Environmental Factors Affecting Growth and Occurrence of Testicular Cancer in Childhood: An Overview of the Current Epidemiological Evidence
Abstract
:1. Introduction
2. Testicular Germ Cell Development in the Infancy Period
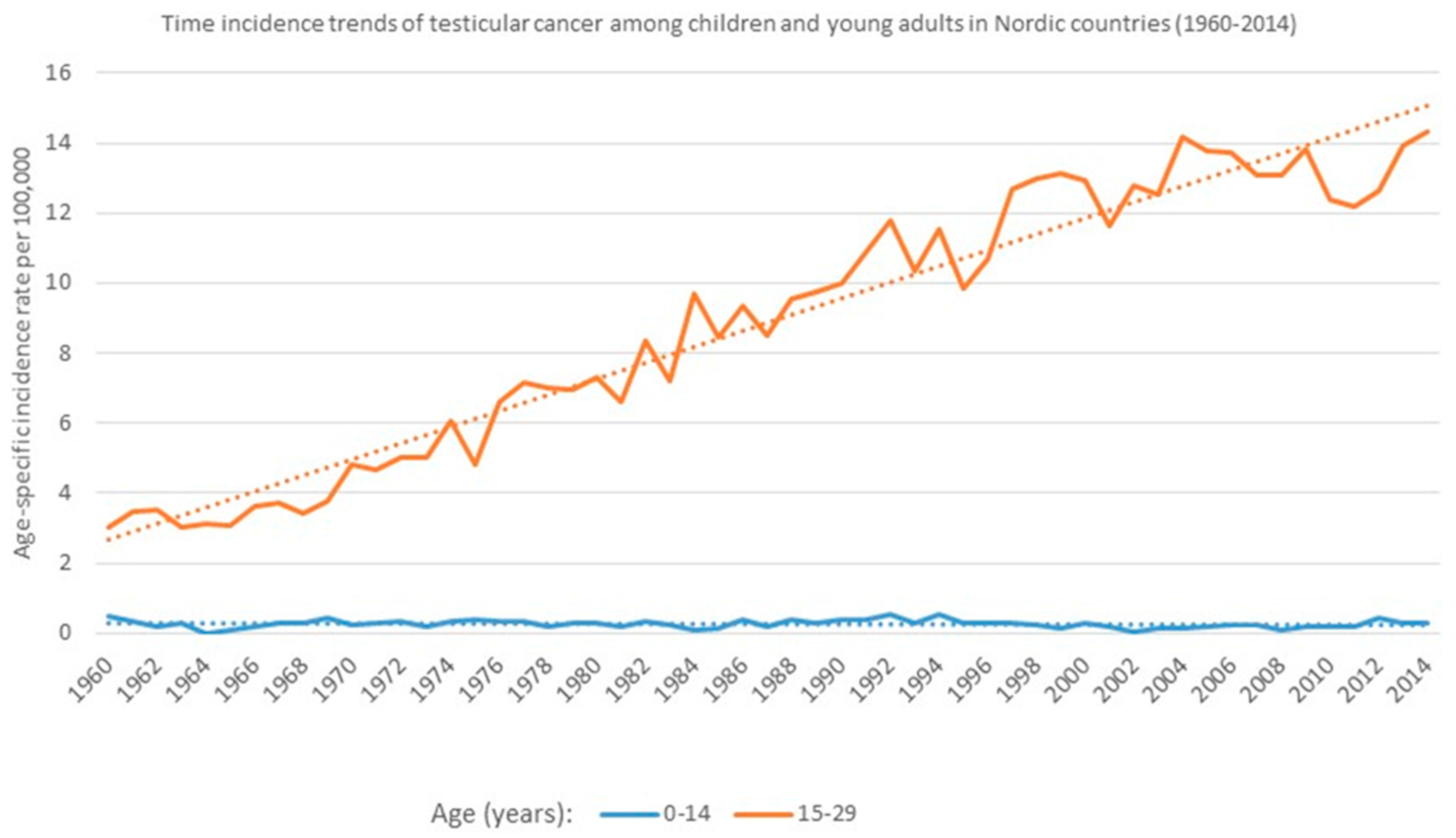
3. Time Incidence Trends of Testicular Cancer (TC): Age-Specific Differences
4. Domestic and Residential Exposures to Pesticides in Childhood
5. Diet and Nutrition in Childhood
6. Height and Child Growth
7. Environmental Exposure to Endocrine-Disrupting Chemicals (EDCs) in Early Life Period
7.1. Dichloro-diphenyl-trichloroethylene (p,p’-DDE)
7.2. Hexachlorobenzene (HCB)
7.3. Chlordane and Its Derivates
7.4. Polychlorinated Biphenyls (PCBs)
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, S.; Semenciw, R.; Waters, C.; Wen, S.W.; Mery, L.S.; Mao, Y. Clues to the aetiological heterogeneity of testicular seminomas and non-seminomas: Time trends and age-period-cohort effects. Int. J. Epidemiol. 2000, 29, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Devesa, S.S.; Sigurdson, A.J.; Mcglynn, K.A. International patterns and trends in testis cancer incidence. Int. J. Cancer 2005, 115, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Richiardi, L.; Ekbom, A.; Pukkala, E.; Cuninkova, M.; Møller, H. Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. Int. J. Cancer 2006, 118, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; Skakkebaek, N.E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 1993, 341, 1392–1395. [Google Scholar] [CrossRef]
- Sharpe, R.M. The ‘oestrogen hypothesis’: Where do we stand now? Int. J. Androl. 2003, 26, 2–15. [Google Scholar] [CrossRef] [PubMed]
- James, W.H. Further grounds for abandoning the concept of testicular dysgenesis syndrome: A response to the paper of Akre and Richiardi. Hum.Reprod. 2010, 25, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Sigurdson, A.J.; Sweeney, A.M.; Amato, R.J.; Strom, S.S.; Mcglynn, K.A. Baldness, acne and testicular germ cell tumours. Int. J. Androl. 2011, 34, e59–e67. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E.; Berthelsen, J.G.; Giwercman, A.; Müller, J. Carcinoma-in-situ of the testis: Possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int. J. Androl. 1987, 10, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Almstrup, K.; Sonne, S.B.; Hoei-Hansen, C.E.; Ottesen, A.M.; Nielsen, J.E.; Skakkebaek, N.E.; Leffers, H.; Rajpert-De Meyts, E. From embryonic stem cells to testicular germ cell cancer: Should we beconcerned? Int. J. Androl. 2006, 29, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Grassetti, D.; Giannandrea, F.; Paoli, D.; Masciandaro, P.; Figura, V.; Carlini, T.; Rizzo, F.; Lombardo, F.; Lenzi, A.; Gandini, L. Androgen receptor polymorphisms and testicular cancer risk. Andrology 2015, 3, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, F.; Paoli, D.; Figà-Talamanca, I.; Lombardo, F.; Lenzi, A.; Gandini, L. Effect of endogenous and exogenous hormones on testicular cancer: The epidemiological evidence. Int. J. Dev. Biol. 2013, 57, 255–263. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Devesa, S.S.; Sigurdson, A.J.; Brown, L.M.; Tsao, L.; Tarone, R.E. Trends in the incidence of testicular germ cell tumors in the United States. Cancer 2003, 97, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, H.M.; Akre, O.; Merletti, F.; Richiardi, L. Time trends in the incidence of testicular cancer in childhood and young adulthood. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2042–2045. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, F.; Gandini, L.; Paoli, D.; Turci, R.; Figà-Talamanca, I. Pesticide exposure and serum organochlorine residuals among testicular cancer patients and healthy controls. J. Environ. Sci. Health B 2011, 46, 780–787. [Google Scholar] [PubMed]
- Møller, H. Work in agriculture, childhood residence, nitrate exposure, and testicular cancer risk: A case‑control study in Denmark. Cancer Epidemiol. Biomark. Prev. 1997, 6, 141–144. [Google Scholar]
- Kristensen, P.; Andersen, A.; Irgens, L.M.; Bye, A.S.; Vagstad, N. Testicular cancer and parental use of fertilizers in agriculture. Cancer Epidemiol. Biomark. Prev. 1996, 5, 3–9. [Google Scholar]
- Sonneveld, D.J.; Schaapveld, M.; Sleijfer, D.T.; Meerman, G.J.; van der Graaf, W.T.; Sijmons, R.H.; Koops, H.S.; Hoekstra, H.J. Geographic clustering of testicular cancer incidence in the northern part of The Netherlands. Br. J. Cancer 1999, 81, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Walschaerts, M.; Muller, A.; Auger, J.; Bujan, L.; Guérin, J.F.; Le Lannou, D.; Clavert, A.; Spira, A.; Jouannet, P.; Thonneau, P. Environmental, occupational and familial risks for testicular cancer: A hospital-based case-control study. Int. J. Androl. 2007, 30, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Nori, F.; Carbone, P.; Giordano, F.; Osborn, J.; Figà-Talamanca, I. Endocrine-disrupting chemicals and testicular cancer: A case-control study. Arch. Environ. Occup. Health 2006, 61, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Aschim, E.L.; Grotmol, T.; Tretli, S.; Haugen, T.B. Is there an association between maternal weight and the risk of testicular cancer? An epidemiologic study of Norwegian data with emphasis on World War II. Int. J. Cancer 2005, 116, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Frankel, S.; Gunnell, D.J.; Peters, T.J.; Maynard, M.; Davey Smith, G. Childhood energy intake and adult mortality from cancer: The Boyd Orr Cohort Study. Br. J. Cancer 1998, 316, 499–504. [Google Scholar] [CrossRef]
- Uauy, R.; Solomons, N. Diet, nutrition, and the life-course approach to cancer prevention. J. Nutr. 2005, 135 (12 Suppl.), 2934S–2945S. [Google Scholar] [PubMed]
- Decarli, A.; La Vecchia, C. Environmental factors and cancer mortality in Italy: Correlational exercise. Oncology 1986, 43, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.W.; Palmer, C.R.; Ruja, E.; Lipscombe, J.M. Adolescent milk, dairy product and fruit consumption and testicular cancer. Br. J. Cancer 1996, 74, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Ganmaa, D.; Li, X.M.; Wang, J.; Qin, L.Q.; Wang, P.Y.; Sato, A. Incidence and mortality of testicular and prostatic cancers in relation to world dietary practices. Int. J. Cancer 2002, 98, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Ahrens, W.; Baumgardt-Elms, C.; Stegmaier, C.; Merzenich, H.; De Vrese, M.; Schrezenmeir, J.; Jöckel, K.H. Adolescent milk fat and galactose consumption and testicular germ cell cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.J.; Birkett, N.J.; Johnson, K.C.; Shatenstein, B.; Ghadirian, P.; Krewski, D.; Canadian Cancer Registries Epidemiology Research Group. Dietary risk factors for testicular carcinoma. Int. J. Cancer 2003, 106, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Giannandrea, F.; Gallo, M.; Turci, R.; Cattaruzza, M.S.; Lombardo, F.; Lenzi, A.; Gandini, L. Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk. J. Endocrinol. Investig. 2015, 38, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.P.; Hartmann, J.T.; Classen, J.; Lüdde, R.; Diederichs, M.; Pichlmeier, U. Tallness is associated with risk of testicular cancer: Evidence for the nutrition hypothesis. Br. J. Cancer 2008, 99, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Lerro, C.C.; Mcglynn, K.A.; Cook, M.B. A systematic review and meta-analysis of the relationship between body size and testicular cancer. Br. J. Cancer 2010, 103, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, F.; Paoli, D.; Lombardo, F.; Lenzi, A.; Gandini, L. Case-control study of anthropometric measures and testicular cancer risk. Front. Endocrinol. (Lausanne) 2012, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Richiardi, L.; Vizzini, L.; Pastore, G.; Segnan, N.; Gillio-Tos, A.; Fiano, V.; Grasso, C.; Ciuffreda, L.; Lista, P.; Pearce, N.; et al. Lifetime growth and risk of testicular cancer. Int. J. Cancer 2014, 135, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; Trabert, B.; Mcglynn, K.A. Organochlorine compounds and testicular dysgenesis syndrome: Human data. Int. J.Androl. 2011, 34, e68–e84. [Google Scholar] [CrossRef] [PubMed]
- Axmon, A.; Hagmar, L.; Jönsson, B.A. Rapid decline of persistent organochlorine pollutants in serum among young Swedish males. Chemosphere 2008, 70, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Hanke, W.; Jurewicz, J. The risk of adverse reproductive and developmental disorders due to occupational pesticide exposure: An overview of current epidemiological evidence. Int. J. Occup. Med. Environ. Health 2004, 17, 223–243. [Google Scholar] [PubMed]
- Giannandrea, F.; Settimi, L.; Figà Talamanca, I. The use of personal protective equipment in pregnant greenhouse workers. Occup. Med. 2008, 58, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Engel, L.S.; Langseth, H.; Needham, L.L.; Andersen, A.; Barr, D.B.; Blair, A.; Rothman, N.; Mcglynn, K.A. Prediagnostic serum concentrations of organochlorine compounds and risk of testicular germ cell tumors. Environ. Health. Perspect. 2009, 117, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Mcglynn, K.A.; Quraishi, S.M.; Graubard, B.I.; Weber, J.P.; Rubertone, M.V.; Erickson, R.L. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J. Natl. Cancer Inst. 2008, 100, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Van Bavel, B.; Lindström, G.; Carlberg, M.; Dreifaldt, A.C.; Wijkström, H.; Starkhammar, H.; Eriksson, M.; Hallquist, A.; Kolmert, T. Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ. Health Perspect. 2003, 111, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.L.; Davis, M.D.; Eaton, D.L.; Weiss, N.S.; Barr, D.B.; Doody, D.R.; Fish, S.; Needham, L.L.; Chen, C.; AndSchwartz, S.M. Serum organochlorine pesticide residues and risk of testicular germ cell carcinoma: A population-based case-control study. CancerEpidemiol. Biomark. Prev. 2008, 17, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Van Bavel, B.; Lindström, G.; Carlberg, M.; Eriksson, M.; Dreifaldt, A.C.; Wijkström, H.; Starkhammar, H.; Hallquist, A.; Kolmert, T. Concentrations of polychlorinated biphenyls in blood and the risk for testicular cancer. Int. J. Androl. 2004, 27, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dong, C.; Chen, M.; Chen, Y.; Gu, A.; Xia, Y.; Sun, H.; Li, Z.; Wang, Y. Effects of monobutyl phthalate on steroidogenesis through steroidogenic acute regulatory protein regulated by transcription factors in mouse Leydigtumor cells. J. Endocrinol. Investig. 2015, 38, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Rouiller-Fabre, V.; Habert, R.; Livera, G. Effects of endocrine disruptors on the human fetal testis. Ann. Endocrinol. (Paris) 2014, 75, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.P.; Flachs, E.M.; Rimborg, S.; Glazer, C.H.; Giwercman, A.; Ramlau-Hansen, C.H.; Hougaard, K.S.; Høyer, B.B.; Hærvig, K.K.; Petersen, S.B.; et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: A systematic review and meta-analysis. Hum. Reprod. Update 2016, 23, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Yin, L.; Yu, K.S.; Hofmann, M.C.; Yu, X. High-content analysis provides mechanistic insights into the testicular toxicity of bisphenol a and selected analogues in mouse spermatogonial cells. Toxicol. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]

| Environmental Childhood Risk Factors That May Be Linked to Testicular Cancer | |
|---|---|
| Domestic and residential exposure to pesticides | +/− |
| Exposure to endocrine-disrupting chemicals (EDCs) | + |
| Height/child growth | ++ |
| Dairy consumption | + |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannandrea, F.; Fargnoli, S. Environmental Factors Affecting Growth and Occurrence of Testicular Cancer in Childhood: An Overview of the Current Epidemiological Evidence. Children 2017, 4, 1. https://doi.org/10.3390/children4010001
Giannandrea F, Fargnoli S. Environmental Factors Affecting Growth and Occurrence of Testicular Cancer in Childhood: An Overview of the Current Epidemiological Evidence. Children. 2017; 4(1):1. https://doi.org/10.3390/children4010001
Chicago/Turabian StyleGiannandrea, Fabrizio, and Stefania Fargnoli. 2017. "Environmental Factors Affecting Growth and Occurrence of Testicular Cancer in Childhood: An Overview of the Current Epidemiological Evidence" Children 4, no. 1: 1. https://doi.org/10.3390/children4010001
APA StyleGiannandrea, F., & Fargnoli, S. (2017). Environmental Factors Affecting Growth and Occurrence of Testicular Cancer in Childhood: An Overview of the Current Epidemiological Evidence. Children, 4(1), 1. https://doi.org/10.3390/children4010001





