well, without medications, and normal somatic growth. All but one had normal neuropsychological development. Conclusion: HandS ECMO model for neonates with life-threatening conditions is effective and successful. A specialized multidisciplinary team and close cooperation between Hub and Spoke centers are essential for success.
1. Introduction
Extracorporeal membrane oxygenation (ECMO) can provide valuable life support for severe acute respiratory and circulatory failure. However, since ECMO is a high-risk and complex therapy, current literature suggests that this treatment should be performed in high-volume ECMO centers [1,2,3,4]. This is even more true in the pediatric field, where neonatal ECMO and appropriate surgical and technical expertise are available in very few tertiary hospitals [3].
As described elsewhere, conventional methods of transport of critically unstable patients may be unsafe or even dangerous [5,6,7]. However, a multidisciplinary team deployed from a high-volume ECMO center (hub) can effectively initiate ECMO at the referring (spoke) hospital and transport the patient on extracorporeal support back to the hub center [8].
Currently, neonates with acute respiratory distress (ARDS) who are unresponsive to conventional therapy can be treated successfully with ECMO [9,10]. Survival in meconium aspiration syndrome (MAS) after ECMO treatment is highly satisfactory [2,11]. However, about 10% of such neonates may need a prompt transfer to a highly specialized hospital for ECMO implantation [11,12,13].
On the basis of these facts, in 2014, we started a neonatal “Hub and Spoke” (HandS) ECMO program [14], intending to provide an ECMO service in multiple sites, where the “hub” was our ECMO center, and the “spokes” were the secondary connecting centers in North-Eastern Italy. Here, we report our seven years’ experience with neonatal HandS ECMO to evaluate results and outline safeguards and pitfalls.
2. Methods
This is a retrospective clinical analysis, including all neonates who required HandS ECMO support between January 2014 and January 2020. The study was approved by the Ethics Committee of the Azienda Ospedaliera-Università di Padova (ref. Prot n. 0021321/20.02.2020). Demographic and clinical data were collected from our institutional database. A follow-up assessment was performed by our neonatal intensive care physicians, including the Denver Developmental Screening Test revised (DDST-R), used for neurodevelopmental assessment at one year [15]. A descriptive analysis of data was performed, and continuous variables were expressed in mean ± SD, median, and range.
Of note, we evaluated our organization’s effectiveness and evolution by calculating our response times to calls from spokes hospital. There are multifactorial parameters, depending on the spoke center’s distance, climate/environmental issues, and spoke center competencies. Among these, the most relevant parameters were the following: team activation time (TAT), i.e., the time elapsed from the first alert call to leave from the hub) and ECMO initiation time (EIT), i.e., the time elapsed from the first alert call to the beginning of ECMO support).
2.1. Hub and Spoke (HandS) Extracorporeal Membrane Ooxygenation (ECMO) Organization
Our neonatal health-care referral area is North-Eastern Italy (seven million people, with about 53,000 deliveries/year). The critical neonatal-and-pediatric transport program (CNaPTP) to spoke hospitals, created in 1999, has been utilized and easily adapted for HandS ECMO pediatric program. Our regional ECMO network includes patients admitted from level III spoke hospitals (Figure 1). Close cooperation between Hub and Spoke centers is encouraged (Supplementary Table S1) and spoke facilities usually repatriate their patients who have been weaned off ECMO and deliver the necessary long-term care to ensure a good turn-over at the hub center.
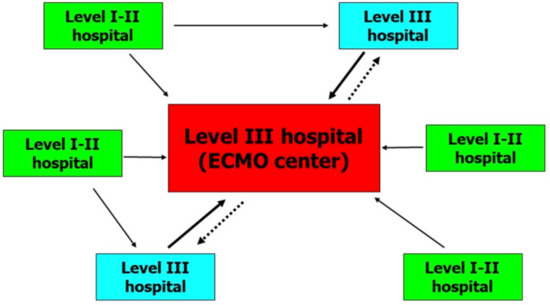
Figure 1.
Organizational model for a regional extracorporeal membrane oxygenation (ECMO) network. Continuous lines indicate urgent transports and dashed lines indicate back transports.
An ECMO team is available 24/7 and includes seven members with ECMO expertise (two surgeons, a neonatologist, a perfusionist, a neonatal intensive care nurse, and two drivers, Table 1). An operating room scrub technician is asked to be provided by the spoke center. Last, a dedicated neonatal/pediatric ambulance and a second assisting vehicle are used for ECMO transports to allocate the patient, the team, and the equipment (Figure 2).

Table 1.
Personnel and roles of the ECMO team.
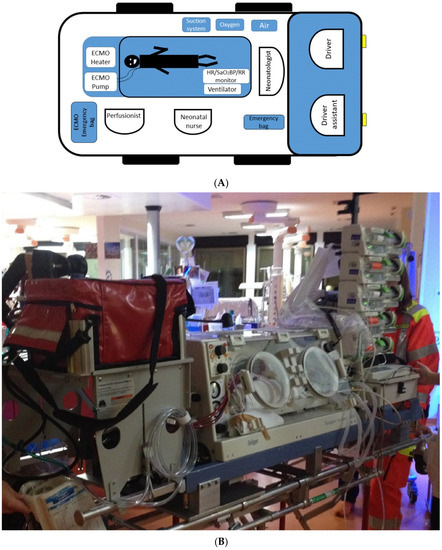
Figure 2.
(A) Diagram showing allocation of the team members in relation to the patient and equipment positioning in the ambulance; (B) Picture showing our current setting.
2.2. HandS ECMO by Steps
As we previously described [14], all HandS ECMO followed some precise steps, which were modified throughout the years, as we gained experience. These steps are as follows:
- Step 1: Start at the hub
The HandS ECMO starts with the referral from the spoke center. A neonatal ECMO consulting hotline is active 24/7, and the patient is evaluated according to selection criteria for HandS ECMO candidates. These are derived from current international guidelines [7,16] and include severe cardiac and respiratory failure, refractory to maximal medical management, with a potentially reversible etiology, and oxygenation index (OI) > 40. Exclusion criteria from HandS ECMO were severe chromosomal or other lethal anomalies, irreversible pulmonary failure or brain damage, uncontrolled bleeding, grade III or greater intraventricular hemorrhage, low birth weight (<2.0 kg), and a distance from spoke center >200 km.
After the evaluation process, the decision to decline, accept a transfer, or activate our HandS ECMO team is made (Supplementary Table S2). If the patient is considered eligible for ECMO transport, the on-call HandS team and the ambulance drivers are alerted.
- Step 2: Organization of transportation
After activation, ground transportation (2 vehicles for the patient, team, and ECMO supplies and materials) is arranged. Upon leaving the hub center, the patient’s conditions are monitored in real time with the requesting spoke center (Supplementary Table S3). At this point, the TAT is recorded.
- Step 3: Spoke center cooperation
The Spoke Center is required to prepare the appropriate field (Supplementary Table S1) for a successful ECMO initiation, in the following 3 ways: (a) optimizing the patient clinical and hemodynamic conditions until the traveling team arrives; (b) providing an adequate surgical setting, such as clearing the right neck for cannulation, alerting the scrub technician, and procuring blood products for priming for the emergent ECMO implantation; (c) parental information and a complete description of risks and benefits of ECMO, to sort out all questions and doubts, and to obtain informed written consent.
- Step 4: Arrival at the spoke center
Upon arrival, a final assessment of the patient’s clinical conditions is warranted to confirm ECMO indications’ persistence. Then, the patient is prepared on the bed in the upside-down position to have the neck well exposed and enough space to operate. After prepping the sterile field and surgically taking down of neck vessels (usually right carotid artery (RCA) and right jugular vein (RJV)), a veno-arterial ECMO is established in the usual fashion. At this point, the EIT is recorded (Supplementary Table S4).
- Step 5: ECMO initiation and stabilization
ECMO support is started. Blood flow is gradually increased until the full flow is reached. A chest X-ray is performed to check the cannulas’ position. Inhaled nitric oxide and high-frequency oscillatory ventilation are weaned off and switched to conventional mechanical ventilation for transportation. Further therapeutic maneuvers include ventilator management during transfer (low ventilation settings to allow lung rest, PEEP 7–8 cmH2O, PIP 15–22), inotropes adjustment, and sedation with narcotic and benzodiazepine, while chemical paralysis is utilized only if the patient has a congenital diaphragmatic hernia (CDH).
- Step 6: Transport and care at the hub
The patient is safely moved to the ground ambulance and transferred to the hub hospital for clinical management. In the ambulance, the positioning of the equipment and the team members is based on the specific roles and tasks (Figure 2).
When a successful transport to the hub center is achieved, and the patient is admitted into the ICU, a chest X-ray is usually repeated to check cannula position and rule out any cannulas’ dislocation during transportation. The following clinical care follows the treatment algorithms that we have previously described [17], developed by an internal multidisciplinary team, and modified according to technical improvement, recent guidelines, and all ECMO providers’ feedback. The weaning off ECMO is usually performed utilizing the so-called “bridge technique” [18], which, in our experience, has facilitated safe weaning despite the absence of a bedside specialist.
3. Results
From January 2014 to January 2020, 15 pediatric patients required HandS ECMO support in our center. Among them, 11 neonates with severe ARDS (MAS in eight neonates, CDH in two neonates, and salicylate poisoning in one neonate, Table 2) were referred from six different spoke centers in North-Eastern Italy. Of note, due to simultaneous requests, on one occasion, a second HandS ECMO team activation was required on the same day. Hub and Spoke distances ranged from 25 to 183 km (median 128), and we used ground transportation in all cases. The median TAT and EIT were 100 (30–150) and 105 (35–230) minutes, respectively. All patients underwent uneventful surgical neck vessel cannulation using pediatric 8 Fr arterial and 10 Fr venous cannula (Medtronic Inc, Minneapolis, MN, USA). After ECMO implantation and clinical stabilization, all patients were uneventfully transferred to our cardiac ICU, where nine patients were weaned off ECMO support without complications after a median time of four days (range 2–32). Soon after decannulation, patients were transferred to our neonatal intensive care unit for further care before repatriation or discharge.

Table 2.
Patients characteristics at Hub and Spoke (HandS) ECMO implantation, and modes of ventilation before (at the spoke) and after (at the hub).
One patient with ARDS caused by salicylate poisoning presented left hemisphere cerebral stroke two days after weaning off ECMO, due to occlusion of the left internal carotid artery, despite full anticoagulation during ECMO and after weaning. The precise cause was not identified. Full coagulation protein screening was within normal limits. The right carotid artery (where the arterial cannula was previously placed for ECMO cannulation) was partially patent.
Two patients with a severe degree of CDH (upward liver dislocation) did not survive. The first presented with severe pulmonary hypoplasia and was weaned off ECMO after 32 days and died immediately after compassionate care. After successful CDH repair and ECMO weaning, the other patient developed refractory pulmonary hypertension, was not responsive to pulmonary vasodilators, and required a redo ECMO, but died from retroperitoneal hemorrhage two days thereafter.
All remaining nine survivors were discharged from the hub after a median stay of 30 days (8–50); eight survivors were repatriated to the spoke center, while one was discharged home.
At a median follow-up of 14.4 months (range 1.3–74.8), all survivors were at home alive and well, with average growth and normal respiratory conditions. Neuropsychological development was normal in all but one patient, who had a stroke after ECMO, and is currently being treated with antiepileptic therapy. Postoperative data and Hands ECMO mission times are summarized in Table 3 and Table 4, respectively.

Table 3.
Postoperative and follow-up data.

Table 4.
Times of HandS ECMO mission.
4. Discussion
Neonatal ARDS is a life-threatening condition that may require emergency respiratory ECMO support when conventional treatment options fail [9,10,11,12]. As these patients can fully recover if prompt treatment is established, it is essential to arrange a HandS ECMO service to provide assistance even in peripheral hospitals. Our experience has proven that this can be highly effective, with a 100% survival in neonates with severe MAS.
It is widely known that candidates of ECMO support have an estimated probability of death of 80–100%, despite maximal conventional therapies. According to the Extracorporeal Life Support Organization (ELSO) Registry [16], current ECMO survival rates are highly satisfactory, ranging from 41% in children with heart failure to 74% in newborns with any ARDS [16]. However, due to resource allocation and costs, specialized ECMO interventions are usually provided in tertiary, high-volume, dedicated centers with proven expertise that can provide the best care and optimize results [2,3,4,16,19,20].
The concept of a mobile ECMO team has been reported either for adults [1,5,7,8,11,19,20,21,22,23,24,25] and children [9,14,26], and highly successful transportation of patients on ECMO has been described for short and long distances by ambulance, helicopter, and airplane [25,27,28]. As stated by Coombs [1], “each ECMO network should ideally create mobile ECMO teams to retrieve patients and to deal with patients who have critical cardiopulmonary failure refractory to conventional therapy”. Hospital networks at the local, regional, or interregional level have been successfully created around tertiary referral hospital with ECMO expertise in the UK [22], Italy [26], and Australia [27] and have been associated with encouraging results for the treatment of the most severe forms of influenza A (H1N1)-associated ARDS [29].
Following this concept, in 2014, we started our HandS ECMO program, supported by the preexisting CNaPTP, which has been essential for success. The CNaPTP covers more than 20 hospitals in our institution and has the capacities and equipment (including HFOV, inhaled nitric oxide, and therapeutic hypothermia) to provide care to high-risk neonates during transportation.
The entire equipment, surgical technique, and management protocols during transportation and afterward do not differ from our standard. In our practice, we have been using our usual protocol modalities for managing ECMO, which are characterized by a limited number of human resources, as previously described [17]. Despite this “basic” arrangement, which has never reduced ECMO effectiveness [17], a successful organizational framework of the HandS ECMO model for neonates has been a viable and highly successful option for peripheral spoke hospitals, as demonstrated by the activation of two simultaneous teams.
Nonetheless, for this arrangement to succeed, it is essential that close cooperation between Hub and Spoke centers is established (Supplementary Table S1). In fact, in our experience, the spoke centers could achieve hemodynamic stabilization of these high-risk neonates and promptly recognized ECMO indication and timing. In order to do this, spoke hospitals in such a network should be trained and adhere to written standardized protocols that detail criteria for both the initiation of ECMO (indications and exclusions) [7,16], as well as optimization of conventional treatments to be undertaken before considering ECMO (such as low-volume, low-pressure, lung-protective ventilation or the use of prone positioning in patients with severe ARDS) [30]. Comprehensive plans regarding access to mobile ECMO should be created within networks. Referral centers and other network members should hold regular meetings to discuss network activity, including a review of ECMO cases. as well as those patients who were deemed inappropriate for ECMO. The preoperative stabilization and optimal timing, and multi-organ care of the patients are essential to minimize postoperative complications related to anticoagulation and ECMO circuit. One of our patients, who had been hemodynamically unstable and hypoxic for a more extended time than other patients, and experienced prolonged hypoxemia, had the only neurological complication we experienced in our series.
Furthermore, to ensure reasonable outcomes at the hub center, spoke facilities must repatriate patients and deliver the necessary long-term care. This kind of collaboration is vital and extremely rewarding because every medical center cannot offer the same degree of expertise in neonatal ECMO implants. However, the HandS model permits a tertiary institution to act as a hub center for peripheral hospitals without advanced cardiac or respiratory care, improving critically ill neonates’ survival.
When we deal with ECMO patients, timing is crucial; if too late, ECMO is useless. As practiced elsewhere [8], minimizing the time to reach and secure the patient is the main priority in planning the primary transportation. For this reason, we created some simple parameters (TAT and EIT) in order to evaluate our organization’s improvement and causes of failures, and that we expect to guide future decisions when a HandS ECMO program is growing. For this reason, the distance limit (<200 km) that we currently refer to may be modified in the future or even not applicable if using a different vehicle. In our experience, ground transportation was a more feasible and practical form of transport in our global organization. In addition, all ECMO transfers were uneventful, since we could benefit from the former CNaPTP experience. The potential risk of ECMO cannulas dislocation and the need to repositioning them during transportation remains possible but less probable when a highly specialized and expert team is provided.
Although the veno-venous (V-V) ECMO support is often indicated because of its simplicity and effectiveness in ARDS [31], in our experience, we have not used it, since ARDS can often significantly affect hemodynamic function in neonates. In addition, our emergency experience has been mostly with V-A ECMO rather than V-V ECMO, and we did not report increased complications as compared with the literature.
In Figure 1, we describe an organizational model for a regional ECMO network. All patients who received ECMO were admitted from level III hospitals where maximal cardiorespiratory support was already in place. Two out of 11 patients were born in a level I hospital and were transferred to level III hospitals unable to offer ECMO. About 12 h later, these critical patients met ECMO treatment criteria, and our HandS ECMO team was activated. This suggests that, although clinical conditions initially appear stable, it would be better for neonates with MAS to be quickly admitted to an ECMO center, because acute and rapid respiratory deterioration can occur during the first hours of life.
On the one hand, our experience has proven that HandS ECMO can be highly effective, with a 100% survival in neonates with severe MAS. The onset of post-ECMO complications in this series was not higher than that reported in the literature [16] and did not differ in quality from complications that could occur during standard ECMO management. On the other hand, in our series, HandS ECMO for CDH has been highly unsuccessful. Certainly, CDH is the first cause of neonatal ARDS requiring ECMO support [32] and causes pulmonary hypoplasia and hypertension, leading to cardiorespiratory failure with high mortality and long-term morbidity [33,34]. Survival, even with ECMO, is not more than 50% [35,36,37]. However, similar survival rates both with and without utilizing ECMO have been reported, and questions about the utility of ECMO support in the CDH population remain. Treatment of CDH is known to be difficult, and the analysis of repair outcomes on or off ECMO is prone to confounding factors, including variability in disease severity and overall management [36]. This supports our impression that there are no particular problems related to HandS ECMO support that affect the outcome. The severity of CDH and complicated management of postoperative pulmonary hypertension and prolonged ventilation-related complications may have played a significant role in the unsuccessful outcome. An anticipatory strategy should be considered in these cases. CDH may be considered to be an indication that the mother should be referred to a tertiary center with an ECMO facility to minimize additional perinatal lethal or invalidating complications for such a delicate neonate.
5. Conclusions
The HandS ECMO model for neonates with life-threatening conditions is effective and successful. Appropriate patient selection, proven ECMO and neonatal transportation expertise, and use of validated protocols and checklists, together with close cooperation among Hub and Spoke centers, are essential for success.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9067/8/1/24/s1, Table S1: “SPOKE” CENTER ECMO CHECKLIST, Table S2: Pre-HandS demographic data form (for physisican), Table S3: Pediatric HandS ECMO transfer form (for Pediatric ICU nurse), Table S4: Pediatric HandS ECMO data form (for the pefusionist).
Author Contributions
Conceptualization, M.A.P., E.B., V.L.V. and D.T.; Data curation, M.A.P., N.D. and D.N.; Formal analysis, E.B. and D.T.; Investigation, M.A.P., N.D., E.B. and D.T.; Methodology, M.A.P., E.B. and D.T; Project administration, D.N. and E.B.; Resources, M.A.P.; Supervision, M.A.P. and N.D.; Validation, M.A.P.; Writing—original draft, M.A.P., E.B. and D.T.; Writing—review & editing, M.A.P., N.D., D.N., E.B. and D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Azienda Ospedaliera-Università di Padova (ref. Prot n. 0021321/20.02.2020).
Informed Consent Statement
Patient consent was waived due to the observational nature of the study.
Data Availability Statement
Data is contained within the article or Supplementary Material. The data presented in this study are available in Supplementary Material here.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ECMO | Extracorporeal membrane oxygenation |
| HandS | Hub and Spoke |
| MAS | Meconium aspiration syndrome |
| ARDS | Acute respiratory distress |
References
- Combes, A.; Brodie, D.; Bartlett, R.; Laurent, B.; Roy, B.; Steve, C.; Daniel, B.; Eddy, F.; Niall, F.; James, F.; et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am. J. Respir. Crit. Care Med. 2014, 190, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Karamlou, T.; Vafaeezadeh, M.; Parrish, A.M.; Gordon, A.C.; Karl, F.W.; Lester, P.; Michael, M. Increased extra- corporeal membrane oxygenation center case volume is associated with improved extracorporeal membrane oxygenation survival among pediatric patients. J. Thorac. Cardiovasc. Surg. 2013, 145, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.L.; Bennett, T.D.; Casper, T.C.; Gitte, Y.L.; Ania, H.; Jacob, W.; Susan, L.B. Pediatric and neonatal extracorporeal membrane oxygenation: Does center volume impact mortality? Crit. Care Med. 2014, 42, 512–519. [Google Scholar] [CrossRef]
- Barbaro, R.P.; Odetola, F.O.; Kidwell, K.M.; Matthew, L.P.; Robert, H.B.; Matthew, M.D.; Gail, M.A. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the Extracorporeal Life Support Organization registry. Am. J. Respir. Crit. Care Med. 2015, 191, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Javidfar, J.; Brodie, D.; Takayama, H.; Mongero, L.; Zwischenberger, J.; Sonett, J.; Bacchetta, M. Safe transport of critically ill adult patients on extracorporeal membrane oxygenation support to a regional extracorporeal membrane oxygenation center. ASAIO J. 2011, 57, 421–425. [Google Scholar] [CrossRef]
- Michaels, A.J.; Hill, J.G.; Long, W.B.; Brian, P.; Young, M.D.; Bernie, P.; Sperley, D.O.; Tanya, R.; Shanks, R.N.; Lori, J.; et al. Adult refractory hypoxemic acute respiratory distress syndrome treated with extracorporeal membrane oxygenation: The role of a regional referral center. Am. J. Surg. 2013, 205, 492–498. [Google Scholar] [CrossRef]
- Guidelines for ECMO Transport. Ann Arbor, MI, Extracorporeal Life Support Organization (ELSO). Available online: https://www.elso.org/Portals/0/Files/ELSOGUIDELINESFORECMOTRANSPORT2015–2018 (accessed on 20 December 2020).
- Broman, L.M.; Holzgraefe, B.; Palmér, K.; Frenckner, B. The Stockholm experience: Interhospital transports on extracorporeal membrane oxygenation. Crit. Care 2015, 19, 278. [Google Scholar] [CrossRef] [PubMed]
- Dargaville, P.A. Respiratory Support in Meconium Aspiration Syndrome: A Practical Guide. Int. J. Pediatrics 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paranka, M.S.; Clark, R.H.; Yoder, B.A.; Null, D.M., Jr. Predictors of failure of high-frequency oscillatory ventilation in term infants with severe respiratory failure. Pediatrics 1995, 95, 400–404. [Google Scholar] [PubMed]
- Singh, B.S.; Clark, R.H.; Powers, R.J.; Spitzer, A.R. Meconium aspiration syndrome remains a significant problem in the NICU: Outcomes and treatment patterns in term neonates admitted for intensive care during a ten-year period. J. Perinatol. 2009, 29, 497–503. [Google Scholar] [CrossRef]
- Broman, L.M.; Frenckner, B. Transportation of critically ill patients on extracorporeal membrane oxygenation. Front. Pediatrics 2016, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Short, B.L. Extracorporeal membrane oxygenation: Use in meconium aspiration syndrome. J. Perinatol. 2008, 28, S79–S83. [Google Scholar] [CrossRef] [PubMed]
- Fichera, D.; Zanella, F.; Fabozzo, A.; Doglioni, N.; Trevisanuto, D.; Lolli, E.; Vida, V.; Ceccherini, E.; Ebraico, A.; Stellin, G.; et al. HandS ECMO: Preliminary Experience with “Hub and Spoke” Model in Neonates with Meconium Aspiration Syndrome. Artif. Organs 2019, 43, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Frankenburg, W.K.; Dodds, J.; Archer, P.; Shapiro, H.; Bresnick, B. The Denver II: Technical Manual; Denver Developmental Materials: Denver, CO, USA, 1990. [Google Scholar]
- Thiagarajan, R.R.; Barbaro, R.P.; Mcmullan, D.; Michael, C.; Steven, A.; Fortenberry, J.D.; Paden, M.L. Extracorporeal Life Support Organization Registry International Report. ASAJO J. 2017, 63, 60–67. [Google Scholar] [CrossRef]
- Padalino, M.A.; Tessari, C.; Guariento, A.; Frigo, A.C.; Vida, V.L.; Marcolongo, A.; Zanella, F.; Harvey, M.J.; Thiagarajan, R.R.; Stellin, G. The “basic” approach: A single-centre experience with a cost-reducing model for paediatric cardiac extracorporeal membrane oxygenation. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Vida, V.L.; Lo, R.M.; Padalino, M.A.; Stellin, G. Extracorporeal membrane oxygenation: The simplified weaning bridge. J. Thorac. Cardiovasc. Surg. 2012, 143, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Noah, M.A.; Peek, G.J.; Finney, S.J.; Griffiths, M.J.; Harrison, D.A.; Grieve, R.; Sadique, M.Z.; Sekhon, J.S.; Auley, D.F.; Firmin, R.K.; et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011, 306, 1659–1668. [Google Scholar] [CrossRef]
- Jaroszewski, D.E.; Kleisli, T.; Staley, L.; Pierce, C.; Scott, R.; Steidley, D.E.; Valeria, P.; Arabia, F.A. A traveling team concept to expedite the transfer and management of unstable patients in cardiopulmonary shock. J. Heart Lung Transpl. 2011, 30, 618–623. [Google Scholar] [CrossRef]
- Bryner, B.; Cooley, E.; Copenhaver, W.; Kristin, B.B.S.; Nicholas, T.M.D.; Denise, L.R.N.; Peter, R.; Mark, H.; Lena, M.N.; Jonathan, H.; et al. Two decades’experience with interfacility transport on extracorporeal membrane oxygenation. Ann. Thor. Surg. 2014, 98, 1363–1370. [Google Scholar] [CrossRef]
- Coppola, C.P.; Tyree, M.; Larry, K.; Geronimo, R. A 22 year experience in global extracorporeal membrane oxygenation. J. Pediatrics Surg. 2008, 43, 46–52. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization. ELSO Neonatal Respiratory Failure Supplement to the ELSO General Guidelines. Version 1.4. Available online: http://www.elso.org (accessed on 10 December 2017).
- ECMOnet, Censimento delle Terapie Intensive Italiane. Available online: http://www.ecmonet.org/index.php?pag=cartina&id_sezione=68&id_supersezione=65 (accessed on 4 September 2017).
- Lucchini, A.; Felippis, C.; Elli, S.; Gariboldi, R.; Vimercati, S.; Tundo, P.; Bondi, H.; Costa, M.C. Mobile ECMO team for inter-hospital transportation of patients with ARDS: A retrospective case series. Heart Lung Vessel 2014, 6, 262–273. [Google Scholar] [PubMed]
- Nardo, M.; Lonero, M.; Pasotti, E.; Cancani, F.; Perrotta, D.; Cecchetti, C.; Stoppa, F.; Pirozzi, N.; Salvia, O.; Nicolini, A.; et al. The first five years of neonatal and pediatric transports on extracorporeal membrane oxygenation in the center and south of Italy: The pediatric branch of the Italian “Rete Respira” network. Perfusion 2018, 33, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Forrest, P.; Ratchford, J.; Burns, B.; Herkes, R.; Jackson, A.; Plunkett, B.; Torzillo, P.; Nair, P.; Granger, E.; Wilson, M.; et al. Retrieval of critically ill adults using extracorporeal membrane oxygenation: An Australian experience. Intensive Care Med. 2011, 37, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, G.; Sanchez, B.; Hennequin, J.L.; Resiere, D.; Hommel, D.; Leonard, C.; Mehdaoui, H.; Roques, F. The French airbridge for circulatory support in the Carribean. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Patroniti, N.; Zangrillo, A.; Pappalardo, F.; Peris, A.; Cianchi, G.; Braschi, A.; Iotti, G.A.; Arcadipane, A.; Panarello, G.; Ranieri, V.M.; et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: Preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011, 37, 1447–1457. [Google Scholar] [CrossRef]
- Guerin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Speggiorin, S.; Robinson, S.G.; Harvey, C.; Westrope, C.; Faulkner, G.M.; Kirkland, P.; Peek, G.J. Experience with the Avalon® bicaval double-lumen veno-venous cannula for neonatal respiratory ECMO. Perfusion 2015, 30, 250–254. [Google Scholar] [CrossRef]
- ELSO Registry Report. Available online: www.elso.org (accessed on 4 July 2020).
- Wang, Y.; Honeyford, K.; Aylin, P.; Bottle, A.; Giuliani, S. One-year outcomes for congenital diaphragmatic hernia. BJS Open 2019, 3, 305–313. [Google Scholar] [CrossRef]
- Vaja, R.; Bakr, A.; Sharkey, A.; Joshi, V.; Faulkner, G.; Westrope, C.; Harvey, C. The use of extracorporeal membrane oxygenation in neonates with severe congenital diaphragmatic hernia: A 26-year experience from a tertiary centre. Eur. J. Cardiothorac. Surg. 2017, 52, 552–557. [Google Scholar] [CrossRef]
- Grover, T.R.; Rintoul, N.E.; Hedrick, H.L. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia. Semin. Perinatol. 2018, 42, 96–103. [Google Scholar] [CrossRef]
- Kays, D.W. ECMO in CDH: Is there a role? Semin. Pediatrics Surg. 2017, 26, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Delaplain, P.T.; Jancelewicz, T.; Nardo, M.; Zhang, L.; Yu, P.T.; Cleary, J.P.; Francesco, M.; Matthew, T.H.; Danh, V.N.; Yigit, S.G. Management preferences in ECMO mode for congenital diaphragmatic hernia. J. Pediatrics Surg. 2019, 54, 903–908. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).