Quantitative Sensory Testing in Adolescents with Co-Occurring Chronic Pain and Obesity: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Participants
2.3. Quantitative Sensory Testing
2.3.1. Heat Pain Threshold (HPT)
2.3.2. Heat Perceptual Sensitization (HPS)
2.3.3. Mechanical Pain Threshold (MPT)
2.3.4. Mechanical Perceptual Sensitization (MPS)
2.4. Power Analysis
2.5. Statistical Analysis
3. Results
3.1. Recruitment
3.2. Participants
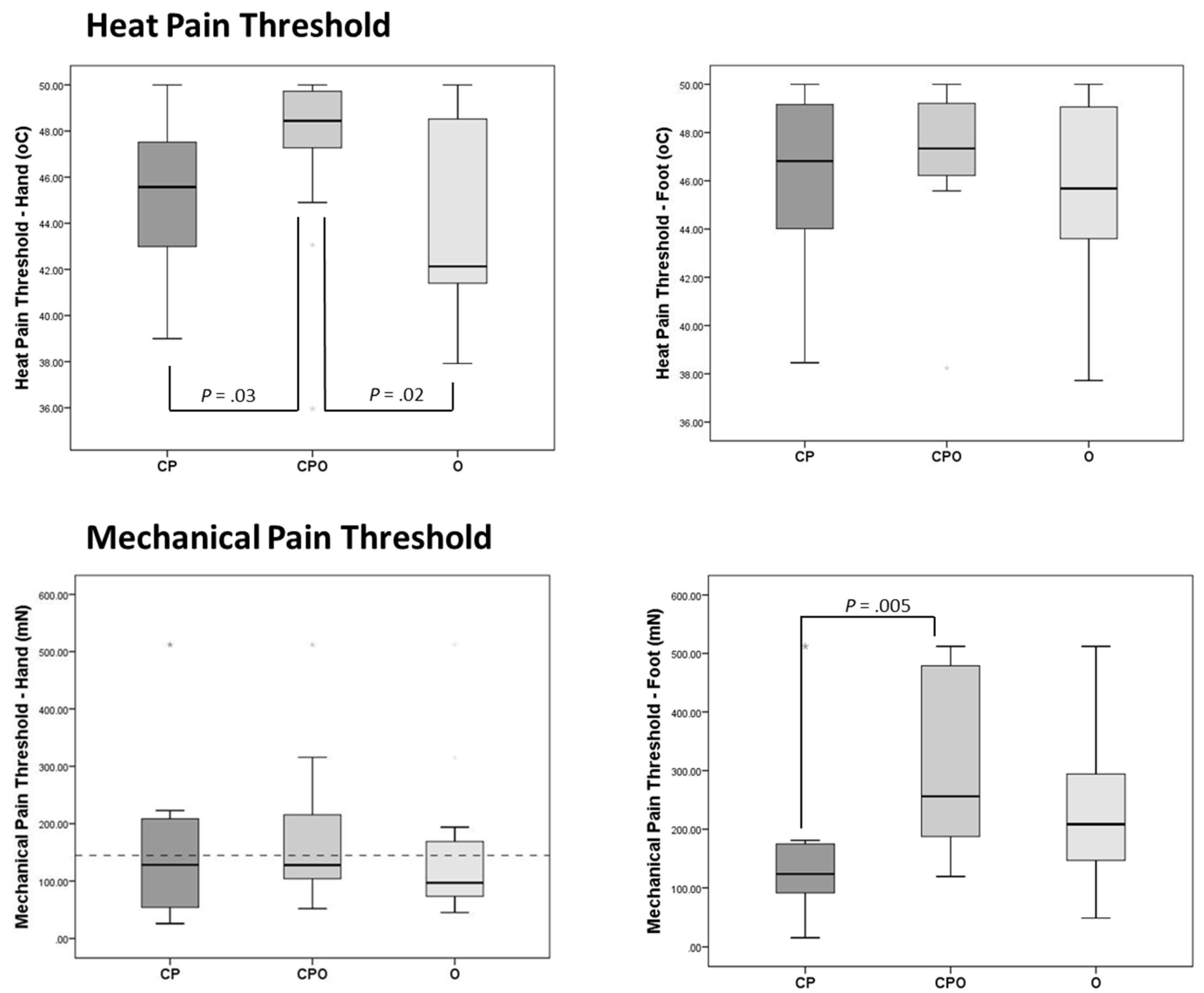
3.3. Heat Pain Threshold
3.4. Mechanical Pain Threshold
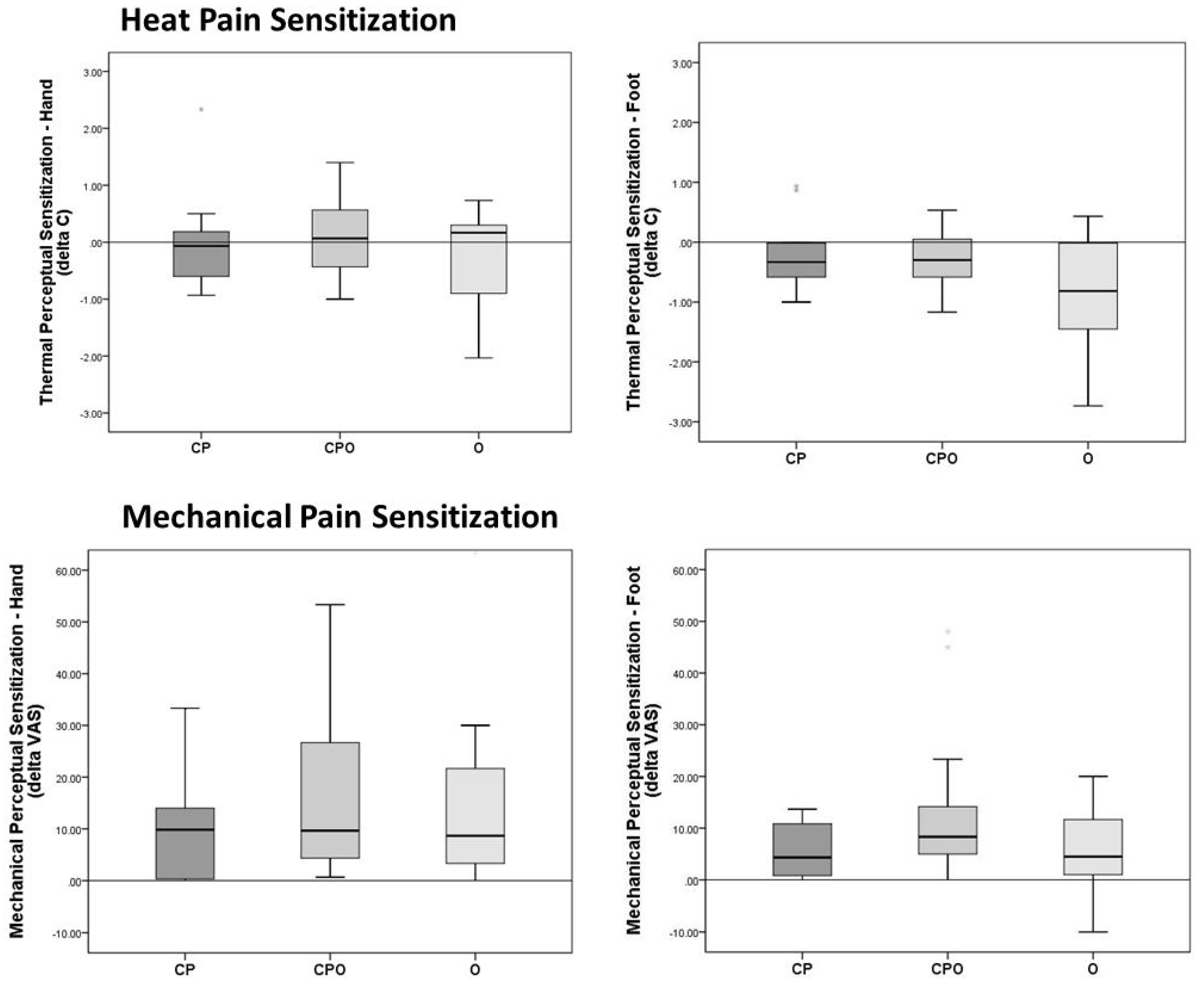
3.5. Heat Perceptual Sensitization
3.6. Mechanical Perceptual Sensitization
3.7. Correlations between Experimental Pain and Clinical Pain in Participants with Chronic Pain
4. Discussion
4.1. Obesity Might Exacerbate the Effects of Chronic Pain on Sensory Functioning
4.2. Obesity Should not Be Overlooked in Pediatric Studies of Somatosensory Functioning
4.3. These Preliminary Findings Warrant Follow-Up, Despite the Inconsistencies in the Literature
4.4. As in Other Areas of Science, Overcoming Methodological Hurdles may Result in New Understanding for the Population under Study
4.5. The Implications Demand Continued Pursuit of Definitive Answers
4.6. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perquin, C.W.; Hazebroek-Kampschreur, A.A.; Hunfeld, J.A.; Bohnen, A.M.; van Suijlekom-Smit, L.W.; Passchier, J.; van der Wouden, J.C. Pain in children and adolescents: A common experience. Pain 2000, 87, 51–58. [Google Scholar] [CrossRef]
- King, S.; Chambers, C.T.; Huguet, A.; MacNevin, R.C.; McGrath, P.J.; Parker, L.; MacDonald, A.J. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 2011, 152, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.; Miro, J. The severity of chronic pediatric pain: An epidemiological study. J. Pain 2008, 9, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, K.R.; Davies, W.H.; Khan, K.A.; Weisman, S.J. Development and preliminary validation of the child activity limitations questionnaire: Flexible and efficient assessment of pain-related functional disability. J. Pain 2007, 8, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M. Impact of recurrent and chronic pain on child and family daily functioning: A critical review of the literature. J. Dev. Behav. Pediatr. 2000, 21, 58–69. [Google Scholar] [CrossRef]
- Kashikar-Zuck, S.; Goldschneider, K.R.; Powers, S.W.; Vaught, M.H.; Hershey, A.D. Depression and functional disability in chronic pediatric pain. Clin. J. Pain 2001, 17, 341–349. [Google Scholar] [CrossRef]
- Hainsworth, K.R.; Davies, W.H.; Khan, K.A.; Weisman, S.J. Co-occurring chronic pain and obesity in children and adolescents: The impact on health-related quality of life. Clin. J. Pain 2009, 25, 715–721. [Google Scholar] [CrossRef]
- Wilson, A.C.; Samuelson, B.; Palermo, T.M. Obesity in children and adolescents with chronic pain: Associations with pain and activity limitations. Clin. J. Pain 2010, 26, 705–711. [Google Scholar] [CrossRef]
- Santos, M.; Murtaugh, T.; Pantaleao, A.; Zempsky, W.T.; Guite, J.W. Chronic Pain and Obesity Within a Pediatric Interdisciplinary Pain Clinic Setting: A Preliminary Examination of Current Relationships and Future Directions. Clin. J. Pain 2017, 33, 738–745. [Google Scholar] [CrossRef]
- Stoner, A.M.; Jastrowski Mano, K.; Weisman, S.J.; Hainsworth, K.R. Obesity impedes functional improvement in youth with chronic pain: An initial investigation. Eur. J. Pain 2017, 21, 1495–1504. [Google Scholar] [CrossRef]
- Dodet, P.; Perrot, S.; Auvergne, L.; Hajj, A.; Simoneau, G.; Decleves, X.; Poitou, C.; Oppert, J.M.; Peoc’h, K.; Mouly, S.; et al. Sensory impairment in obese patients? Sensitivity and pain detection thresholds for electrical stimulation after surgery-induced weight loss, and comparison with a nonobese population. Clin. J. Pain 2013, 29, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Price, R.C.; Asenjo, J.F.; Christou, N.V.; Backman, S.B.; Schweinhardt, P. The role of excess subcutaneous fat in pain and sensory sensitivity in obesity. Eur. J. Pain 2013, 17, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P.A.; Brown, S.C. Quantitative Sensory Testing in children: Practical considerations for research and clinical practice. Pain 2006, 123, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.M.; Berde, C.B.; DiCanzio, J.; Zurakowski, D.; Sethna, N.F. Quantitative assessment of cutaneous thermal and vibration sensation and thermal pain detection thresholds in healthy children and adolescents. Muscle Nerve 2001, 24, 1339–1345. [Google Scholar] [CrossRef]
- Blankenburg, M.; Boekens, H.; Hechler, T.; Maier, C.; Krumova, E.; Scherens, A.; Magerl, W.; Aksu, F.; Zernikow, B. Reference values for quantitative sensory testing in children and adolescents: Developmental and gender differences in somatosensory perception. Schmerz 2010, 24, 380–382. [Google Scholar] [CrossRef]
- Sethna, N.F.; Meier, P.M.; Zurakowski, D.; Berde, C.B. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain 2007, 131, 153–161. [Google Scholar] [CrossRef]
- Zohsel, K.; Hohmeister, J.; Oelkers-Ax, R.; Flor, H.; Hermann, C. Quantitative sensory testing in children with migraine: Preliminary evidence for enhanced sensitivity to painful stimuli especially in girls. Pain 2006, 123, 10–18. [Google Scholar] [CrossRef]
- Duarte, M.A.; Goulart, E.M.; Penna, F.J. Pressure pain threshold in children with recurrent abdominal pain. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 280–285. [Google Scholar] [CrossRef]
- Brandow, A.M.; Stucky, C.L.; Hillery, C.A.; Hoffmann, R.G.; Panepinto, J.A. Patients with sickle cell disease have increased sensitivity to cold and heat. Am. J. Hematol. 2012, 88, 37–43. [Google Scholar] [CrossRef]
- O’Leary, J.D.; Crawford, M.W.; Odame, I.; Shorten, G.D.; McGrath, P.A. Thermal pain and sensory processing in children with sickle cell disease. Clin. J. Pain 2014, 30, 244–250. [Google Scholar] [CrossRef]
- King, C.D.; Jastrowski Mano, K.E.; Barnett, K.A.; Pfeiffer, M.; Ting, T.V.; Kashikar-Zuck, S. Pressure pain threshold and anxiety in adolescent females with and without juvenile fibromyalgia: A pilot study. Clin. J. Pain 2017, 33, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Blankenburg, M.; Junker, J.; Hirschfeld, G.; Michel, E.; Aksu, F.; Wager, J.; Zernikow, B. Quantitative sensory testing profiles in children, adolescents and young adults (6-20 years) with cerebral palsy: Hints for a neuropathic genesis of pain syndromes. Eur. J. Paediatr. Neurol. 2018, 22, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Tham, S.W.; Palermo, T.M.; Holley, A.L.; Zhou, C.; Stubhaug, A.; Furberg, A.S.; Nielsen, C.S. A population-based study of quantitative sensory testing in adolescents with and without chronic pain. Pain 2016, 157, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Neumann, L.; Lerner, E.; Glazer, Y.; Bolotin, A.; Shefer, A.; Buskila, D. A cross-sectional study of the relationship between body mass index and clinical characteristics, tenderness measures, quality of life, and physical functioning in fibromyalgia patients. Clin. Rheumatol. 2008, 27, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Pradalier, A.; Willer, J.C.; Boureau, F.; Dry, J. Relationship between pain and obesity: An electrophysiological study. Physiol. Behav. 1981, 27, 961–964. [Google Scholar] [CrossRef]
- McKendall, M.J.; Haier, R.J. Pain sensitivity and obesity. Psychiatry Res. 1983, 8, 119–125. [Google Scholar] [CrossRef]
- Dorfman, L.J.; Robinson, L.R. AAEM minimonograph #47: Normative data in electrodiagnostic medicine. ff. Muscle Nerve 1997, 20, 4–14. [Google Scholar] [PubMed]
- Zahorska-Markiewicz, B.; Zych, P.; Kucio, C. Pain sensitivity in obesity. Acta Physiol. Pol. 1988, 39, 183–187. [Google Scholar]
- Torensma, B.; Oudejans, L.; van Velzen, M.; Swank, D.; Niesters, M.; Dahan, A. Pain sensitivity and pain scoring in patients with morbid obesity. Surg. Obes. Relat. Dis. 2017, 13, 788–795. [Google Scholar] [CrossRef]
- Miscio, G.; Guastamacchia, G.; Brunani, A.; Priano, L.; Baudo, S.; Mauro, A. Obesity and peripheral neuropathy risk: A dangerous liaison. J. Peripher. Nerv. Syst. 2005, 10, 354–358. [Google Scholar] [CrossRef]
- Stolzman, S.; Danduran, M.; Hunter, S.K.; Bement, M.H. Pain Response after Maximal Aerobic Exercise in Adolescents across Weight Status. Med. Sci. Sports Exerc. 2015, 47, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Okifuji, A.; Donaldson, G.W.; Barck, L.; Fine, P.G. Relationship between fibromyalgia and obesity in pain, function, mood, and sleep. J. Pain 2010, 11, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Dick, I.E.; Brochu, R.M.; Purohit, Y.; Kaczorowski, G.J.; Martin, W.J.; Priest, B.T. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J. Pain 2007, 8, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Codd, E.E.; Shank, R.P.; Schupsky, J.J.; Raffa, R.B. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: Structural determinants and role in antinociception. J. Pharmacol. Exp. Ther. 1995, 274, 1263–1270. [Google Scholar] [PubMed]
- Dharmshaktu, P.; Tayal, V.; Kalra, B.S. Efficacy of antidepressants as analgesics: A review. J. Clin. Pharmacol. 2012, 52, 6–17. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Grummer-Strawn, L.M.; Flegal, K.M.; Guo, S.S.; Wei, R.; Mei, Z.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. CDC growth charts: United States. Adv. Data 2000, 314, 1–27. [Google Scholar]
- Paley, C.A.; Johnson, M.I. Physical Activity to Reduce Systemic Inflammation Associated with Chronic Pain and Obesity: A Narrative Review. Clin. J. Pain 2016, 32, 365–370. [Google Scholar] [CrossRef]
- Boerner, K.E.; Birnie, K.A.; Caes, L.; Schinkel, M.; Chambers, C.T. Sex differences in experimental pain among healthy children: A systematic review and meta-analysis. Pain 2014, 155, 983–993. [Google Scholar] [CrossRef]
- Lu, Q.; Zeltzer, L.; Tsao, J. Multiethnic differences in responses to laboratory pain stimuli among children. Health Psychol. 2013, 32, 905–914. [Google Scholar] [CrossRef]
- Gandhi, R.; Dhotar, H.; Tsvetkov, D.; Mahomed, N.N. The relation between body mass index and waist-hip ratio in knee osteoarthritis. Can. J. Surg. 2010, 53, 151–154. [Google Scholar]
- Gandhi, R.; Perruccio, A.V.; Rizek, R.; Dessouki, O.; Evans, H.M.; Mahomed, N.N. Obesity-related adipokines predict patient-reported shoulder pain. Obes. Facts 2013, 6, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Tukker, A.; Visscher, T.; Picavet, H. Overweight and health problems of the lower extremities: Osteoarthritis, pain and disability. Public Health Nutr. 2009, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Adams, M.C.; Vincent, K.R.; Hurley, R.W. Musculoskeletal pain, fear avoidance behaviors, and functional decline in obesity: Potential interventions to manage pain and maintain function. Reg. Anesth. Pain Med. 2013, 38, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Seay, A.N.; Montero, C.; Conrad, B.P.; Hurley, R.W.; Vincent, K.R. Functional pain severity and mobility in overweight older men and women with chronic low-back pain—Part I. Am. J. Phys. Med. Rehabil. 2013, 92, 430–438. [Google Scholar] [CrossRef]
- Atchison, J.W.; Vincent, H.K. Obesity and low back pain: Relationships and treatment. Pain Manag. 2012, 2, 79–86. [Google Scholar] [CrossRef]
- Bigal, M.E.; Lipton, R.B. Obesity and chronic daily headache. Curr. Pain Headache Rep. 2008, 12, 56–61. [Google Scholar] [CrossRef]
- Peterlin, B.L.; Rapoport, A.M.; Kurth, T. Migraine and obesity: Epidemiology, mechanisms, and implications. Headache 2010, 50, 631–648. [Google Scholar] [CrossRef]
- Janal, M.N.; Glusman, M.; Kuhl, J.P.; Clark, W.C. On the absence of correlation between responses to noxious heat, cold, electrical and ischemic stimulation. Pain 1994, 58, 403–411. [Google Scholar] [CrossRef]
- Taylor, E.D.; Theim, K.R.; Mirch, M.C.; Ghorbani, S.; Tanofsky-Kraff, M.; Adler-Wailes, D.C.; Brady, S.; Reynolds, J.C.; Calis, K.A.; Yanovski, J.A. Orthopedic complications of overweight in children and adolescents. Pediatrics 2006, 117, 2167–2174. [Google Scholar] [CrossRef]
- Shultz, S.P.; Browning, R.C.; Schutz, Y.; Maffeis, C.; Hills, A.P. Childhood obesity and walking: Guidelines and challenges. Int. J. Pediatr. Obes. 2011, 6, 332–341. [Google Scholar] [CrossRef]
- Shultz, S.P.; Sitler, M.R.; Tierney, R.T.; Hillstrom, H.J.; Song, J. Consequences of pediatric obesity on the foot and ankle complex. J. Am. Podiatr. Med Assoc. 2012, 102, 5–12. [Google Scholar] [CrossRef] [PubMed]
| CP | CPO | O | p Value | |
|---|---|---|---|---|
| (n = 12) | (n = 19) | (n = 14) | ||
| Age (years) | ||||
| Mean (± SD) | 15.3 (± 1.6) | 16.0 (± 1.2) | 15.1 (± 1.4) | 0.21 |
| Range | 13.0–17.5 | 13.8–17.8 | 13.1–17.3 | |
| Race | 0.14 | |||
| AA | 41.70% | 31.60% | 71.40% | |
| Caucasian | 50% | 57.90% | 21.40% | |
| Hispanic | 8.30% | -- | 7.10% | |
| Asian | -- | 10.50% | -- | |
| Female | 58.30% | 52.60% | 71.40% | 0.56 |
| BMI %ile | ||||
| Median | 52 | 95 | 99 | <0.001 a |
| (IQR) | (38.8–65.0) | (92.0–99.0) | (98.0–99.0) | |
| Days with pain in past 2 weeks | 8 | 14 | 3 | 0.001 b |
| (2.8–13.3) | (7.0–14.0) | (2.0–7.0) | ||
| Usual Pain Intensity | 6.5 | 5 | 5.5 | 0.78 |
| (4.5–7.8) | (4.0–7.0) | (4.0–7.8) | ||
| Worst Pain Intensity | 8.5 | 8.5 | 7 | 0.4 |
| (7.3–10.0) | (7.8–9.3) | (6.0–9.0) | ||
| Duration of Pain Problem (months) | 11.5 | 12 | 2.5 | 0.54 |
| (6.3–24.0) | (5.0–28.0) | (0.0–27.0) |
| CP | CPO | O | p Value | η2 | |
| (n = 12) | (n = 19) | (n = 14) | |||
| Heat Pain | |||||
| Hand | 45.6 (42.5–47.9) | 48.4 (46.6–49.8) | 42.1 (41.4–48.7) | 0.02 a | 0.13 c |
| Foot | 46.8 (43.9–49.6) | 47.3 (46.1–49.5) | 45.7 (42.7–49.1) | 0.3 | 0.01 |
| Mechanical Pain | |||||
| Hand | 128.3 (51.3–215.6) | 128.0 (104.0–222.9) | 97.0 (72.3–175.2) | 0.47 | 0.012 |
| Foot | 123.7 (85.1–178.0) | 256.0 (181.0–512.0) | 208.4 (134.5–305.0) | 0.005 b | 0.15 c |
| CP | CPO | O | pValue | η² | |
|---|---|---|---|---|---|
| Heat Sensitization | (n = 12) a | (n = 17) a | (n = 11) a | ||
| Hand | −0.1 (−0.7–0.2) | 0.1 (−0.5–0.6) | 0.2 (−1.0–0.4) | 0.6 | 0.026 |
| (n = 11) a | (n = 13) a | (n = 15) a | |||
| Foot | −0.3 (−0.6–0.0) | −0.3 (−0.6–0.1) | −0.8 (−1.6–0.0) | 0.29 | 0.013 |
| Mechanical Sensitization | (n = 12) | (n = 19) | (n = 14) | 0.47 | |
| Hand | 9.8 (0.2–14.3) | 9.7 (4.0–26.7) | 8.7 (3.3–22.9) | 0.012 | |
| Foot | 4.3 (0.4–11.3) | 8.3 (4.7–15.0) | 4.5 (0.8–12.1) | 0.15 | 0.043 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hainsworth, K.R.; Simpson, P.M.; Ali, O.; Varadarajan, J.; Rusy, L.; Weisman, S.J. Quantitative Sensory Testing in Adolescents with Co-Occurring Chronic Pain and Obesity: A Pilot Study. Children 2020, 7, 55. https://doi.org/10.3390/children7060055
Hainsworth KR, Simpson PM, Ali O, Varadarajan J, Rusy L, Weisman SJ. Quantitative Sensory Testing in Adolescents with Co-Occurring Chronic Pain and Obesity: A Pilot Study. Children. 2020; 7(6):55. https://doi.org/10.3390/children7060055
Chicago/Turabian StyleHainsworth, Keri R., Pippa M. Simpson, Omar Ali, Jaya Varadarajan, Lynn Rusy, and Steven J. Weisman. 2020. "Quantitative Sensory Testing in Adolescents with Co-Occurring Chronic Pain and Obesity: A Pilot Study" Children 7, no. 6: 55. https://doi.org/10.3390/children7060055
APA StyleHainsworth, K. R., Simpson, P. M., Ali, O., Varadarajan, J., Rusy, L., & Weisman, S. J. (2020). Quantitative Sensory Testing in Adolescents with Co-Occurring Chronic Pain and Obesity: A Pilot Study. Children, 7(6), 55. https://doi.org/10.3390/children7060055





