Pulmonary Hypertension with Prolonged Patency of the Ductus Arteriosus in Preterm Infants
Abstract
1. Introduction
2. Pathophysiology
2.1. Magnitude of PDA and Prematurity
- (i)
- Faster development of pulmonary overcirculation: The intrinsic tone in their pulmonary arteries is relatively lesser due to a smaller amount of muscle in the media. This precludes appropriate pulmonary constriction and leads to pulmonary overcirculation and cardiac volume overload earlier (2–3 weeks of age) than in term infants (3–6 weeks) [3].
- (ii)
- Delayed pulmonary vascular maturation: Similar to respiratory distress syndrome, preterm infants have differential perfusion, i.e, poorly aerated lung segments have decreased PBF and well-aerated segments have good PBF. A left-to-right shunt increases PBF to the already well perfused segments and can lead to pulmonary edema. These pre-capillary vessels are poorly reactive and transmit arterial pressure. A persistent increase in PBF over a prolonged period delays the normal maturation of pulmonary blood vessels. This leads to persistence of smooth muscle with proliferation and hypertrophy and the development of PVD.
- (iii)
- Risk of BPD: Preterm infants with a chronic hsPDA are observed to have an increased incidence of BPD [9]. There is equalization of aorto-pulmonary pressures in a large hsPDA. This leads to an increase in the pulmonary venous pressure and the left atrial pressure. The pulmonary capillaries in preterm infants are more permeable. This leads to the leakage of plasma proteins into air-sacs, which affects the function of the surfactant and reduces lung compliance. The mean airway pressures rise to provide adequate oxygenation to non-compliant lungs. This can cause lung damage and possibly lead to BPD [3].
2.2. Impact of an hsPDA on Pulmonary Vasculature
2.3. Consequence of Prolonged Patency of the PDA on Pulmonary Vasculature
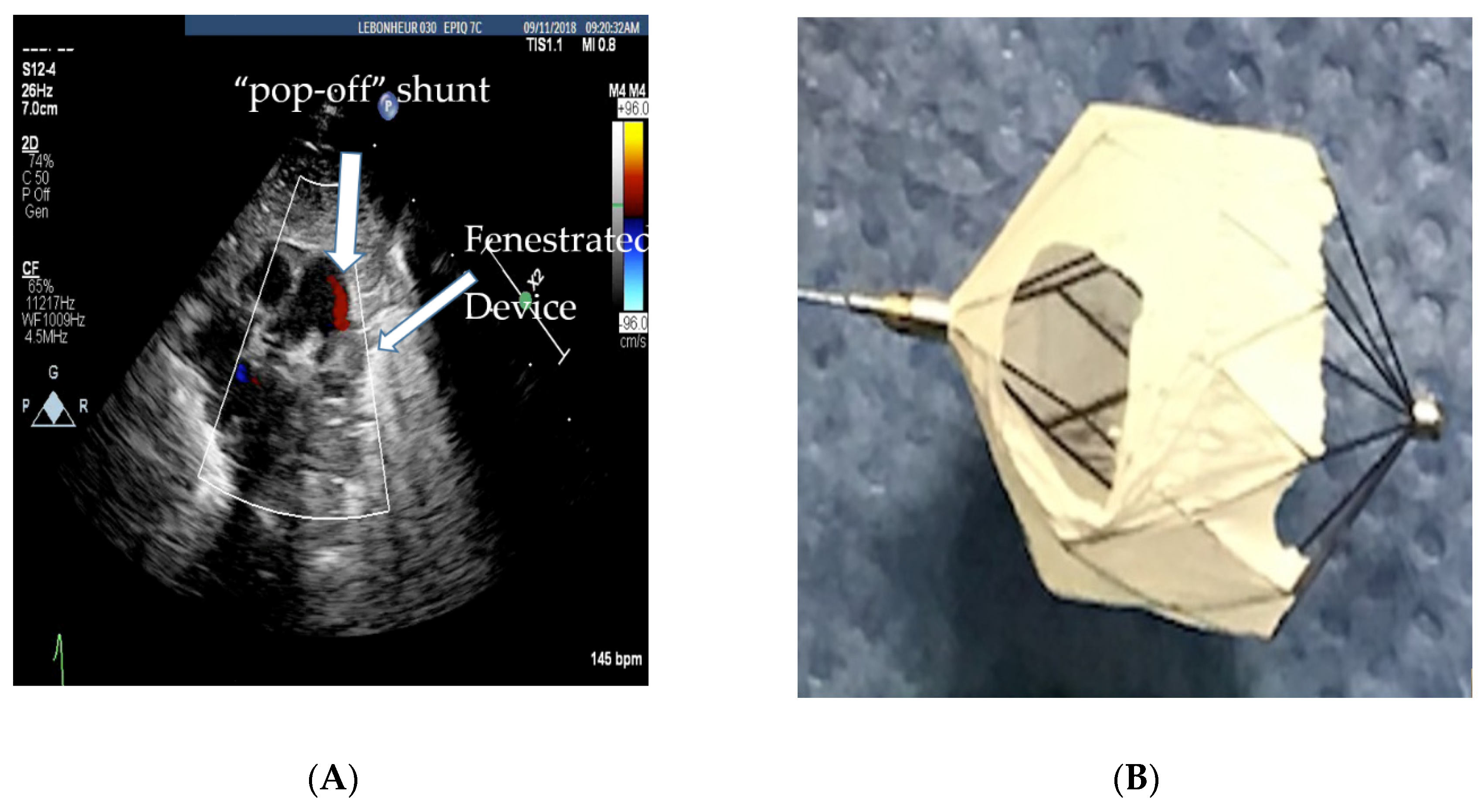
3. Management of a Large PDA with Pulmonary Hypertension
Role of Cardiac Catheterization
- (a)
- 20% drop in the baseline systolic PAP or at least no increase in systolic PAP.
- (b)
- No drop in the systemic pressure. This suggests that the PDA was not required for providing cardiac output.
- (c)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhat, R.; Salas, A.A.; Foster, C.; Carlo, W.A.; Ambalavanan, N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 2012, 129, e682–e689. [Google Scholar] [CrossRef] [PubMed]
- Mourani, P.M.; Sontag, M.K.; Younoszai, A.; Miller, J.I.; Kinsella, J.P.; Baker, C.D.; Poindexter, B.B.; Ingram, D.A.; Abman, S.H. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2015, 191, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.; Towbin, J.A.; Sathanandam, S.; Goldberg, J.; Yohannan, T.; Swaminathan, N.; Johnson, J.N. Effect of patent ductus arteriosus on the heart in preterm infants. Congenit. Heart Dis. 2019, 14, 33–36. [Google Scholar] [CrossRef] [PubMed]
- D’Alto, M.; Mahadevan, V.S. Pulmonary arterial hypertension associated with congenital heart disease. Eur. Respir. Rev. 2012, 21, 328–337. [Google Scholar] [CrossRef]
- Bando, K.; Turrentine, M.W.; Sharp, T.G.; Sekine, Y.; Aufiero, T.X.; Sun, K.; Brown, J.W. Pulmonary hypertension after operation for congenital heart disease: Analysis of risk factors and management. J. Thorac. Cardiovasc. Surg. 1996, 112, 1600–1609. [Google Scholar] [CrossRef]
- Tharakan, J.S. Large patent ductus arteriosus: To close or not to close. Ann. Pediatric Cardiol. 2012, 5, 141–144. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Saugstad, O.D. The fetal circulation, pathophysiology of hypoxemic respiratory failure and pulmonary hypertension in neonates, and the role of oxygen therapy. J. Perinatol. 2016, 36 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef]
- Philip, R.; Waller, B.R., 3rd; Agrawal, V.; Wright, D.; Arevalo, A.; Zurakowski, D.; Sathanandam, S. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc. Interv. 2016, 87, 310–317. [Google Scholar] [CrossRef]
- Rudolph, A.M. Congenital Diseases of the Heart: Clinical-Physiologic Considerations, 3rd ed.; Wiley-Blackwell: San Francisco, CA, USA, 2009. [Google Scholar]
- Rondelet, B.; Kerbaul, F.; Van Beneden, R.; Hubloue, I.; Huez, S.; Fesler, P.; Remmelink, M.; Brimioulle, S.; Salmon, I.; Naeije, R. Prevention of pulmonary vascular remodeling and of decreased BMPR-2 expression by losartan therapy in shunt-induced pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2319–H2324. [Google Scholar] [CrossRef]
- Lam, C.-F.; Peterson, T.E.; Croatt, A.J.; Nath, K.A.; Katusic, Z.S. Functional adaptation and remodeling of pulmonary artery in flow-induced pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2334–H2341. [Google Scholar] [CrossRef]
- Adatia, I.; Kothari, S.S.; Feinstein, J.A. Pulmonary hypertension associated with congenital heart disease: Pulmonary vascular disease: The global perspective. Chest 2010, 137 (Suppl. S6), 52S–61S. [Google Scholar] [CrossRef]
- Rosenzweig, E.B.; Barst, R.J. Congenital heart disease and pulmonary hypertension: Pharmacology and feasibility of late surgery. Prog. Cardiovasc. Dis. 2012, 55, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Pereiras, L.; Head, T.; Dupuis, G.; Sessums, J.; Corder, G.; Graves, K.; Tipton, J.; Sathanandam, S. Transport of extremely low birth weight neonates for persistent ductus arteriosus closure in the catheterization lab. Congenit. Heart Dis. 2019, 14, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Balduf, K.; Chilakala, S.; Washington, K.; Allen, K.; Knott-Craig, C. Role of Transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter Cardiovasc. Interv. 2019, 93, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Agrawal, H.; Chilakala, S.; Johnson, J.; Allen, K.; Knott-Craig, C.; Waller, B.R.; Philip, R. Can Transcatheter PDA closure be performed in neonates ≤1000 grams? The Memphis experience. Congenit. Heart Dis. 2019, 14, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Sathanandam, S.; Justino, H.; Waller, B.R., 3rd; Radtke, W.; Qureshi, A.M. Initial clinical experience with the Medtronic Micro Vascular Plug™ in transcatheter occlusion of PDAs in extremely premature infants. Catheter Cardiovasc. Interv. 2017, 89, 1051–1058. [Google Scholar] [CrossRef]
- Sathanandam, S.K.; Gutfinger, D.; O’Brien, L.; Forbes, T.J.; Gillespie, M.J.; Berman, D.P.; Armstrong, A.K.; Shahanavaz, S.; Jones, T.K.; Morray, B.H.; et al. Amplatzer Piccolo Occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter Cardiovasc. Interv. 2020. [Google Scholar] [CrossRef]
- Krishnan, U.; Feinstein, J.A.; Adatia, I.; Austin, E.D.; Mullen, M.P.; Hopper, R.K.; Hanna, B.; Romer, L.; Keller, R.L.; Fineman, J.; et al. Pediatric Pulmonary Hypertension Network (PPHNet).Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia. J. Pediatrics 2017, 188, 24–34. [Google Scholar] [CrossRef]
- Niu, M.C.; Mallory, G.B.; Justino, H.; Ruiz, F.E.; Petit, C.J. Treatment of severe pulmonary hypertension in the setting of the large patent ductus arteriosus. Pediatrics 2013, 131, e1643–e1649. [Google Scholar] [CrossRef]
- Yan, C.; Zhao, S.; Jiang, S.; Xu, Z.; Huang, L.; Zheng, H.; Ling, J.; Wang, C.; Wu, W.; Hu, H.; et al. Transcatheter closure of patent ductus arteriosus with severe pulmonary arterial hypertension in adults. Heart 2007, 93, 514–518. [Google Scholar] [CrossRef]
- Feng, J.; Kong, X.; Sheng, Y.; Yang, R. Patent ductus arteriosus with persistent pulmonary artery hypertension after transcatheter closure. Clin. Risk Manag. 2016, 12, 1609–1613. [Google Scholar] [CrossRef]
- Khan, A.H.; Hoskoppal, D.; Kumar, T.K.S.; Bird, L.; Allen, K.; Lloyd, H.; Knott-Craig, C.J.; Waller, B.R.; Sathanandam, S. Utility of the Medtronic microvascular plug™ as a transcatheter implantable and explantable pulmonary artery flow restrictor in a swine model. Catheter Cardiovasc. Interv. 2019, 93, 1320–1328. [Google Scholar] [CrossRef]
- Apalodimas, L.; Waller, I.I.I.B.R.; Philip, R.; Crawford, J.; Cunningham, J.; Sathanandam, S. A comprehensive program for preterm infants with patent ductus arteriosus. Congenit. Heart Dis. 2019, 14, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Philip, R.; Waller, B.; Chilakala, S.; Graham, B.; Stecchi, N.; Apalodimas, L.; Cunningham, J.; Washington, K.; Sathanandam, S. Hemodynamic and Clinical Consequences of Early Versus Delayed Closure of Patent Ductus Arteriosus in Extremely Low Birth Weight Infants. J. Perinatol. 2020. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philip, R.; Lamba, V.; Talati, A.; Sathanandam, S. Pulmonary Hypertension with Prolonged Patency of the Ductus Arteriosus in Preterm Infants. Children 2020, 7, 139. https://doi.org/10.3390/children7090139
Philip R, Lamba V, Talati A, Sathanandam S. Pulmonary Hypertension with Prolonged Patency of the Ductus Arteriosus in Preterm Infants. Children. 2020; 7(9):139. https://doi.org/10.3390/children7090139
Chicago/Turabian StylePhilip, Ranjit, Vineet Lamba, Ajay Talati, and Shyam Sathanandam. 2020. "Pulmonary Hypertension with Prolonged Patency of the Ductus Arteriosus in Preterm Infants" Children 7, no. 9: 139. https://doi.org/10.3390/children7090139
APA StylePhilip, R., Lamba, V., Talati, A., & Sathanandam, S. (2020). Pulmonary Hypertension with Prolonged Patency of the Ductus Arteriosus in Preterm Infants. Children, 7(9), 139. https://doi.org/10.3390/children7090139




