Egg Food Challenges are Associated with More Gastrointestinal Reactions
Abstract
:1. Introduction
2. Methods: Study Population and Oral Challenges
3. Classification of Reactions
4. Skin Testing and Serum IgE
5. Statistical Methods
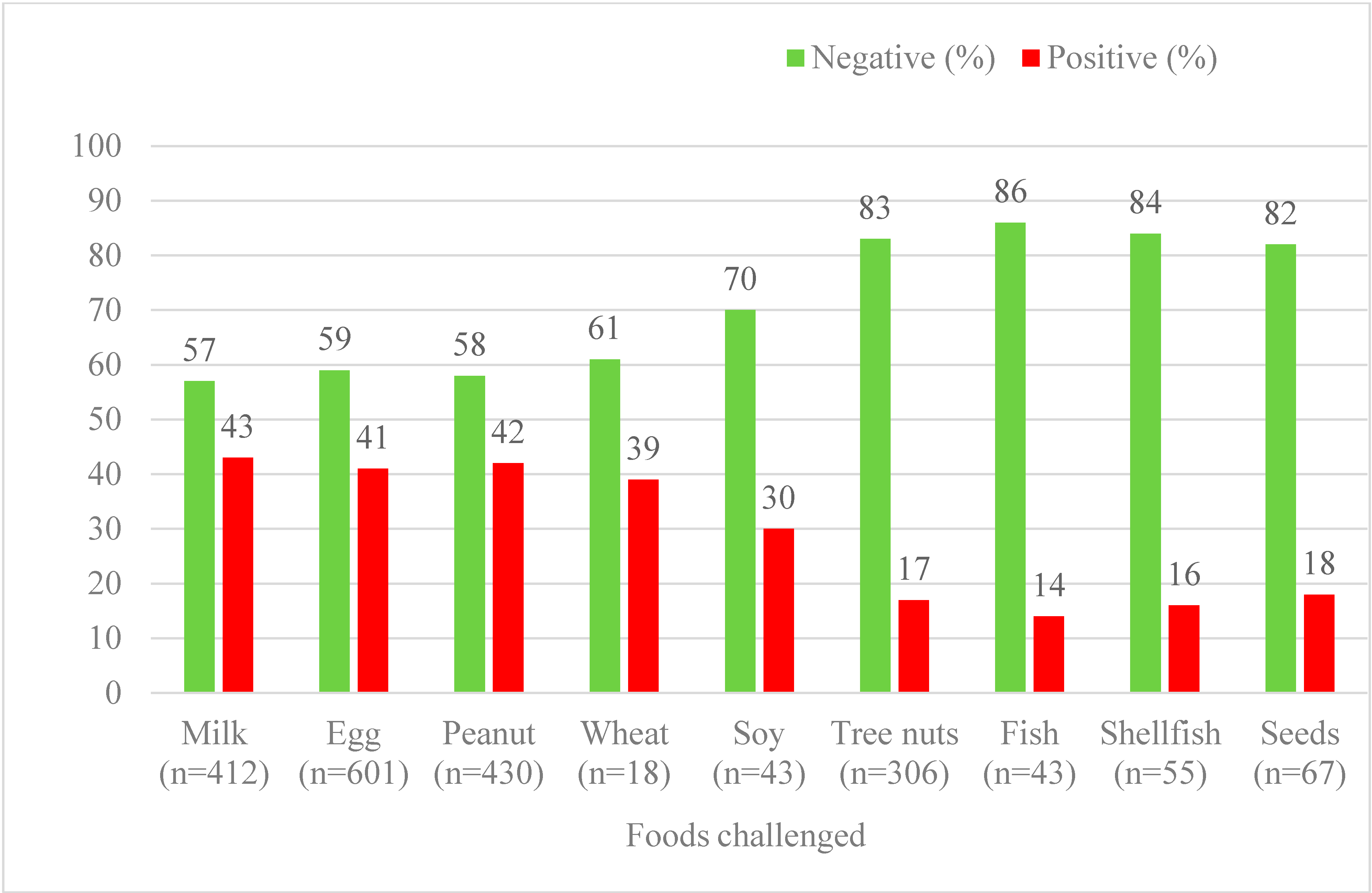
6. Results
| All Foods (Not Egg) | Egg | |
|---|---|---|
| Male, no. (%) | 1171/1703 (69) | 424/601 (70) |
| Atopic history, no. (%) | ||
| Asthma | 1021/1703 (60) | 341/601 (57) |
| Atopic Dermatitis | 80/1703 (46) | 315/601 (52) |
| Seasonal Allergic Rhinitis | 719/1703 (42) | 99/601 (16) |
| Previous reaction | 936/1434 (65%) | 374/542 (69%)# |
| Age (years), mean ± SD | 6.7 ± 3.4 | 5.1 ± 2.9 * |
| Positive OFCs; age (years), mean ± SD | 6.3 ± 3.1 | 5.1 ± 2.8 * |
| Wheal (mm) Mean + Range | 95% Confidence Interval | |
|---|---|---|
| Negative egg challenges | 4.4 + 2.8 * | 4.1–4.7 |
| All positive egg challenges | 6.5 + 3.4 * | 6.0–6.9 |
| Positive egg challenges, not requiring epinephrine | 6.5 + 3.1 | 6.0–7.1 |
| Positive egg challenges, requiring epinephrine | 6.9 + 4.2 | 5.8–7.9 |
| Egg, No. (%) | Milk | Peanut | Tree Nuts | |
|---|---|---|---|---|
| GI only | 37/244 (15%) | 15/178 (8%) * | 14/180 (8%) * | 3/53 (7%) * |
| GI + skin | 32/244 (13%) | 23/178 (13%) | 20/180 (11%) | 6/53 (11%) |
| Any GI | 69/244 (28) | 42/178 (23%) | 35/180 (20%) * | 9/53 (17%) |
| Lower respiratory | 65/244 (27%) | 45/151 (30%) | 60/139 (43%) | 16/18 (88%) # |
| Multi-system | 144/244 (59%) | 111/178 (62%) | 116/180 (64%) | 38/53 (71.7%) |
| Egg, No. (%) | Milk, No. (%) | Peanut, No. (%) | Tree nuts, No. (%) | |
|---|---|---|---|---|
| GI only | 1/37 (3%) | 2/19 (10%) | 1/15 (8%) | 0/3 (0%) |
| GI + skin | 7/32(22%) | 4/23 (17%) | 8/20 (40%) | 3/6 (50%) |
| Any GI | 8/69 (11%) | 8/42 (19%) | 9/35 (25%) | 3/9 (33%) |
| Any reaction | 66/244 (27%) | 54/178 (30%) | 76/180 (42.2%) # | 29/53 (55%) # |
7. Discussion
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prescott, S.L.; Pawankar, R.; Allen, K.J.; Campbell, D.E.; Sinn, J.; Fiocchi, A.; Ebisawa, M.; Sampson, H.A.; Beyer, K.; Lee, B.W.; et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.J.; Koplin, J.J.; Martin, P.E.; Gurrin, L.C.; Lowe, A.J.; Matheson, M.C.; Ponsonby, A.L.; Wake, M.; Tang, M.L.; Dharmage, S.C.; et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J. Allergy Clin. Immunol. 2011, 127, 668 e1–676 e2. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H. Epidemiology of food allergy. J. Allergy Clin. Immunol. 2011, 127, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Bock, S.A.; Spears, G.C.; Wilson, C.G.; Miyazawa, N.K.; Gleason, M.C.; Gyorkos, E.A.; Murphy, J.R.; Atkins, D.; Leung, D.Y.M.; et al. Oral food challenges in children with a diagnosis of food allergy. J. Pediatr. 2011, 158, 578 e1–583 e1. [Google Scholar] [CrossRef] [PubMed]
- Christie, L.; Hine, R.J.; Parker, J.G.; Burks, W. Food allergies in children affect nutrient intake and growth. J. Am. Diet. Assoc. 2002, 102, 1648–1651. [Google Scholar] [CrossRef]
- Flammarion, S.; Santos, C.; Guimber, D.; Jouannic, L.; Thumerelle, C.; Gottrand, F.; Deschildre, A. Diet and nutritional status of children with food allergies. Pediatr. Allergy Immunol. 2011, 22, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.; Groetch, M.; Wang, J. Growth and nutritional concerns in children with food allergy. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.A.; Guerrerio, A.L.; Hauck, S.A.; Henry, B.J.; Keet, C.A.; Brereton, N.H.; Oh, S.; Stasinopoulos, D.M.; Wood, R.A. Growth and nutrition in children with food allergy requiring amino acid-based nutritional formulas. J. Allergy Clin. Immunol. 2014, 134, 1463 e5–1466 e5. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A. The natural history of food allergy. Pediatrics 2003, 111, Part 3. 1631–1637. [Google Scholar] [PubMed]
- Sicherer, S.H.; Wood, R.A.; Vickery, B.P.; Jones, S.M.; Liu, A.H.; Fleischer, D.M.; Dawson, P.; Mayer, L.; Burks, A.W.; Grishin, A.; et al. The natural history of egg allergy in an observational cohort. J. Allergy Clin. Immunol. 2014, 133, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.H.; Matsui, E.C.; Skripak, J.M.; Wood, R.A. The natural history of egg allergy. J. Allergy Clin. Immunol. 2007, 120, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Boyano-Martinez, T.; Garcia-Ara, C.; Diaz-Pena, J.M.; Martin-Esteban, M. Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. J. Allergy Clin. Immunol. 2002, 110, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A.; Assa’a, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Bahna, S.L.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. Nutrition 2011, 27, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Garrett, J.P.; Naimi, D.R.; Khullar, K.; Spergel, J.M. Predictive values for food challenge-induced severe reactions: Development of a simple food challenge score. Isr. Med. Assoc. J. 2012, 14, 24–28. [Google Scholar] [PubMed]
- Fleischer, D.M.; Burks, A.W. Oral food challenges in children with a diagnosis of food allergy. J. Pediatr. 2011, 158, 578 e1–583 e1. [Google Scholar] [CrossRef] [PubMed]
- Noimark, L.; Cox, H.E. Nutritional problems related to food allergy in childhood. Pediatr. Allergy Immunol. 2008, 19, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Gerth van Wijk, R.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.J.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar] [PubMed]
- Nowak-Wegrzyn, A.; Assa’ad, A.H.; Bahna, S.L.; Bock, S.A.; Sicherer, S.H.; Teuber, S.S. Work Group report: Oral food challenge testing. J. Allergy Clin. Immunol. 2009, 123, S365–S383. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.T.; Matsui, E.C.; Conover-Walker, M.K.; Wood, R.A. Risk of oral food challenges. J. Allergy Clin. Immunol. 2004, 114, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Khullar, K.; Saltzman, R.; Fiedler, J.; Garrett, J.P.; Naimi, D.R.; Spergel, J.M. Oral food challenge to wheat: A near-fatal anaphylaxis and review of 93 food challenges in children. World Allergy Organ. J. 2013, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Sporik, R.; Hill, D.J.; Hosking, C.S. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin. Exp. Allergy 2000, 30, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A. Use of food-challenge tests in children. Lancet 2001, 358, 1832–1833. [Google Scholar] [CrossRef]
- Sampson, H.A.; Ho, D.G. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J. Allergy Clin. Immunol. 1997, 100, 444–451. [Google Scholar] [CrossRef]
- Gupta, R.S.; Lau, C.H.; Hamilton, R.G.; Donnell, A.; Newhall, K.K. Predicting outcomes of oral food challenges by using the allergen-specific IgE-total IgE ratio. J. Allergy Clin. Immunol. Pract. 2014, 2, 300–305. [Google Scholar] [CrossRef] [PubMed]
- DunnGalvin, A.; Daly, D.; Cullinane, C.; Stenke, E.; Keeton, D.; Erlewyn-Lajeunesse, M.; Roberts, G.C.; Lucas, J.; Hourihane, J.O’B. Highly accurate prediction of food challenge outcome using routinely available clinical data. J. Allergy Clin. Immunol. 2011, 127, 633 e1–639 e3. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Mendelson, L.; Rosen, J.P. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N. Engl. J. Med. 1992, 327, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Pumphrey, R.S. Fatal anaphylaxis in the UK, 1992–2001. Novartis Found. Symp. 2004, 257, 116–128, discussion 128–132, 157–160, 276–285. [Google Scholar] [PubMed]
- Bock, S.A.; Munoz-Furlong, A.; Sampson, H.A. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J. Allergy Clin. Immunol. 2007, 119, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A.; Munoz-Furlong, A.; Sampson, H.A. Fatalities due to anaphylactic reactions to foods. J. Allergy Clin. Immunol. 2001, 107, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Soares-Weiser, K.; Takwoingi, Y.; Panesar, S.S.; Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Halken, S.; Poulsen, L.; van Ree, R.; et al. The diagnosis of food allergy: A systematic review and meta-analysis. Allergy 2014, 69, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, M.; Garcia-Paba, M.B.; Giner, M.T.; Piquer, M.; Dominguez, O.; Lozano, J.; Jiménez, R.; Machinena, A.; Martín-Mateos, M.A.; Plaza, A.M. Tolerance to egg proteins in egg-sensitized infants without previous consumption. Allergy 2014, 69, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Islam, S.; King, Y.; Deighton, J.; Szun, S.; Anagnostou, K.; Ewan, P. A longitudinal study of resolution of allergy to well-cooked and uncooked egg. Clin. Exp. Allergy 2011, 41, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, A.H.; Zamora, S.A.; Eigenmann, P.A. Correlation between specific immunoglobulin E levels and the severity of reactions in egg allergic patients. Pediatr. Allergy Immunol. 2008, 19, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Rolinck-Werninghaus, C.; Niggemann, B.; Grabenhenrich, L.; Wahn, U.; Beyer, K. Outcome of oral food challenges in children in relation to symptom-eliciting allergen dose and allergen-specific IgE. Allergy 2012, 67, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Ortiz, M.; Alvaro, M.; Piquer, M.; Dominguez, O.; Machinena, A.; Martin-Mateos, M.A.; Plaza, A.M. Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg-allergic children. Clin. Exp. Allergy 2014, 44, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Novembre, E.; Mugnaini, L.; Lombardi, E.; Bernardini, R.; Pucci, N.; Vierucci, A. Clinical features of acute anaphylaxis in patients admitted to a university hospital: An 11-year retrospective review (1985–1996). Ann. Allergy Asthma Immunol. 2001, 87, 27–32. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 14: Logistic regression. Crit. Care (Lond. Engl.) 2005, 9, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Langeland, T. Allergy to hen’s egg white in atopic dermatitis. Acta Derm. Venereol. Suppl. (Stockh) 1985, 114, 109–112. [Google Scholar] [PubMed]
- Sampson, H.A. Role of immediate food hypersensitivity in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 1983, 71, 473–480. [Google Scholar] [CrossRef]
- Spergel, J.M. Epidemiology of atopic dermatitis and atopic march in children. Immunol. Allergy Clin. N. Am. 2010, 30, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Beausoleil, J.L.; Fiedler, J.M.; Ginsberg, J.; Wagner, K.; Pawlowski, N.A. Correlation of initial food reactions to observed reactions on challenges. Ann. Allergy Asthma Immunol. 2004, 92, 217–224. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, M.; Grossmann, L.D.; Spergel, J.M.; Cianferoni, A. Egg Food Challenges are Associated with More Gastrointestinal Reactions. Children 2015, 2, 371-381. https://doi.org/10.3390/children2030371
Gupta M, Grossmann LD, Spergel JM, Cianferoni A. Egg Food Challenges are Associated with More Gastrointestinal Reactions. Children. 2015; 2(3):371-381. https://doi.org/10.3390/children2030371
Chicago/Turabian StyleGupta, Malika, Liron D. Grossmann, Jonathan M. Spergel, and Antonella Cianferoni. 2015. "Egg Food Challenges are Associated with More Gastrointestinal Reactions" Children 2, no. 3: 371-381. https://doi.org/10.3390/children2030371
APA StyleGupta, M., Grossmann, L. D., Spergel, J. M., & Cianferoni, A. (2015). Egg Food Challenges are Associated with More Gastrointestinal Reactions. Children, 2(3), 371-381. https://doi.org/10.3390/children2030371





