Prevalence, Spectrum, and Management of Thyroid Dysfunction in Children with Down Syndrome: A Retrospective Study from Southern Saudi Arabia
Highlights
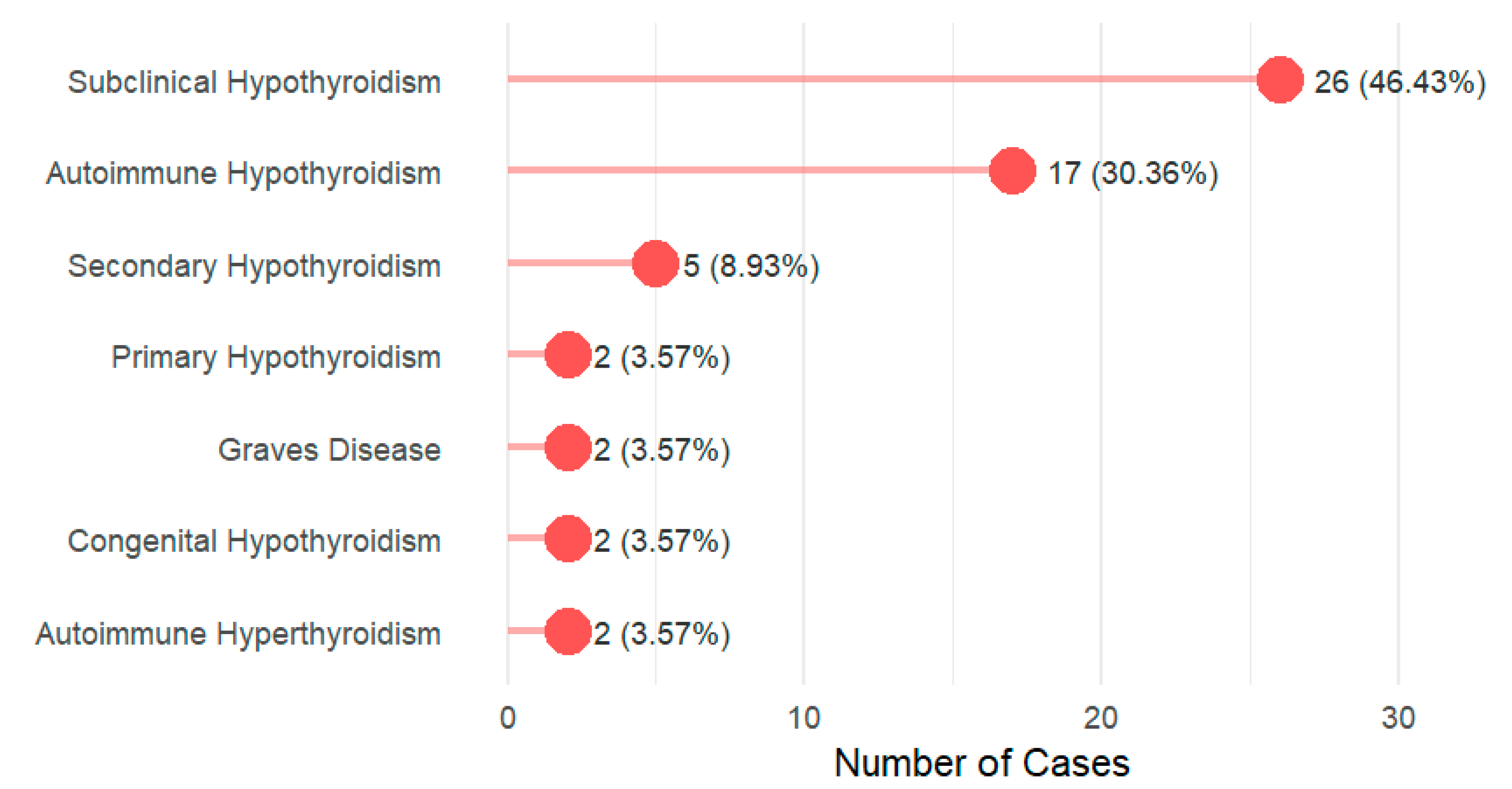
- Thyroid dysfunction is very common, mainly subclinical and autoimmune hypothyroidism.
- Family history is the strongest risk factor, and most treated children need levothyroxine dose adjustment.
- Treatment optimization is a must to improve disease management.
- Guidelines should emphasize stricter monitoring and standardized management to close the care gap.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Sample Size Calculation
2.3. Data Collection Instrument
2.4. Data Quality Assurance
2.5. Variable Coding and Transformation
- Euthyroid: Normal TSH and Free T4 for age, no history or symptoms of thyroid disease.
- Isolated hyperthyrotropinemia (IHT): Mildly elevated TSH with normal Free T4, usually asymptomatic, often transient or physiologic.
- Subclinical hypothyroidism: Elevated TSH with normal Free T4, may have mild/non-specific symptoms, often associated with autoimmune thyroiditis (i.e., Anti-TPO positive).
- Congenital hypothyroidism: Thyroid hormone deficiency present at birth, detected on newborn screening.
- Autoimmune hypothyroidism (Hashimoto’s thyroiditis): Hypothyroidism with positive Anti-TPO antibodies and/or autoimmune thyroid destruction.
- Graves’ disease: Hyperthyroidism due to TRAb/TSI, with clinical features of hyperthyroidism.
- Other/Overt hyperthyroidism: Hyperthyroidism not caused by Graves’ disease (e.g., toxic nodule, toxic multinodular goiter, thyroiditis).
- Secondary (Central) hypothyroidism: Low FT4 with low or inappropriately normal TSH due to pituitary or hypothalamic dysfunction.
2.5.1. Symptom Coding
- Total symptom score: The sum of all individual symptom indicators, representing the overall symptom burden.
- Metabolic symptom score: A composite of fatigue, weight changes, and cold intolerance.
- Neurodevelopmental symptom score: A composite of developmental delay and behavioral changes.
- Dermatological symptom score: Represented by the presence of dry skin.
- Gastrointestinal symptom score: Represented by the presence of constipation.
2.5.2. Comorbidity Assessment
2.5.3. Laboratory Tests
- 0–1 month: 0.7–18.1 mU/L.
- 1–12 months: 1.12–8.21 mU/L.
- 1–5 years: 0.80–6.26 mU/L.
- 6–10 years: 0.80–5.40 mU/L.
- 11–14 years: 0.70–4.61 mU/L.
- 15–18 years: 0.50–4.33 mU/L.
- 1 month to <1 year: 1.3–2.8.
- 1 to <3 years: 1.3–2.4.
- 3 to <8 years: 1.3–2.4.
- 8 to <18 years: majority 1.3–2.4.
- Autoimmune positive: Elevated level of either Anti-TPO or Anti-TG antibody.
- Autoimmune negative: Normal levels of both Anti-TPO and Anti-TG antibodies.
- Not tested: Absence of results for both antibody tests.
- Partially tested: Only one of the two antibody tests was performed.
2.5.4. Care Gap Identification
Caregivers’ Perspective and Awareness-Care Gap
Screening Adherence (For Non-Thyroid Patients)
- Adherent: Last thyroid screen conducted within ≤15 months, allowing a 3-month grace period beyond the annual recommendation.
- Moderately adherent: Last thyroid screen conducted 15–24 months ago.
- Non-adherent: Last thyroid screen conducted >24 months ago.
Follow-Up Adequacy Assessment
- Severe or moderate thyroid dysfunction, e.g., overt hypothyroidism, hyperthyroidism: Appropriate follow-up: ≤6 months
- Mild thyroid dysfunction: (e.g., subclinical hypothyroidism): Appropriate follow-up: ≤12 months
- Patients whose follow-up intervals exceeded these timeframes were classified as having inadequate monitoring.
Biochemical Improvement
2.6. Statistical Analysis Plan
2.7. Ethical Considerations
3. Results
4. Discussion
4.1. Summary of the Main Findings
4.2. Interpretation of the Main Findings
4.3. Factors Associated with Thyroid Dysfunction
4.4. Awareness Gap
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Abbreviation | Full Term |
| AAP | American Academy of Pediatrics |
| Anti-TG | Anti-Thyroglobulin Antibody |
| Anti-TPO | Anti-Thyroid Peroxidase Antibody |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| DALYs | Disability-Adjusted Life Years |
| DS | Down Syndrome |
| FT4 | Free Thyroxine |
| IRB | Institutional Review Board |
| IQR | Interquartile Range |
| OR | Odds Ratio |
| SCH | Subclinical Hypothyroidism |
| SD | Standard Deviation |
| TRAb | TSH Receptor Antibodies |
| TSH | Thyroid-Stimulating Hormone |
| CHD | Congenital Heart Disease |
| FT4/Free T4 | Free Thyroxine |
| SDI | Socio-Demographic Index |
| TD | Thyroid Dysfunction |
References
- Domino, F.J.; Baldor, R.A.; Golding, J.; Stephens, M.B. The 5-Minute Clinical Consult 2020; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2019. [Google Scholar]
- Baruffi, M.R.; Souza, D.H.; Silva, R.A.; Ramos, E.S.; Moretti-Ferreira, D. A rare non-Robertsonian translocation involving chromosomes 15 and 21. Sao Paulo Med. J. 2013, 131, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Chromosomal Abnormalities: Trisomy 21 (Down Syndrome). 2024. Available online: https://archive.cdc.gov/www_cdc_gov/ncbddd/birthdefects/surveillancemanual/quick-reference-handbook/trisomy-21-down-syndrome.html (accessed on 13 December 2025).
- Asim, A.; Kumar, A.; Muthuswamy, S.; Jain, S.; Agarwal, S. Down syndrome: An insight of the disease. J. Biomed. Sci. 2015, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Bittles, A.H.; Glasson, E.J. Clinical, social, and ethical implications of changing life expectancy in Down syndrome. Dev. Med. Child. Neurol. 2004, 46, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Wang, Y.; Hu, H.; Zhan, Y.; Zeng, Z.; Liu, L. Global, Regional, and National Burden and Trends of Down Syndrome From 1990 to 2019. Front. Genet. 2022, 13, 908482. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Sanyal, D.; Roy, K.; Bhattacharyya, S. Correlation between physical anomaly and behavioral abnormalities in Down syndrome. J. Pediatr. Neurosci. 2010, 5, 105–110. [Google Scholar] [CrossRef]
- Amiel, J.; Sproat-Emison, E.; Garcia-Barcelo, M.; Lantieri, F.; Burzynski, G.; Borrego, S.; Pelet, A.; Arnold, S.; Miao, X.; Griseri, P.; et al. Hirschsprung disease, associated syndromes and genetics: A review. J. Med. Genet. 2008, 45, 1–14. [Google Scholar] [CrossRef]
- Wallace, R.A. Clinical audit of gastrointestinal conditions occurring among adults with Down syndrome attending a specialist clinic. J. Intellect. Dev. Disabil. 2007, 32, 45–50. [Google Scholar] [CrossRef]
- Henry, E.; Walker, D.; Wiedmeier, S.E.; Christensen, R.D. Hematological abnormalities during the first week of life among neonates with Down syndrome: Data from a multihospital healthcare system. Am. J. Med. Genet. A 2007, 143a, 42–50. [Google Scholar] [CrossRef]
- Lott, I.T. Neurological phenotypes for Down syndrome across the life span. Prog. Brain Res. 2012, 197, 101–121. [Google Scholar]
- Hawli, Y.; Nasrallah, M.; El-Hajj Fuleihan, G. Endocrine and musculoskeletal abnormalities in patients with Down syndrome. Nat. Rev. Endocrinol. 2009, 5, 327–334. [Google Scholar] [CrossRef]
- Merrick, J.; Koslowe, K. Refractive errors and visual anomalies in Down syndrome. Down Syndr. Res. Pract. 2001, 6, 131–133. [Google Scholar] [CrossRef]
- Salehi, A.; Ashford, J.W.; Mufson, E.J. The Link between Alzheimer’s Disease and Down Syndrome. A Historical Perspective. Curr. Alzheimer Res. 2016, 13, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, C.S.; Madsen, C.D.; Toftager, M.; Amholt, T.T.; Schipperijn, J. The role of playgrounds in the development of children’s fundamental movement skills: A scoping review. PLoS ONE 2023, 18, e0294296. [Google Scholar] [CrossRef] [PubMed]
- Matitu-Untalan, L.A.; Estrada, S.C. Prevalence of thyroid disorders among children with Down syndrome seen in the out-patient clinics of the Philippine general hospital. Int. J. Pediatr. Endocrinol. 2015, 2015, P101. [Google Scholar] [CrossRef]
- Bull, M.J.; The Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics 2011, 128, 393–406. [Google Scholar] [CrossRef]
- Amr, N.H. Thyroid Disorders in Subjects with Down Syndrome: An Update. Acta Biomed. 2018, 89, 132–139. [Google Scholar]
- Tomer, Y. Genetic susceptibility to autoimmune thyroid disease: Past, present, and future. Thyroid 2010, 20, 715–725. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Boelaert, K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef]
- Kapelari, K.; Kirchlechner, C.; Högler, W.; Schweitzer, K.; Virgolini, I.; Moncayo, R. Pediatric reference intervals for thyroid hormone levels from birth to adulthood: A retrospective study. BMC Endocr. Disord. 2008, 8, 15. [Google Scholar] [CrossRef]
- Bull, M.J.; Trotter, T.; Santoro, S.L.; Christensen, C.; Grout, R.W.; Burke, L.W.; Berry, S.A.; Geleske, T.A.; Holm, I.; Hopkin, R.J.; et al. Health Supervision for Children and Adolescents With Down Syndrome. Pediatrics 2022, 149, e2022057010. [Google Scholar] [CrossRef]
- Pierce, M.J.; LaFranchi, S.H.; Pinter, J.D. Characterization of Thyroid Abnormalities in a Large Cohort of Children with Down Syndrome. Horm. Res. Paediatr. 2017, 87, 170–178. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics 1994, 93, 855–859. [Google Scholar] [CrossRef]
- Van Cleve, S.N.; Cohen, W.I. Part I: Clinical practice guidelines for children with Down syndrome from birth to 12 years. J. Pediatr. Health Care 2006, 20, 47–54. [Google Scholar] [CrossRef]
- Van Cleve, S.N.; Cannon, S.; Cohen, W.I. Part II: Clinical Practice Guidelines for adolescents and young adults with Down Syndrome: 12 to 21 Years. J. Pediatr. Health Care 2006, 20, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Erlichman, I.; Mimouni, F.B.; Erlichman, M.; Schimmel, M.S. Thyroxine-Based Screening for Congenital Hypothyroidism in Neonates with Down Syndrome. J. Pediatr. 2016, 173, 165–168. [Google Scholar] [CrossRef]
- Cutler, A.T.; Benezra-Obeiter, R.; Brink, S.J. Thyroid function in young children with Down syndrome. Am. J. Dis. Child. 1986, 140, 479–483. [Google Scholar] [CrossRef]
- King, K.; O’Gorman, C.; Gallagher, S. Thyroid dysfunction in children with Down syndrome: A literature review. Ir. J. Med. Sci. 2014, 183, 1–6. [Google Scholar] [CrossRef]
- World Population Review. Down Syndrom Rates by Country 2025. Available online: https://worldpopulationreview.com/country-rankings/down-syndrome-rates-by-country (accessed on 23 November 2025).
- Mitha, K. Conceptualising and addressing mental disorders amongst Muslim communities: Approaches from the Islamic Golden Age. Transcult. Psychiatry 2020, 57, 763–774. [Google Scholar] [CrossRef]
- Bentley, J.A.; Feeny, N.C.; Dolezal, M.L.; Klein, A.; Marks, L.H.; Graham, B.; Zoellner, L.A. Islamic Trauma Healing: Integrating Faith and Empirically Supported Principles in a Community-Based Program. Cogn. Behav. Pract. 2021, 28, 167–192. [Google Scholar] [CrossRef]
- Alasiri, A.A.; Mohammed, V. Healthcare Transformation in Saudi Arabia: An Overview Since the Launch of Vision 2030. Health Serv. Insights 2022, 15, 11786329221121214. [Google Scholar] [CrossRef]
- van den Driessen Mareeuw, F.A.; Coppus, A.M.W.; Delnoij, D.M.J.; de Vries, E. Quality of health care according to people with Down syndrome, their parents and support staff-A qualitative exploration. J. Appl. Res. Intellect. Disabil. 2020, 33, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Jang, M.; Guo, T.; Soldin, S.J. Pediatric reference intervals for free thyroxine and free triiodothyronine. Thyroid 2009, 19, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, R.A. Thyroid disorder in children and young people with Down syndrome: DSMIG guideline review. Arch. Dis. Child.-Educ. Pract. 2022, 107, 34–35. [Google Scholar] [CrossRef]
- Feldman, P.M.; Rodriguez, N.; Morrison, E.; Barton, B.; Lee, M.M. Prospective study of thyroid function in the first year of life in infants with Down syndrome. Eur. J. Pediatr. 2023, 182, 2903–2911. [Google Scholar] [CrossRef]
- Desai, S.S. Down syndrome: A review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 279–285. [Google Scholar] [CrossRef]
- Maphumulo, S.; Honey, E.; Abdelatif, N.; Karsas, M. The prevalence and spectrum of thyroid dysfunction among children with Down syndrome attending the paediatric services at two tertiary hospitals in Pretoria, South Africa. S. Afr. J. Child Health 2023, 17, 192–195. [Google Scholar] [CrossRef]
- Moosa, S.; Segal, D.G.; Christianson, A.L.; Gregersen, N.E. Thyroid dysfunction in a cohort of South African children with Down syndrome. S. Afr. Med. J. 2013, 103, 966–970. [Google Scholar] [CrossRef]
- Mulu, B.; Fantahun, B. Thyroid abnormalities in children with Down syndrome at St. Paul’s hospital millennium medical college, Ethiopia. Endocrinol. Diabetes Metab. 2022, 5, e00337. [Google Scholar] [CrossRef]
- Purdy, I.B.; Singh, N.; Brown, W.L.; Vangala, S.; Devaskar, U.P. Revisiting early hypothyroidism screening in infants with Down syndrome. J. Perinatol. 2014, 34, 936–940. [Google Scholar] [CrossRef]
- Prasher, V.P. Down syndrome and thyroid disorders: A review. Downs Syndr. Res. Pract. 1999, 6, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.; Sharr, C.; Elsharkawi, I.; Ozonoff, A.; Baumer, N.; Brasington, C.; Cannon, S.; Crissman, B.; Davidson, E.; Florez, J.C.; et al. Thyroid dysfunction in patients with Down syndrome: Results from a multi-institutional registry study. Am. J. Med. Genet. A 2017, 173, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Sankar, H.V.; Anjukrishna, K.; Riaz, I. Thyroid stimulating hormone level at diagnosis as a predictor of persistent subclinical hypothyroidism in children with Down syndrome. Indian Pediatr. 2018, 55, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Pascanu, I.; Banescu, C.; Benedek, T.; Duicu, C.; Csep, K.; Dema, A. Thyroid dysfunction in children with down’s syndrome. Acta Endocrinol. 2009, 5, 85–92. [Google Scholar] [CrossRef]
- Gibson, P.A.; Newton, R.W.; Selby, K.; Price, D.A.; Leyland, K.; Addison, G.M. Longitudinal study of thyroid function in Down’s syndrome in the first two decades. Arch. Dis. Child. 2005, 90, 574–578. [Google Scholar] [CrossRef]
- Aversa, T.; Crisafulli, G.; Zirilli, G.; De Luca, F.; Gallizzi, R.; Valenzise, M. Epidemiological and clinical aspects of autoimmune thyroid diseases in children with Down’s syndrome. Ital. J. Pediatr. 2018, 44, 39. [Google Scholar] [CrossRef]
- Pepe, G.; Corica, D.; De Sanctis, L.; Salerno, M.; Faienza, M.F.; Tessaris, D.; Tuli, G.; Scala, I.; Penta, L.; Alibrandi, A.; et al. Prospective evaluation of autoimmune and non-autoimmune subclinical hypothyroidism in Down syndrome children. Eur. J. Endocrinol. 2020, 182, 385–392. [Google Scholar] [CrossRef]
- Fort, P.; Lifshitz, F.; Bellisario, R.; Davis, J.; Lanes, R.; Pugliese, M.; Richman, R.; Post, E.M.; David, R. Abnormalities of thyroid function in infants with Down syndrome. J. Pediatr. 1984, 104, 545–549. [Google Scholar] [CrossRef]
- Calcaterra, V.; Nappi, R.E.; Regalbuto, C.; De Silvestri, A.; Incardona, A.; Amariti, R.; Bassanese, F.; Clemente, A.M.; Vinci, F.; Albertini, R.; et al. Gender Differences at the Onset of Autoimmune Thyroid Diseases in Children and Adolescents. Front. Endocrinol. 2020, 11, 229. [Google Scholar] [CrossRef]
- Hossen, M.S.; Islam, M.M.; Das, A.; Ripon, M.A.R.; Tohidul Amin, M.; Basher, M.A.; Rashid, M.M.O. Thyroid Dysfunction Prevalence and Risk Factors in the Southeastern Part of Bangladesh: A Cross-Sectional Study. Health Sci. Rep. 2025, 8, e70329. [Google Scholar] [CrossRef] [PubMed]
- Al Qassimi, A.M.; Al Marzooq, R.A.; Alfaraj, L.H.; Al Radhwan, N.M.; Al-Askari, Z.A.; AlKhalifah, A.S. Prevalence and impact of endocrinopathies on growth in pediatric down syndrome patients: A retrospective analysis. Saudi Med. J. 2025, 46, 364. [Google Scholar] [CrossRef] [PubMed]
- Corona-Rivera, J.R.; Andrade-Romo, T.O.; Aguirre-Salas, L.M.; Bobadilla-Morales, L.; Aranda-Sánchez, C.I.; Corona-Rivera, A.; Pérez-Ramírez, R.O. Family history of thyroid disease and risk of congenital hypothyroidism in neonates with Down síndrome. Gac. Med. Mex. 2021, 157, 133–139. [Google Scholar] [PubMed]
- Guaraldi, F.; Rossetto Giaccherino, R.; Lanfranco, F.; Motta, G.; Gori, D.; Arvat, E.; Ghigo, E.; Giordano, R. Endocrine Autoimmunity in Down’s Syndrome. Front. Horm. Res. 2017, 48, 133–146. [Google Scholar]
- Sabel, G.; Ahmadi, A.; Podder, D.; Stala, O.; Hirani, R.; Etienne, M. Association Between Hypothyroidism and Depression in Individuals with Down Syndrome: A Retrospective Analysis. Life 2025, 15, 1199. [Google Scholar] [CrossRef]
- Shahid, M.A.; Ashraf, M.A.; Sharma, S. Physiology, Thyroid Hormone; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Oliveira, A.T.; Longui, C.A.; Calliari, E.P.; Ferone Ede, A.; Kawaguti, F.S.; Monte, O. Evaluation of the hypothalamic-pituitary-thyroid axis in children with Down syndrome. J. Pediatr. 2002, 78, 295–300. [Google Scholar] [CrossRef]
- Jasim, S.; Papaleontiou, M. Considerations in the Diagnosis and Management of Thyroid Dysfunction in Older Adults. Thyroid 2025, 35, 624–632. [Google Scholar] [CrossRef]
| Variable | Characteristic | Overall N = 106 |
|---|---|---|
| Gender | Female | 59.0 (55.7%) |
| Male | 47.0 (44.3%) | |
| Age (years) | Median (Q1, Q3) | 8.0 (5.9, 10.7) |
| Min, Max | 1.9, 17.0 | |
| Type of DS | Trisomy 21 | 95 (89.6%) |
| Translocation | 6 (5.6%) | |
| Mosaic | 5 (4.7%) | |
| Total symptoms | 0 | 2.0 (1.9%) |
| 1 | 39.0 (36.8%) | |
| 2 | 37.0 (34.9%) | |
| 3 | 20.0 (18.9%) | |
| 4 | 4.0 (3.8%) | |
| 5 | 2.0 (1.9%) | |
| 6 | 2.0 (1.9%) | |
| Total comorbidities | 0 | 39.0 (36.8%) |
| 1 | 52.0 (49.1%) | |
| 2 | 15.0 (14.2%) | |
| TSH level | Median (Q1, Q3) | 4.4 (3.4, 6.0) |
| Min, Max | 0.1, 19.0 | |
| Free T4 | Mean ± SD | 14.2 ± 2.5 |
| Min, Max | 9.8, 22.0 | |
| Height (cm) | Mean ± SD | 122.0 ± 23.8 |
| Min, Max | 14.0, 162.0 | |
| Weight (kg) | Mean ± SD | 40.5 ± 17.4 |
| Min, Max | 12.0, 100.0 | |
| Body mass index | Median (Q1, Q3) | 25.4 (22.4, 28.4) |
| Min, Max | 15.0, 46.5 | |
| Ultrasound performed | 4 (3.8%) | |
| Family history of thyroid disease | 49.0 (46.2%) | |
| Awareness of high risk of thyroid disease | 76.0 (71.7%) |
| Characteristic | Category | No Thyroid Dysfunction (n = 50) | With Thyroid Dysfunction (n = 56) | p-Value |
|---|---|---|---|---|
| Gender | Female | 33 (66.0%) | 26 (46.4%) | 0.043 |
| Male | 17 (34.0%) | 30 (53.6%) | ||
| Age (years) | Median (Q1, Q3) | 7.4 (5.4, 11.0) | 8.4 (6.2, 10.4) | 0.2 |
| Min, Max | 2.2, 15.0 | 1.9, 17.0 | ||
| Total symptoms | 0 | 2 (4.0%) | 0 | 0.005 |
| 1 | 25 (50.0%) | 14 (25.0%) | ||
| 2 | 17 (34.0%) | 20 (35.7%) | ||
| 3 | 4 (8.0%) | 16 (28.6%) | ||
| 4 | 2 (4.0%) | 2 (3.6%) | ||
| 5 | 0 | 2 (3.6%) | ||
| 6 | 0 | 2 (3.6%) | ||
| Metabolic symptoms | Median (IQR) | 1 (0–1) | 1 (1–1) | 0.0058 |
| Neurodevelopmental symptoms | Median (IQR) | 0 (0–1) | 0 (0–1) | 0.2 |
| Dermatological symptoms | n (%) | 4/50 (8%) | 17/56 (30.4%) | 0.0063 |
| Gastrointestinal symptoms | n (%) | 16/50 (32%) | 51/56 (91.1%) | <0.0001 |
| Total Comorbidities | 0 | 21 (42.0%) | 18 (32.1%) | 0.6 |
| 1 | 23 (46.0%) | 29 (51.8%) | ||
| 2 | 6 (12.0%) | 9 (16.1%) | ||
| TSH Level | Median (Q1, Q3) | 4.0 (3.0, 4.2) | 6.0 (4.6, 7.6) | <0.001 |
| Min, Max | 1.9, 5.1 | 0.0, 19.0 | ||
| Free T4 | Median (Q1, Q3) | 14.0 (13.0, 16.0) | 13.9 (12.0, 15.6) | 0.02 |
| Min, Max | 11.0, 19.0 | 9.8, 22.0 | ||
| Height (cm) | Median (Q1, Q3) | 126.5 (107–135) | 129.0 (119–139.5) | 0.2 |
| Min, Max | 70–159 | 65–162 | ||
| Weight (kg) | Median (Q1, Q3) | 35.0 (24–47) | 39.5 (32–49) | 0.077 |
| Min, Max | 14–86 | 12–100 | ||
| Body mass index | Median (Q1, Q3) | 25.4 (21–28.3) | 25.5 (22.6–28.6) | 0.4 |
| Min, Max | 15–46.5 | 17.1–44 | ||
| Family history of thyroid disease | No | 38 (76.0%) | 19 (33.9%) | <0.001 |
| Yes | 12 (24.0%) | 37 (66.1%) | ||
| Awareness that children with down syndrome are at higher risk for thyroid dysfunction | No | 18 (36.0%) | 12 (21.4%) | 0.1 |
| Yes | 32 (64.0%) | 44 (78.6%) |
| Characteristic | OR | 95% CI | p-Value |
|---|---|---|---|
| Gender | |||
| Female | - | - | |
| Male | 1.70 | 0.69, 4.24 | 0.2 |
| Total symptoms | 1.92 | 1.22, 3.25 | 0.008 |
| Family history of thyroid disease | |||
| No | - | - | |
| Yes | 4.57 | 1.89, 11.6 | <0.001 |
| Category | Variable | Value/Description | Count (n) | Percentage (%) |
|---|---|---|---|---|
| Follow-up and Monitoring | Appropriate follow-up | Appropriate | 46 | 82.1% |
| Inadequate | 10 | 17.9% | ||
| Appropriate dosing of levothyroxine (n = 41) * | May need adjustment | 30 | 73.2% | |
| Appropriate | 11 | 26.8% | ||
| Clinical Status | Autoimmune testing | Fully tested | 41 | 73.2% |
| Partially tested | 4 | 7.1% | ||
| Not tested | 11 | 19.6% | ||
| Patient and Caregiver Perspective | Care satisfaction | Dissatisfied | 17 | 30.4% |
| Aware of high thyroid risk | Aware | 44 | 78.6% | |
| Awareness-care gap | Aware but not satisfied | 14 | 25.0% | |
| Awareness-care gap prevalence | (Among aware patients, n = 44) | 14 | 31.8% | |
| Willing to participate in future studies | 52 | 92.9% | ||
| Metric | Category | n | % | |
|---|---|---|---|---|
| Screening Adherence | Total patients without thyroid dysfunction | 50 | 100% | |
| Adherent (≤15 months) | 39 | 78.0% | ||
| Moderately Adherent (15–24 months) | 6 | 12.0% | ||
| Non-Adherent (>24 months) | 5 | 10.0% | ||
| Patient and Caregiver Perspective | Awareness and Satisfaction | Aware of high thyroid risk | 32 | 64.0% |
| Dissatisfied with thyroid care | 6 | 12.0% | ||
| Awareness-care gap (Aware but dissatisfied) | 3 | 6.0% | ||
| Awareness-care gap prevalence | Among aware patients (n = 32) | 3 | 9.4% | |
| Willing to participate in future studies | 47 | 94.0% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Alqahtani, Y.A.; Shati, A.A.; Alshaikh, A.A.; Alshahrani, A.T.; Bin Qaed, S.A.; Alqahtani, M.A.; Alotaibi, O.A.; Alharthi, M.O.; Sarhan, M.H.; Alrasheed, A.M.; et al. Prevalence, Spectrum, and Management of Thyroid Dysfunction in Children with Down Syndrome: A Retrospective Study from Southern Saudi Arabia. Children 2026, 13, 6. https://doi.org/10.3390/children13010006
Alqahtani YA, Shati AA, Alshaikh AA, Alshahrani AT, Bin Qaed SA, Alqahtani MA, Alotaibi OA, Alharthi MO, Sarhan MH, Alrasheed AM, et al. Prevalence, Spectrum, and Management of Thyroid Dysfunction in Children with Down Syndrome: A Retrospective Study from Southern Saudi Arabia. Children. 2026; 13(1):6. https://doi.org/10.3390/children13010006
Chicago/Turabian StyleAlqahtani, Youssef Ali, Ayed A. Shati, Ayoub Ali Alshaikh, Ali Thamer Alshahrani, Salwa Abdullah Bin Qaed, Manar Ali Alqahtani, Omar Ayidh Alotaibi, Muteb Obaid Alharthi, Mohamed Hassan Sarhan, Abdulaziz Mohammed Alrasheed, and et al. 2026. "Prevalence, Spectrum, and Management of Thyroid Dysfunction in Children with Down Syndrome: A Retrospective Study from Southern Saudi Arabia" Children 13, no. 1: 6. https://doi.org/10.3390/children13010006
APA StyleAlqahtani, Y. A., Shati, A. A., Alshaikh, A. A., Alshahrani, A. T., Bin Qaed, S. A., Alqahtani, M. A., Alotaibi, O. A., Alharthi, M. O., Sarhan, M. H., Alrasheed, A. M., & Ghazy, R. M. (2026). Prevalence, Spectrum, and Management of Thyroid Dysfunction in Children with Down Syndrome: A Retrospective Study from Southern Saudi Arabia. Children, 13(1), 6. https://doi.org/10.3390/children13010006








