Efficacy, Safety, and Usability of Remifentanil as Premedication for INSURE in Preterm Neonates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Retrospective Review
2.2. Staff Survey
2.3. Analyses
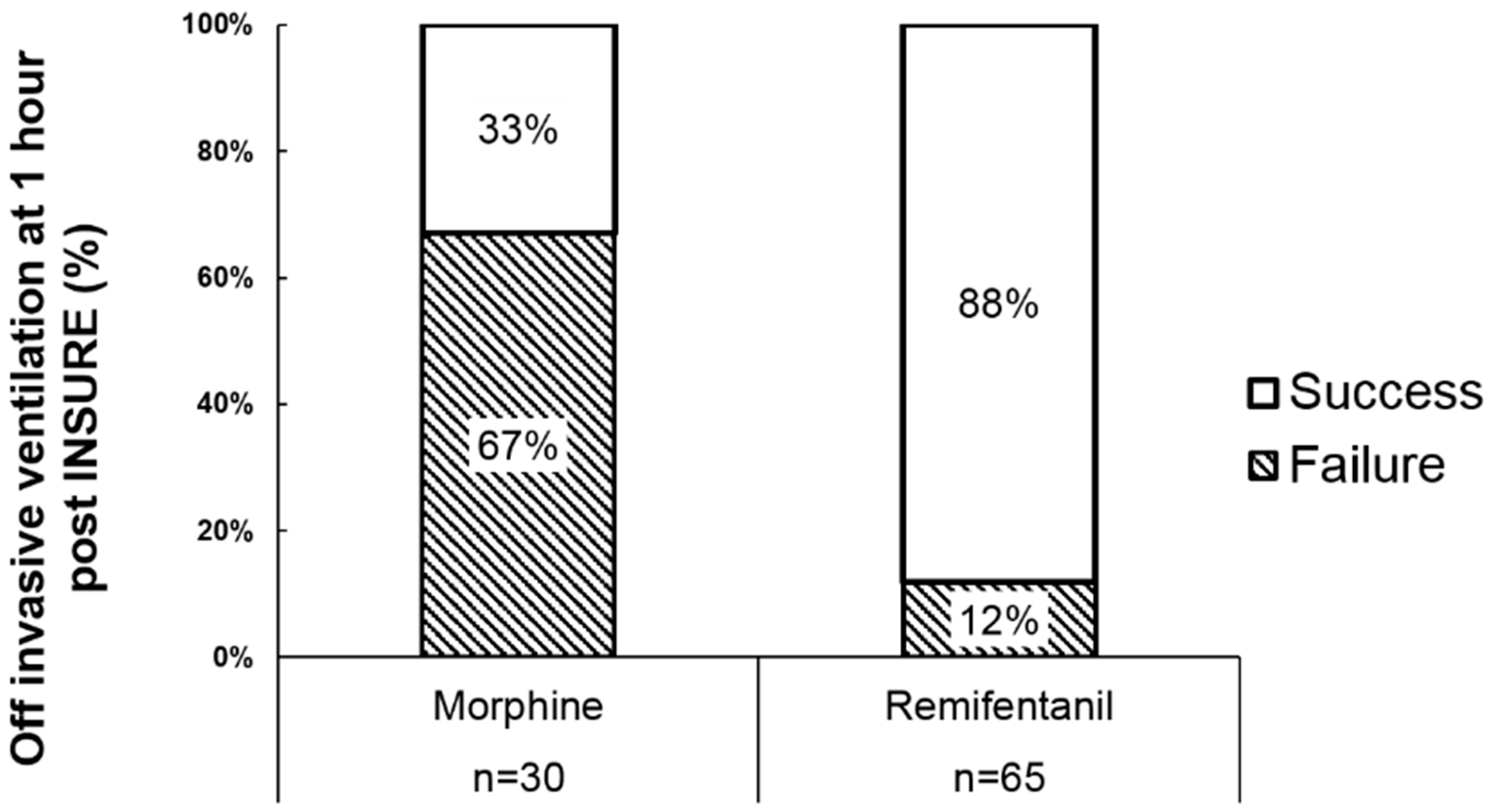
3. Results
3.1. Retrospective Review
3.2. Staff Survey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| REMIFENTANIL FOR INSURE PREMEDICATION SURVEY |
- 1. Approximately how many cases have you been involved in, either as observer or as care provider, for using remifentanil as induction premedication to INSURE (Intubation, Surfactant, Rapid Extubation)?
(a) 0 (b) 1–2 (c) 3+ - 2. What is your work title or function?
(a) Resident (e) Attending (b) NP/PA (f) RN (c) NP or PA student (g) Respiratory therapist (d) Fellow (h) Pharmacist - 3. What role(s) have you played in the INSURE procedures? (check all that apply)
(a) Intubator (c) Supervisor (e) None of these (b) Other direct care provider (d) Observer only - 4. Do you feel remifentanil premedication provides effective analgesia/sedation for neonatal intubation?(a) Yes(b) Maybe(c) No—If no explain: ____________________________________________________________________________________________________________________________________________________________
- 5. Were there any adverse effects or logistical problems with remifentanil administration during the INSURE procedures?(a) No(b) Yes—if yes explain: ___________________________________________________________________________________________________________________________________________________________
- 6. What is your overall satisfaction with remifentanil as an induction premedication to INSURE?(a) Very satisfied(b) Somewhat satisfied(c) Neither satisfied nor dissatisfied(d) Somewhat dissatisfied(e) Very dissatisfied
- 7. Based on your experiences please provide any additional feedback or comments regarding remifentanil as an induction premedication to INSURE (both positive and negative).________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
References
- Jobe, A.; Ikegami, M. Surfactant for the treatment of respiratory distress syndrome. Am. Rev. Respir. Dis. 1987, 136, 1256–1275. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.P.; Harrington, E.W.; Blennow, M.; Soll, R.F. Early surfactant administration with brief ventilation vs. Selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef] [PubMed]
- Gortner, L.; Wauer, R.R.; Hammer, H.; Stock, G.J.; Heitmann, F.; Reiter, H.L.; Kuhl, P.G.; Moller, J.C.; Friedrich, H.J.; Reiss, I.; et al. Early versus late surfactant treatment in preterm infants of 27 to 32 weeks’ gestational age: A multicenter controlled clinical trial. Pediatrics 1998, 102, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Brew, N.; Hooper, S.B.; Allison, B.J.; Wallace, M.J.; Harding, R. Injury and repair in the very immature lung following brief mechanical ventilation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L917–L926. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Plavka, R.; Saugstad, O.D.; Simeoni, U.; Speer, C.P.; Vento, M.; et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants—2013 update. Neonatology 2013, 103, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Schmölzer, G.M.; Kumar, M.; Pichler, G.; Aziz, K.; O’Reilly, M.; Cheung, P.-Y. Non-invasive versus invasive respiratory support in preterm infants at birth: Systematic review and meta-analysis. BMJ Br. Med. J. 2013, 347. [Google Scholar] [CrossRef] [PubMed]
- Verder, H.; Robertson, B.; Greisen, G.; Ebbesen, F.; Albertsen, P.; Lundstrom, K.; Jacobsen, T. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish multicenter study group. N. Engl. J. Med. 1994, 331, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Bertini, G.; Pezzati, M.; Cecchi, A.; Caviglioli, C.; Rubaltelli, F.F. Early extubation and nasal continuous positive airway pressure after surfactant treatment for respiratory distress syndrome among preterm infants <30 weeks’ gestation. Pediatrics 2004, 113, e560–e563. [Google Scholar] [PubMed]
- Aldana-Aguirre, J.C.; Pinto, M.; Featherstone, R.M.; Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F17–F23. [Google Scholar] [CrossRef] [PubMed]
- Trevisanuto, D.; Grazzina, N.; Ferrarese, P.; Micaglio, M.; Verghese, C.; Zanardo, V. Laryngeal mask airway used as a delivery conduit for the administration of surfactant to preterm infants with respiratory distress syndrome. Biol. Neonate 2005, 87, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Muniraman, H.K.; Yaari, J.; Hand, I. Premedication use before nonemergent intubation in the newborn infant. Am. J. Perinatol. 2015, 32, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Lago, P. Premedication for non-emergency intubation in the neonate. Minerva Pediatr. 2010, 62, 61–63. [Google Scholar] [PubMed]
- Allen, K.A. Premedication for neonatal intubation: Which medications are recommended and why. Adv. Neonatal Care 2012, 12, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Denson, S.E.; Mancuso, T.J. Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics 2010, 125, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, J.; Mallya, P.; Wyllie, J. Premedication before intubation in UK neonatal units: A decade of change? Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F332–F335. [Google Scholar] [CrossRef] [PubMed]
- Norman, E.; Wikstrom, S.; Hellstrom-Westas, L.; Turpeinen, U.; Hamalainen, E.; Fellman, V. Rapid sequence induction is superior to morphine for intubation of preterm infants: A randomized controlled trial. J. Pediatr. 2011, 159, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, V.; Ponnusamy, V.; Anandaraj, J.; Chaudhary, R.; Malviya, M.; Clarke, P.; Arasu, A.; Curley, A. Endotracheal intubation in a neonatal population remains associated with a high risk of adverse events. Eur. J. Pediatr. 2011, 170, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Avino, D.; Zhang, W.H.; De Villé, A.; Johansson, A.B. Remifentanil versus morphine-midazolam premedication on the quality of endotracheal intubation in neonates: A noninferiority randomized trial. J. Pediatr. 2014, 164, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Pereira e Silva, Y.; Gomez, R.S.; Marcatto, J.O.; Maximo, T.A.; Barbosa, R.F.; Simoes e Silva, A.C. Morphine versus remifentanil for intubating preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F293–F294. [Google Scholar] [CrossRef] [PubMed]
- Welzing, L.; Kribs, A.; Huenseler, C.; Eifinger, F.; Mehler, K.; Roth, B. Remifentanil for insure in preterm infants: A pilot study for evaluation of efficacy and safety aspects. Acta Paediatr. 2009, 98, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Badiee, Z.; Vakiliamini, M.; Mohammadizadeh, M. Remifentanil for endotracheal intubation in premature infants: A randomized controlled trial. J. Res. Pharm. Pract. 2013, 2, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Choong, K.; AlFaleh, K.; Doucette, J.; Gray, S.; Rich, B.; Verhey, L.; Paes, B. Remifentanil for endotracheal intubation in neonates: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F80–F84. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.M.B.; Santana-Rivas, Q.; Pezzano, C. Randomized trial of laryngeal mask airway versus endotracheal intubation for surfactant delivery. J. Perinatol. 2016, 36, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Thewissen, L.; Allegaert, K. Analgosedation in neonates: Do we still need additional tools after 30 years of clinical research? Arch. Dis. Child. Educ. Pract. Ed. 2011, 96, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Marcatto, J.D.O.; Barbosa, R.F.; Silva, A.C.S. Is remifentanil an option for premedication for neonatal endotracheal intubation? Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F463–F464. [Google Scholar] [CrossRef] [PubMed]
- de Kort, E.H.; Reiss, I.K.; Simons, S.H. Sedation of newborn infants for the INSURE procedure, are we sure? Biomed. Res. Int. 2013, 2013, 892974. [Google Scholar] [CrossRef] [PubMed]
- de Kort, E.H.; Hanff, L.M.; Roofthooft, D.; Reiss, I.K.; Simons, S.H. Insufficient sedation and severe side effects after fast administration of remifentanil during insure in preterm newborns. Neonatology 2017, 111, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Leone, T.A.; Rich, W.; Finer, N.N. Neonatal intubation: Success of pediatric trainees. J. Pediatr. 2005, 146, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Descamps, C.S.; Chevallier, M.; Ego, A.; Pin, I.; Epiard, C.; Debillon, T. Propofol for sedation during less invasive surfactant administration in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F465. [Google Scholar] [CrossRef] [PubMed]
- Nasr, V.G.; Davis, J.M. Anesthetic use in newborn infants: The urgent need for rigorous evaluation. Pediatr. Res. 2015, 78, 2–6. [Google Scholar] [CrossRef] [PubMed]
| Adverse Event Documented | n/N | Percent of Valid Data |
|---|---|---|
| Missing all documentation | 4/73 | 5% |
| Desaturation | 26 | 36% |
| Bradycardia | 6 | 8% |
| Trauma | 3 | 4% |
| Pneumothorax within 2 h | 3 | 4% |
| Pneumothorax beyond 2 h | 2 | 3% |
| Chest wall rigidity | 3 | 4% |
| CPR (post-surfactant) | 1 | 1% |
| Apnea | 6 | 8% |
| Medications/naloxone | 4 | 5% |
| Other adverse event | 19 | 26% |
| Any adverse event | 36 | 49% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audil, H.Y.; Tse, S.; Pezzano, C.; Mitchell-van Steele, A.; Pinheiro, J.M.B. Efficacy, Safety, and Usability of Remifentanil as Premedication for INSURE in Preterm Neonates. Children 2018, 5, 63. https://doi.org/10.3390/children5050063
Audil HY, Tse S, Pezzano C, Mitchell-van Steele A, Pinheiro JMB. Efficacy, Safety, and Usability of Remifentanil as Premedication for INSURE in Preterm Neonates. Children. 2018; 5(5):63. https://doi.org/10.3390/children5050063
Chicago/Turabian StyleAudil, Hadiyah Y., Sara Tse, Chad Pezzano, Amy Mitchell-van Steele, and Joaquim M. B. Pinheiro. 2018. "Efficacy, Safety, and Usability of Remifentanil as Premedication for INSURE in Preterm Neonates" Children 5, no. 5: 63. https://doi.org/10.3390/children5050063






