A Scoping Review of Modifiable Risk Factors in Pediatric Onset Multiple Sclerosis: Building for the Future
Abstract
1. Introduction
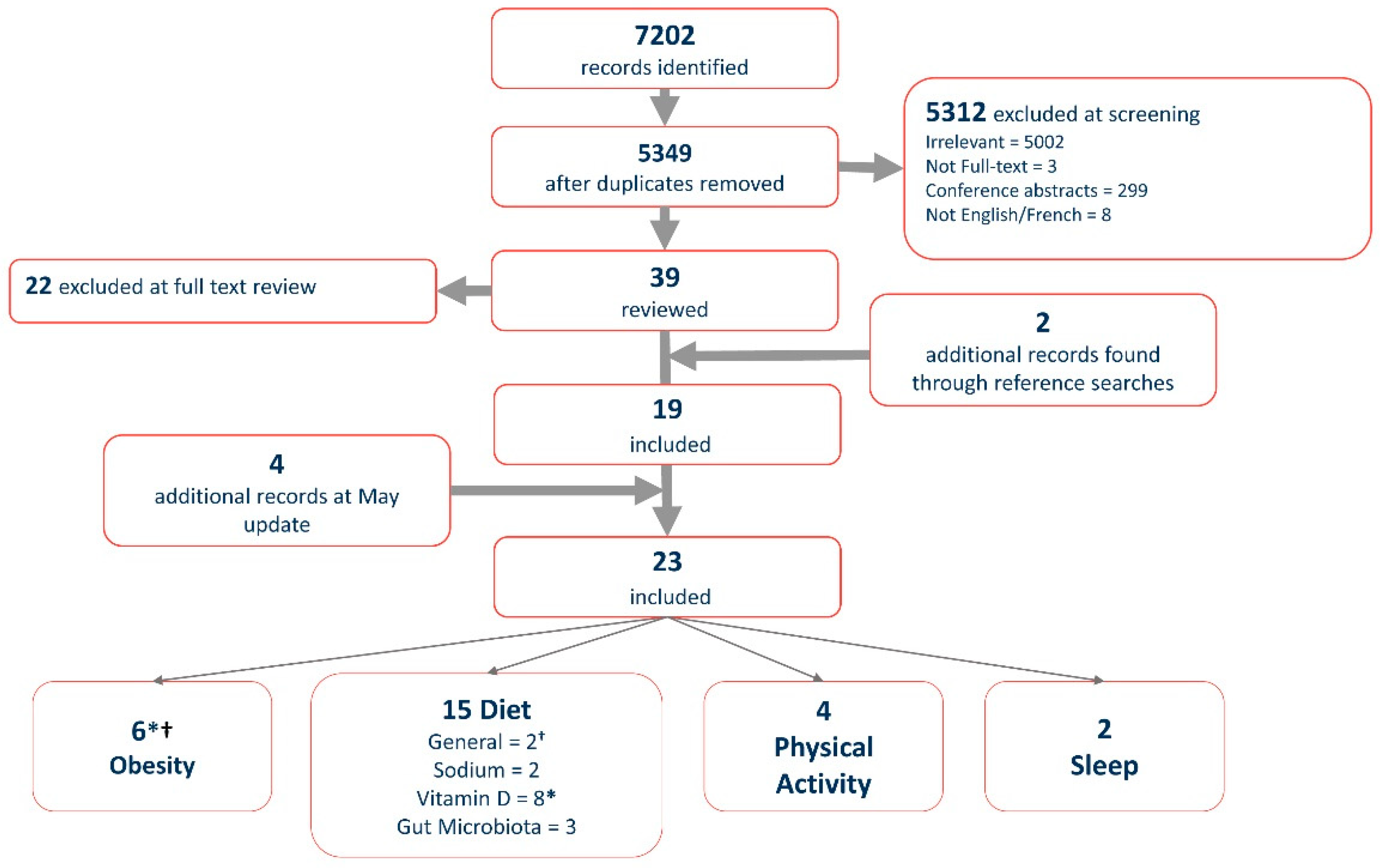
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Charting and Analysis
3. Results
3.1. Dietary Factors
3.1.1. Dietary Micronutrients
Sodium
Vitamin D
3.1.2. Gut Microbiome
3.2. Obesity
3.3. Physical Activity
3.4. Sleep
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krupp, L.B.; Banwell, B.; Tenembaum, S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007, 68, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Bigi, S.; Banwell, B. Pediatric multiple sclerosis. J. Child Neurol. 2012, 27, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Renoux, C.; Vukusic, S.; Confavreux, C. The natural history of multiple sclerosis with childhood onset. Clin. Neurol. Neurosurg. 2008, 110, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Gorman, M.P.; Healy, B.C.; Polgar-Turcsanyi, M.; Chitnis, T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch. Neurol. 2009, 66, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Waubant, E.; Chabas, D.; Okuda, D.T.; Glenn, O.; Mowry, E.; Henry, R.G.; Strober, J.B.; Soares, B.; Wintermark, M.; Pelletier, D. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs. adults. Arch. Neurol. 2009, 66, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Yeshokumar, A.K.; Narula, S.; Banwell, B. Pediatric multiple sclerosis. Curr. Opin. Neurol. 2017, 30, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Renoux, C.; Vukusic, S.; Mikaeloff, Y.; Edan, G.; Clanet, M.; Dubois, B.; Debouverie, M.; Brochet, B.; Lebrun-Frenay, C.; Pelletier, J.; et al. Natural history of multiple sclerosis with childhood onset. N. Engl. J. Med. 2007, 356, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- Jancic, J.; Nikolic, B.; Ivancevic, N.; Hencic, B.; Samardzic, J. Multiple sclerosis therapies in pediatric patients: Challenges and opportunities. In Multiple Sclerosis: Perspectives in Treatment and Pathogenesis; Zagon, I.S., McLaughlin, P.J., Eds.; Codon Publications Copyright: Brisbane, Australia, 2017. [Google Scholar]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E.; Aaen, G.; Belman, A.; et al. Gut microbiota composition and relapse risk in pediatric MS: A pilot study. J. Neurol. Sci. 2016, 363, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, T.; Thompson, A. When are we going to take modifiable risk factors more seriously in multiple sclerosis? Mult. Scler. J. 2017, 23, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Riise, T. Multiple sclerosis: A lifestyle disease? Neurology 2016, 86, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Thannhauser, J.E. Navigating life and loss in pediatric multiple sclerosis. Qual. Health Res. 2014, 24, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.; Kirk, S. Living with uncertainty and hope: A qualitative study exploring parents’ experiences of living with childhood multiple sclerosis. Chronic Illn. 2017, 13, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Pakpoor, J.; Seminatore, B.; Graves, J.S.; Schreiner, T.; Waldman, A.T.; Lotze, T.E.; Belman, A.; Greenberg, B.M.; Weinstock-Guttman, B.; Aaen, G. Dietary factors and pediatric multiple sclerosis: A case-control study. Mult. Scler. J. 2018, 24, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Ann Yeh, E.; Kinnett-Hopkins, D.; Grover, S.A.; Motl, R.W. Physical activity and pediatric multiple sclerosis: Developing a research agenda. Mult. Scler. J. 2015, 21, 1618–1625. [Google Scholar]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.; Stridh, P.P.; Rhead, B.B.S.; Shao, X.M.A.; Xu, E.B.A.; Graves, J.S.M.D.P.; Chitnis, T.M.D.; Waldman, A.M.D.; Lotze, T.M.D.; Schreiner, T.M.D.; et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology 2017, 88, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Ginsberg, M.; Rensel, M.; Moodley, M. Pediatric-onset multiple sclerosis: A single center study. J. Child Neurol. 2018, 33, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Nicholas Brenton, J.; Koenig, S.; Goldman, M.D. Vitamin D status and age of onset of demyelinating disease. Mult. Scler. Relat. Disord. 2014, 3, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Azary, S.; Schreiner, T.; Graves, J.; Waldman, A.; Belman, A.; Guttman, B.W.; Aaen, G.; Tillema, J.-M.; Mar, S.; Hart, J. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Toussaint-Duyster, L.C.; Wong, Y.Y.M.; Van der Cammen-van Zijp, M.H.; Van Pelt-Gravesteijn, D.; Catsman-Berrevoets, C.E.; Hintzen, R.Q.; Neuteboom, R.F. Fatigue and physical functioning in children with multiple sclerosis and acute disseminated encephalomyelitis. Mult. Scler. J. 2018, 24, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, U.; Anlar, B.; Gucuyener, K. Characteristics of pediatric multiple sclerosis: The turkish pediatric multiple sclerosis database. Eur. J. Paediatr. Neurol. 2017, 21, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Hunsberger, M.; O’malley, J.; Block, T.; Norris, J.C. Relative validation of block kids food screener for dietary assessment in children and adolescents. Mater. Child Nutr. 2015, 11, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, B.; Graves, J.; Casper, T.C.; Lulu, S.; Waldman, A.; Belman, A.; Greenberg, B.; Weinstock-Guttman, B.; Aaen, G.; Tillema, J.M.; et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.; Graves, J.; Waldman, A.; Lotze, T.; Schreiner, T.; Belman, A.; Greenberg, B.; Weinstock-Guttman, B.; Aaen, G.; Tillema, J.M.; et al. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 6, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Banwell, B.; Bar-Or, A.; Arnold, D.L.; Sadovnick, D.; Narayanan, S.; McGowan, M.; O’Mahony, J.; Magalhaes, S.; Hanwell, H.; Vieth, R.; et al. Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: A prospective national cohort study. Lancet Neurol. 2011, 10, 436–445. [Google Scholar] [CrossRef]
- Mowry, E.M.; Krupp, L.B.; Milazzo, M.; Chabas, D.; Strober, J.B.; Belman, A.L.; McDonald, J.C.; Oksenberg, J.R.; Bacchetti, P.; Waubant, E. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann. Neurol. 2010, 67, 618–624. [Google Scholar] [PubMed]
- Mowry, E.M.; James, J.A.; Krupp, L.B.; Waubant, E. Vitamin D status and antibody levels to common viruses in pediatric-onset multiple sclerosis. Mult. Scler. J. 2011, 17, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.S.; Barcellos, L.F.; Shao, X.; Noble, J.; Mowry, E.M.; Quach, H.; Belman, A.; Casper, T.C.; Krupp, L.B.; Waubant, E. Genetic predictors of relapse rate in pediatric MS. Mult. Scler. 2016, 22, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Hart, J.; Roalstad, S.; Graves, J.; Spencer, C.M.; Lynch, S.V.; Zamvil, S.S.; Waubant, E.; et al. Associations between the gut microbiota and host immune markers in pediatric multiple sclerosis and controls. BMC Neurol. 2016, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Zhu, F.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E. Gut microbiota in early pediatric multiple sclerosis: A case-control study. Eur. J. Neurol. 2016, 23, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Langer-Gould, A.; Brara, S.M.; Beaber, B.E.; Koebnick, C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology 2013, 80, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Graves, J.; Weinstock-Guttman, B.; Belman, A.; Olsen, C.; Misra, M.; Aaen, G.; Benson, L.; Candee, M.; Gorman, M.; et al. Distinct effects of obesity and puberty on risk and age at onset of pediatric MS. Ann. Clin. Transl. Neurol. 2016, 3, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Glymour, M.M.; Walter, S.; Rhead, B.; Shao, X.; Shen, L.; Quach, H.; Hubbard, A.; Jonsdottir, I.; Stefansson, K.; et al. Causal effect of genetic variants associated with body mass index on multiple sclerosis susceptibility. Am. J. Epidemiol. 2017, 185, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.A.; Aubert-Broche, B.; Fetco, D.; Collins, D.L.; Arnold, D.L.; Finlayson, M.; Banwell, B.L.; Motl, R.W.; Yeh, E.A. Lower physical activity is associated with higher disease burden in pediatric multiple sclerosis. Neurology 2015, 85, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.A.; Sawicki, C.P.; Kinnett-Hopkins, D.; Finlayson, M.; Schneiderman, J.E.; Banwell, B.; Till, C.; Motl, R.W.; Yeh, E.A. Physical activity and its correlates in youth with multiple sclerosis. J. Pediatr. 2016, 179, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kinnett-Hopkins, D.; Grover, S.A.; Yeh, E.A.; Motl, R.W. Physical activity in pediatric onset multiple sclerosis: Validating a questionnaire for clinical practice and research. Mult. Scler. Relat. Disord. 2016, 10, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Sikes, E.M.; Richardson, E.V.; Cederberg, K.J.; Sasaki, J.E.; Sandroff, B.M.; Motl, R.W. Use of the godin leisure-time exercise questionnaire in multiple sclerosis research: A comprehensive narrative review. Disabil. Rehabil. 2018, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Wilkinson, K.; Marcu, S.; Shapiro, C.M. Center for epidemiological studies depression scale for children (CES-DC). In Stop, That and One Hundred Other Sleep Scales; Springer: New York, NY, USA, 2011; pp. 93–96. [Google Scholar]
- Varni, J.W.; Limbers, C.A.; Bryant, W.P.; Wilson, D.P. The PedsQL multidimensional fatigue scale in pediatric obesity: Feasibility, reliability and validity. Int. J. Pediatr. Obes. 2010, 5, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Rovniak, L.S.; Anderson, E.S.; Winett, R.A.; Stephens, R.S. Social cognitive determinants of physical activity in young adults: A prospective structural equation analysis. Ann. Behav. Med. 2002, 24, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.P.; Pate, R.R.; Felton, G.; Dowda, M.; Weinrich, M.C.; Ward, D.S.; Parsons, M.A.; Baranowski, T. Development of questionnaires to measure psychosocial influences on children’s physical activity. Prev. Med. 1997, 26, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.A.; Kusumi, F.; Hosmer, D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973, 85, 546–562. [Google Scholar] [CrossRef]
- Lewandowski, A.S.; Ward, T.M.; Palermo, T.M. Sleep problems in children and adolescents with common medical conditions. Pediatr. Clin. North Am. 2011, 58, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.B.; Ness, J.; Dowdy, S.; Avis, K.; Bashir, K. Examining sleep, fatigue, and daytime sleepiness in pediatric multiple sclerosis patients. Mult. Scler. J. 2012, 18, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.; Chalder, T.; Hemingway, C.; Heyman, I.; Moss-Morris, R. “It feels like wearing a giant sandbag.” Adolescent and parent perceptions of fatigue in paediatric multiple sclerosis. Eur. J. Paediatr. Neurol. 2016, 20, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Mielcarz, D.W.; Kasper, L.H. The gut microbiome in multiple sclerosis. Curr. Treat. Options Neurol. 2015, 17, 344. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Shi, M.; Lang, Y.; Shen, D.; Jin, T.; Zhu, J.; Cui, L. Gut microbiota in multiple sclerosis and experimental autoimmune encephalomyelitis: Current applications and future perspectives. Mediat. Inflamm. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pétrin, J.; Fiander, M.D.J.; Doss, P.M.I.A.; Yeh, E.A. A Scoping Review of Modifiable Risk Factors in Pediatric Onset Multiple Sclerosis: Building for the Future. Children 2018, 5, 146. https://doi.org/10.3390/children5110146
Pétrin J, Fiander MDJ, Doss PMIA, Yeh EA. A Scoping Review of Modifiable Risk Factors in Pediatric Onset Multiple Sclerosis: Building for the Future. Children. 2018; 5(11):146. https://doi.org/10.3390/children5110146
Chicago/Turabian StylePétrin, Julie, Maximillian D.J. Fiander, Prenitha Mercy Ignatius Arokia Doss, and E. Ann Yeh. 2018. "A Scoping Review of Modifiable Risk Factors in Pediatric Onset Multiple Sclerosis: Building for the Future" Children 5, no. 11: 146. https://doi.org/10.3390/children5110146
APA StylePétrin, J., Fiander, M. D. J., Doss, P. M. I. A., & Yeh, E. A. (2018). A Scoping Review of Modifiable Risk Factors in Pediatric Onset Multiple Sclerosis: Building for the Future. Children, 5(11), 146. https://doi.org/10.3390/children5110146




