Gestational Diabetes and Obesity: Immediate and Late Sequelae for Offspring
Abstract
Highlights
- Gestational diabetes and obesity are followed by an interplacental variety of complications responsible for maternal morbidity and fetal reprogramming.
- Although gestational diabetes is an area of scientific research in the past decade mainly due to short- and long-term maternal and fetal implications, there is a lack of knowledge in regard to therapeutic alternatives such as antioxidant supplementation and its long-term effectiveness.
- We recommend antioxidant supplementation during preconception and gestation for mothers with pregestational diabetes or a previous history of gestational diabetes.
- The use of continuous glucose monitoring systems, dietary modifications during pregnancy and regular mild–moderate exercise against oxidative stress during gestation and as a preconception modification are valuable assets against gestational diabetes- and diabesity (gestational diabetes and obesity)-related complications.
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Gestational Diabetes Mellitus (GDM)
3.1.1. GDM Demographics
3.1.2. GDM Diagnosis
3.1.3. Predisposing Modifiable Factors Related to GDM
3.1.4. Predisposing Unmodifiable Factors Related to GDM
| (a) | |
| Immediate Consequences for the Mother | |
| Increased levels of oxidative stress during gestation Dystocia and/or caesarian section due to neonatal macrosomia Preeclampsia, hypertension and circulatory disorders during gestation Sepsis/perinatal infections Increased maternal morbidity/mortality | |
| Late Consequences for the Mother | |
| Increased risk of T2D Increased risk of metabolic syndrome Increased risk of cardiovascular disease | |
| (b) | |
| Fetal, Neonatal, Offspring, Adult Complications | |
| Fetal complications | Congenital anomalies, fetal death, macrosomia, cardiomyopathy, chorioamnitis, perinatal asphyxia, hydramnios (cause for preterm labor) and large for gestational age |
| Neonatal complications | Traumatic labor, respiratory difficulty, transient tachypnoea, hypoglycemia, polycythemia, jaundice, hypocalcemia, hypomagnesaemia and neonatal death |
| Pediatric complications | Neurodevelopmental complications, obesity, insulin resistance, impaired glucose tolerance, T2D and early onset of puberty |
| Adult complications | Obesity, insulin resistance, impaired glucose tolerance, type 2 diabetes, metabolic syndrome and depression |
3.1.5. Cardiovascular Complications
3.2. Gestational Obesity
3.2.1. Gestational Obesity Demographics and Diagnosis
3.2.2. Diabesity-Related Complications
3.2.3. Epigenetics in Diabesity
3.3. Preventional Measures of GDM and Obesity Complications
- Optimal glycemic control
- b.
- Weight management and healthy lifestyle
- c.
- Administration of Ω3 and folic acid
- d.
- Breastfeeding and prevention of infantile overfeeding
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Nishimura, A.; Matsumura, K.; Kikuno, S.; Nagasawa, K.; Okubo, M.; Mori, Y.; Kobayashi, T. Slowly Progressive Type 1 Diabetes Mellitus: Current Knowledge and Future Perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2461–2477. [Google Scholar] [CrossRef]
- Elias-Assad, G.; Saab, R.; Molnes, J.; Hess, O.; Abu-Ras, R.; Darawshi, H.; Rasmus Njølstad, P.; Tenenbaum-Rakover, Y. Maturity Onset Diabetes of the Young Type 2 (MODY2): Insight from an Extended Family. Diabetes Res. Clin. Pract. 2021, 175, 108791. [Google Scholar] [CrossRef]
- Caughey, A.B.; Turrentine, M. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.A.; Xiang, A.H. Gestational Diabetes Mellitus. J. Clin. Investig. 2005, 115, 485–491. [Google Scholar] [CrossRef]
- Guevara-Ramírez, P.; Paz-Cruz, E.; Cadena-Ullauri, S.; Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Felix, M.L.; Simancas-Racines, D.; Zambrano, A.K. Molecular Pathways and Nutrigenomic Review of Insulin Resistance Development in Gestational Diabetes Mellitus. Front. Nutr. 2023, 10, 1228703. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Prasad, K.; Lemos, J.R.N.; Arevalo, G.; Hirani, K. Unveiling Gestational Diabetes: An Overview of Pathophysiology and Management. Int. J. Mol. Sci. 2025, 26, 2320. [Google Scholar] [CrossRef]
- Wu, L.; Ouyang, J.; Lai, Y.; Wu, P.; Wang, Y.; Ye, Y.; Wang, J.; Hu, M.; Zhang, J.; Xu, J.; et al. Combined Healthy Lifestyle in Early Pregnancy and Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2023, 130, 1611–1619. [Google Scholar] [CrossRef]
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy; World Health Organization: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/WHO-NMH-MND-13.2 (accessed on 1 June 2025).
- Gestational Diabetes-Causes & Treatment|ADA. Available online: https://diabetes.org/about-diabetes/gestational-diabetes (accessed on 19 July 2024).
- IDF-2017.Pdf. Available online: https://diabetesatlas.org/resources/previous-editions/ (accessed on 1 June 2025).
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef]
- American Diabetes Association. Management of Diabetes in Pregnancy. Diabetes Care 2017, 40, S114–S119. [Google Scholar] [CrossRef] [PubMed]
- Bain, E.; Crane, M.; Tieu, J.; Han, S.; Crowther, C.A.; Middleton, P. Diet and Exercise Interventions for Preventing Gestational Diabetes Mellitus. Cochrane Database Syst. Rev. 2015, 4, CD010443, Update in Cochrane Database Syst. Rev. 2017, 11, CD010443. https://doi.org/10.1002/14651858.CD010443.pub3. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 5 November 2023).
- Leddy, M.A.; Power, M.L.; Schulkin, J. The Impact of Maternal Obesity on Maternal and Fetal Health. Rev. Obstet. Gynecol. 2008, 1, 170–178. [Google Scholar]
- Friedrich, M.J. Global Obesity Epidemic Worsening. JAMA 2017, 318, 603. [Google Scholar] [CrossRef] [PubMed]
- Nurul-Farehah, S.; Rohana, A.J. Maternal Obesity and Its Determinants: A Neglected Issue? Malays. Fam. Physician Off. J. Acad. Fam. Physicians Malays. 2020, 15, 34–42. [Google Scholar]
- Kouba, I.; Del Pozzo, J.; Lesser, M.L.; Shahani, D.; Gulersen, M.; Bracero, L.A.; Blitz, M.J. Socioeconomic and Clinical Factors Associated with Excessive Gestational Weight Gain. Arch. Gynecol. Obstet. 2024, 309, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- CDCMMWR QuickStats: Percentage of Mothers with Gestational Diabetes, by Maternal Age—National Vital Statistics System, United States, 2016 and 2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 16. [CrossRef]
- Mazumder, T.; Akter, E.; Rahman, S.M.; Islam, M.T.; Talukder, M.R. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Bangladesh: Findings from Demographic Health Survey 2017–2018. Int. J. Environ. Res. Public. Health 2022, 19, 2583. [Google Scholar] [CrossRef]
- Zhou, T.; Du, S.; Sun, D.; Li, X.; Heianza, Y.; Hu, G.; Sun, L.; Pei, X.; Shang, X.; Qi, L. Prevalence and Trends in Gestational Diabetes Mellitus Among Women in the United States, 2006–2017: A Population-Based Study. Front. Endocrinol. 2022, 13, 868094. [Google Scholar] [CrossRef]
- Jiwani, A.; Marseille, E.; Lohse, N.; Damm, P.; Hod, M.; Kahn, J.G. Gestational Diabetes Mellitus: Results from a Survey of Country Prevalence and Practices. J. Matern. Fetal Neonatal Med. 2012, 25, 600–610. [Google Scholar] [CrossRef]
- Coustan, D.R.; Lowe, L.P.; Metzger, B.E.; Dyer, A.R. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Paving the Way for New Diagnostic Criteria for Gestational Diabetes Mellitus. Am. J. Obstet. Gynecol. 2010, 202, 654.e1–654.e6. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, T.; Chen, L.; Wang, L.; Wang, T.; Zhao, L.; Ye, Z.; Zhang, S.; Luo, L.; Zheng, Z.; et al. Risk of Congenital Heart Defects in Offspring Exposed to Maternal Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2019, 300, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, B.; Sun, Y.; Du, Y.; Santillan, M.K.; Santillan, D.A.; Snetselaar, L.G.; Bao, W. Association of Maternal Prepregnancy Diabetes and Gestational Diabetes Mellitus With Congenital Anomalies of the Newborn. Diabetes Care 2020, 43, 2983–2990. [Google Scholar] [CrossRef]
- Kong, L.; Norstedt, G.; Schalling, M.; Gissler, M.; Lavebratt, C. The Risk of Offspring Psychiatric Disorders in the Setting of Maternal Obesity and Diabetes. Pediatrics 2018, 142, e20180776. [Google Scholar] [CrossRef]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Mouzon, S.H.; Jawerbaum, A. The Role of Oxidative Stress in the Pathophysiology of Gestational Diabetes Mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla Rodriguez, B.S.; Vadakekut, E.S.; Mahdy, H. Gestational Diabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zhang, Y.; Xiao, C.-M.; Zhang, Y.; Chen, Q.; Zhang, X.-Q.; Li, X.-F.; Shao, R.-Y.; Gao, Y.-M. Factors Associated with Gestational Diabetes Mellitus: A Meta-Analysis. J. Diabetes Res. 2021, 2021, 6692695. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y. Gestational Diabetes Mellitus and Subsequent Risks of Diabetes and Cardiovascular Diseases: The Life Course Perspective and Implications of Racial Disparities. Curr. Diab. Rep. 2024, 24, 244–255. [Google Scholar] [CrossRef]
- Alejandro, E.U.; Mamerto, T.P.; Chung, G.; Villavieja, A.; Gaus, N.L.; Morgan, E.; Pineda-Cortel, M.R.B. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int. J. Mol. Sci. 2020, 21, 5003. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 Diabetes Mellitus after Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Sheiner, E. Gestational Diabetes Mellitus: Long-Term Consequences for the Mother and Child Grand Challenge: How to Move on Towards Secondary Prevention? Front. Clin. Diabetes Healthc. 2020, 1, 546256. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Yang, L. Association between Diabetes Mellitus and Miscarriage, Recurrent Miscarriage: A Meta-study. J. Obstet. Gynaecol. Res. 2024, 50, 2029–2037. [Google Scholar] [CrossRef]
- McGrogan, A.; Snowball, J.; de Vries, C.S. Pregnancy Losses in Women with Type 1 or Type 2 Diabetes in the UK: An Investigation Using Primary Care Records. Diabet. Med. J. Br. Diabet. Assoc. 2014, 31, 357–365. [Google Scholar] [CrossRef]
- Gabbay-Benziv, R.; Reece, E.A.; Wang, F.; Yang, P. Birth Defects in Pregestational Diabetes: Defect Range, Glycemic Threshold and Pathogenesis. World J. Diabetes 2015, 6, 481–488. [Google Scholar] [CrossRef]
- Martin, R.B.; Duryea, E.L.; Ambia, A.; Ragsdale, A.; Mcintire, D.; Wells, C.E.; Spong, C.Y.; Dashe, J.S.; Nelson, D.B. Congenital Malformation Risk According to Hemoglobin A1c Values in a Contemporary Cohort with Pregestational Diabetes. Am. J. Perinatol. 2021, 38, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Cortés, Y.I.; Zhang, S.; Hussey, J.M. Pregnancy Loss Is Related to Body Mass Index and Prediabetes in Early Adulthood: Findings from Add Health. PLoS ONE 2022, 17, e0277320. [Google Scholar] [CrossRef] [PubMed]
- Perea, V.; Urquizu, X.; Valverde, M.; Macias, M.; Carmona, A.; Esteve, E.; Escribano, G.; Pons, N.; Giménez, O.; Gironés, T.; et al. Influence of Maternal Diabetes on the Risk of Neurodevelopmental Disorders in Offspring in the Prenatal and Postnatal Periods. Diabetes Metab. J. 2022, 46, 912–922. [Google Scholar] [CrossRef]
- Avci, R.; Whittington, J.R.; Blossom, S.J.; Escalona-Vargas, D.; Siegel, E.R.; Preissl, H.T.; Eswaran, H. Studying the Effect of Maternal Pregestational Diabetes on Fetal Neurodevelopment Using Magnetoencephalography. Clin. EEG Neurosci. 2020, 51, 331–338. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, S.; Dalman, C.; Karlsson, H.; Gardner, R. Association of Maternal Diabetes with Neurodevelopmental Disorders: Autism Spectrum Disorders, Attention-Deficit/Hyperactivity Disorder and Intellectual Disability. Int. J. Epidemiol. 2021, 50, 459–474. [Google Scholar] [CrossRef]
- Ciężki, S.; Odyjewska, E.; Bossowski, A.; Głowińska-Olszewska, B. Not Only Metabolic Complications of Childhood Obesity. Nutrients 2024, 16, 539. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative Stress: Normal Pregnancy versus Preeclampsia. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef]
- Laforgia, N.; Di Mauro, A.; Favia Guarnieri, G.; Varvara, D.; De Cosmo, L.; Panza, R.; Capozza, M.; Baldassarre, M.E.; Resta, N. The Role of Oxidative Stress in the Pathomechanism of Congenital Malformations. Oxid. Med. Cell. Longev. 2018, 2018, 7404082. [Google Scholar] [CrossRef]
- Martini, S.; Aceti, A.; Della Gatta, A.N.; Beghetti, I.; Marsico, C.; Pilu, G.; Corvaglia, L. Antenatal and Postnatal Sequelae of Oxidative Stress in Preterm Infants: A Narrative Review Targeting Pathophysiological Mechanisms. Antioxidants 2023, 12, 422. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. How to Characterize an Antioxidant: An Update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Noguchi, N.; Niki, E. Comparative Study on Dynamics of Antioxidative Action of α-Tocopheryl Hydroquinone, Ubiquinol, and α-Tocopherol against Lipid Peroxidation. Free Radic. Biol. Med. 1999, 27, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Levine, M. Criteria and Recommendations for Vitamin C Intake. JAMA 1999, 281, 1415. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Simán, C.M.; Gittenberger-De Groot, A.C.; Wisse, B.; Eriksson, U.J. Malformations in Offspring of Diabetic Rats: Morphometric Analysis of Neural Crest-Derived Organs and Effects of Maternal Vitamin E Treatment. Teratology 2000, 61, 355–367. [Google Scholar] [CrossRef]
- Mitanchez, D. What Neonatal Complications Should the Pediatrician Be Aware of in Case of Maternal Gestational Diabetes? World J. Diabetes 2015, 6, 734. [Google Scholar] [CrossRef]
- Kc, K.; Shakya, S.; Zhang, H. Gestational Diabetes Mellitus and Macrosomia: A Literature Review. Ann. Nutr. Metab. 2015, 66, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Fasoulakis, Z.; Koutras, A.; Antsaklis, P.; Theodora, M.; Valsamaki, A.; Daskalakis, G.; Kontomanolis, E.N. Intrauterine Growth Restriction Due to Gestational Diabetes: From Pathophysiology to Diagnosis and Management. Medicina 2023, 59, 1139. [Google Scholar] [CrossRef] [PubMed]
- Weintrob, N.; Karp, M.; Hod, M. Short- and Long-Range Complications in Offspring of Diabetic Mothers. J. Diabetes Complications 1996, 10, 294–301. [Google Scholar] [CrossRef]
- Nuwagaba, J.; Dave, D. Management of Neonatal Complications of Macrosomia: A Case Report at a Tertiary Hospital in a Developing Country. Clin. Case Rep. 2022, 10, e05298. [Google Scholar] [CrossRef]
- Negrato, C.A.; Montenegro, R.M.; Mattar, R.; Zajdenverg, L.; Francisco, R.P.; Pereira, B.G.; Sancovski, M.; Torloni, M.R.; Dib, S.A.; Viggiano, C.E.; et al. Dysglycemias in Pregnancy: From Diagnosis to Treatment. Brazilian Consensus Statement. Diabetol. Metab. Syndr. 2010, 2, 27. [Google Scholar] [CrossRef]
- Mello, G.; Parretti, E.; Mecacci, F.; La Torre, P.; Cioni, R.; Cianciulli, D.; Scarselli, G. What Degree of Maternal Metabolic Control in Women with Type 1 Diabetes Is Associated with Normal Body Size and Proportions in Full-Term Infants? Diabetes Care 2000, 23, 1494–1498. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Liu, J.; Li, J.; Liu, J.; Liu, H.; Wang, Y.; Leng, J.; Wang, S.; Chen, H.; Chan, J.C.N.; et al. Interactive Effects of Prepregnancy Overweight and Gestational Diabetes on Macrosomia and Large for Gestational Age: A Population-Based Prospective Cohort in Tianjin, China. Diabetes Res. Clin. Pract. 2019, 154, 82–89. [Google Scholar] [CrossRef]
- Beta, J.; Khan, N.; Fiolna, M.; Khalil, A.; Ramadan, G.; Akolekar, R. Maternal and Neonatal Complications of Fetal Macrosomia: Cohort Study. Ultrasound Obstet. Gynecol. 2019, 54, 319–325. [Google Scholar] [CrossRef]
- Yildiz Atar, H.; Baatz, J.E.; Ryan, R.M. Molecular Mechanisms of Maternal Diabetes Effects on Fetal and Neonatal Surfactant. Children 2021, 8, 281. [Google Scholar] [CrossRef]
- Mitanchez, D.; Yzydorczyk, C.; Siddeek, B.; Boubred, F.; Benahmed, M.; Simeoni, U. The Offspring of the Diabetic Mother—Short- and Long-Term Implications. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 256–269. [Google Scholar] [CrossRef]
- Bashir, B.; Othman, S. Neonatal Polycythaemia. Sudan. J. Paediatr. 2019, 19, 81–83. [Google Scholar] [CrossRef]
- Kallimath, A.; Kolkur, K.; Malshe, N.; Klimek, J.; Suryawanshi, P. Hemodynamics in Neonates with Polycythemia before and after Partial Exchange Transfusion: An Observational Study. Front. Pediatr. 2024, 11, 1296184. [Google Scholar] [CrossRef]
- Wiswell, T.E.; Cornish, J.D.; Northam, R.S. Neonatal Polycythemia: Frequency of Clinical Manifestations and Other Associated Findings. Pediatrics 1986, 78, 26–30. [Google Scholar] [CrossRef]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO Analysis of Causes of Maternal Death: A Systematic Review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez, D.; Burguet, A.; Simeoni, U. Infants Born to Mothers with Gestational Diabetes Mellitus: Mild Neonatal Effects, a Long-Term Threat to Global Health. J. Pediatr. 2014, 164, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, R.A.; Avraam, D.; Guxens, M.; Julvez, J.; Harris, J.R.; Nader, J.T.; Cadman, T.; Elhakeem, A.; Strandberg-Larsen, K.; Marroun, H.E.; et al. Is Maternal Diabetes during Pregnancy Associated with Attention Deficit Hyperactivity Disorder and Autism Spectrum Disorder in Children? Insights from Individual Participant Data Meta-Analysis in Ten Birth Cohorts. BMC Pediatr. 2025, 25, 76. [Google Scholar] [CrossRef] [PubMed]
- E Silva, R.N.A.; Yu, Y.; Liew, Z.; Vested, A.; Sørensen, H.T.; Li, J. Associations of Maternal Diabetes During Pregnancy with Psychiatric Disorders in Offspring During the First 4 Decades of Life in a Population-Based Danish Birth Cohort. JAMA Netw. Open 2021, 4, e2128005. [Google Scholar] [CrossRef]
- Rodolaki, K.; Pergialiotis, V.; Iakovidou, N.; Boutsikou, T.; Iliodromiti, Z.; Kanaka-Gantenbein, C. The Impact of Maternal Diabetes on the Future Health and Neurodevelopment of the Offspring: A Review of the Evidence. Front. Endocrinol. 2023, 14, 1125628. [Google Scholar] [CrossRef]
- Briana, D.D.; Papastavrou, M.; Boutsikou, M.; Marmarinos, A.; Gourgiotis, D.; Malamitsi-Puchner, A. Differential Expression of Cord Blood Neurotrophins in Gestational Diabetes: The Impact of Fetal Growth Abnormalities. J. Matern. Fetal Neonatal Med. 2018, 31, 278–283. [Google Scholar] [CrossRef]
- Jadhav, A.; Khaire, A.; Gundu, S.; Wadhwani, N.; Chandhiok, N.; Gupte, S.; Joshi, S. Placental Neurotrophin Levels in Gestational Diabetes Mellitus. Int. J. Dev. Neurosci. 2021, 81, 352–363. [Google Scholar] [CrossRef]
- Jadhav, A.; Khaire, A.; Joshi, S. Exploring the Role of Oxidative Stress, Fatty Acids and Neurotrophins in Gestational Diabetes Mellitus. Growth Factors 2020, 38, 226–234. [Google Scholar] [CrossRef]
- Rodrigo, N.; Glastras, S.J. Pathophysiology Underpinning Gestational Diabetes Mellitus and the Role of Biomarkers for Its Prediction. EMJ Diabetes 2020, 5, 90–96. [Google Scholar] [CrossRef]
- Raftopoulou, C.; Paltoglou, G.; Charmandari, E. Association between Telomere Length and Pediatric Obesity: A Systematic Review. Nutrients 2022, 14, 1244. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.H.; Kim, Y.R.; Kim, N.; Jung, J.E.; Han, S.H.; Cho, H.Y. Effect of Endogenic and Exogenic Oxidative Stress Triggers on Adverse Pregnancy Outcomes: Preeclampsia, Fetal Growth Restriction, Gestational Diabetes Mellitus and Preterm Birth. Int. J. Mol. Sci. 2021, 22, 10122. [Google Scholar] [CrossRef] [PubMed]
- Kaul, P.; Bowker, S.L.; Savu, A.; Yeung, R.O.; Donovan, L.E.; Ryan, E.A. Association between Maternal Diabetes, Being Large for Gestational Age and Breast-Feeding on Being Overweight or Obese in Childhood. Diabetologia 2019, 62, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, N.M.; Visser, G.H.A.; van Rossem, L.; Biesma, D.H.; Wit, J.M.; de Valk, H.W. Long-Term BMI and Growth Profiles in Offspring of Women with Gestational Diabetes. Diabetologia 2018, 61, 1037–1045. [Google Scholar] [CrossRef]
- Mantzorou, M.; Papandreou, D.; Pavlidou, E.; Papadopoulou, S.K.; Tolia, M.; Mentzelou, M.; Poutsidi, A.; Antasouras, G.; Vasios, G.K.; Giaginis, C. Maternal Gestational Diabetes Is Associated with High Risk of Childhood Overweight and Obesity: A Cross-Sectional Study in Pre-School Children Aged 2–5 Years. Medicina 2023, 59, 455. [Google Scholar] [CrossRef]
- Grunnet, L.G.; Hansen, S.; Hjort, L.; Madsen, C.M.; Kampmann, F.B.; Thuesen, A.C.B.; Granstrømi, C.; Strøm, M.; Maslova, E.; Frikke-Schmidt, R.; et al. Adiposity, Dysmetabolic Traits, and Earlier Onset of Female Puberty in Adolescent Offspring of Women With Gestational Diabetes Mellitus: A Clinical Study Within the Danish National Birth Cohort. Diabetes Care 2017, 40, 1746–1755. [Google Scholar] [CrossRef]
- Wicklow, B.; Retnakaran, R. Gestational Diabetes Mellitus and Its Implications across the Life Span. Diabetes Metab. J. 2023, 47, 333–344. [Google Scholar] [CrossRef]
- Olsen, J.; Melbye, M.; Olsen, S.F.; Sørensen, T.I.A.; Aaby, P.; Nybo Andersen, A.-M.; Taxbøl, D.; Hansen, K.D.; Juhl, M.; Schow, T.B.; et al. The Danish National Birth Cohort—Its Background, Structure and Aim. Scand. J. Public Health 2001, 29, 300–307. [Google Scholar] [CrossRef]
- Nybo Andersen, A.-M.; Olsen, J. The Danish National Birth Cohort: Selected Scientific Contributions within Perinatal Epidemiology and Future Perspectives. Scand. J. Public Health 2011, 39, 115–120. [Google Scholar] [CrossRef]
- Obesity and Pregnancy. Available online: https://www.acog.org/womens-health/faqs/obesity-and-pregnancy (accessed on 9 August 2025).
- Overweight and Pregnant. Available online: https://www.nhs.uk/pregnancy/related-conditions/existing-health-conditions/overweight/ (accessed on 9 August 2025).
- Kent, L.; McGirr, M.; Eastwood, K.-A. Global Trends in Prevalence of Maternal Overweight and Obesity: A Systematic Review and Meta-Analysis of Routinely Collected Data Retrospective Cohorts. Int. J. Popul. Data Sci. 2024, 9, 2401. [Google Scholar] [CrossRef]
- Almutairi, F.S.; Alsaykhan, A.M.; Almatrood, A.A. Obesity Prevalence and Its Impact on Maternal and Neonatal Outcomes in Pregnant Women: A Systematic Review. Cureus 2024, 16, e75262. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.; Yan, Y. Estimated Global Overweight and Obesity Burden in Pregnant Women Based on Panel Data Model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef]
- Dutton, H.; Borengasser, S.J.; Gaudet, L.M.; Barbour, L.A.; Keely, E.J. Obesity in Pregnancy. Med. Clin. N. Am. 2018, 102, 87–106. [Google Scholar] [CrossRef]
- Denison, F.; Aedla, N.; Keag, O.; Hor, K.; Reynolds, R.; Milne, A.; Diamond, A.; the Royal College of Obstetricians and Gynaecologists. Care of Women with Obesity in Pregnancy: Green-top Guideline No. 72. BJOG Int. J. Obstet. Gynaecol. 2019, 126, e64–e106. [Google Scholar] [CrossRef] [PubMed]
- Vats, H.; Saxena, R.; Sachdeva, M.P.; Walia, G.K.; Gupta, V. Impact of Maternal Pre-Pregnancy Body Mass Index on Maternal, Fetal and Neonatal Adverse Outcomes in the Worldwide Populations: A Systematic Review and Meta-Analysis. Obes. Res. Clin. Pract. 2021, 15, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Weir, T.L.; Majumder, M.; Glastras, S.J. A Systematic Review of the Effects of Maternal Obesity on Neonatal Outcomes in Women with Gestational Diabetes. Obes. Rev. 2024, 25, e13747. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; van Poppel, M. The Feto-Placental Dialogue and Diabesity. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 15–23. [Google Scholar] [CrossRef]
- Hay, W.W. Placental-Fetal Glucose Exchange and Fetal Glucose Metabolism. Trans. Am. Clin. Climatol. Assoc. 2006, 117, 321–339, discussion 339-340. [Google Scholar]
- Bianco, M.E.; Josefson, J.L. Hyperglycemia During Pregnancy and Long-Term Offspring Outcomes. Curr. Diab. Rep. 2019, 19, 143. [Google Scholar] [CrossRef]
- Alba-Linares, J.J.; Pérez, R.F.; Tejedor, J.R.; Bastante-Rodríguez, D.; Ponce, F.; Carbonell, N.G.; Zafra, R.G.; Fernández, A.F.; Fraga, M.F.; Lurbe, E. Maternal Obesity and Gestational Diabetes Reprogram the Methylome of Offspring beyond Birth by Inducing Epigenetic Signatures in Metabolic and Developmental Pathways. Cardiovasc. Diabetol. 2023, 22, 44. [Google Scholar] [CrossRef]
- Lehnen, H.; Zechner, U.; Haaf, T. Epigenetics of Gestational Diabetes Mellitus and Offspring Health: The Time for Action Is in Early Stages of Life. Mol. Hum. Reprod. 2013, 19, 415–422. [Google Scholar] [CrossRef]
- Heerwagen, M.J.R.; Miller, M.R.; Barbour, L.A.; Friedman, J.E. Maternal Obesity and Fetal Metabolic Programming: A Fertile Epigenetic Soil. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R711–R722. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Echouffo-Tcheugui, J.B.; Ekhlaspour, L.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S306–S320. [Google Scholar] [CrossRef]
- Alexopoulos, A.-S.; Blair, R.; Peters, A.L. Management of Preexisting Diabetes in Pregnancy: A Review. JAMA 2019, 321, 1811. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, R.J.; Gebretsadik, T.; Jagasia, S.; Shintani, A.; Elasy, T.A. Variation in the Relationship between Gestational Diabetes Diagnosis and Total Gestational Weight Gain by Race/Ethnicity. Diabetes Res. Clin. Pract. 2015, 108, e14–e17. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary Omega-3 Fatty Acids Normalize BDNF Levels, Reduce Oxidative Damage, and Counteract Learning Disability after Traumatic Brain Injury in Rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef]
- Liu, H.; Deng, Y.; Chen, L.; Weng, S.; Xu, D. The Effect of Omega 3 Supplementation on Serum Brain-Derived Neurotrophic Factor: A Systematic Review and Meta-Analysis. Eur. J. Integr. Med. 2023, 61, 102264. [Google Scholar] [CrossRef]
- Rathod, R.S.; Khaire, A.A.; Kale, A.A.; Joshi, S.R. Effect of Vitamin B12 and Omega-3 Fatty Acid Supplementation on Brain Neurotrophins and Cognition in Rats: A Multigeneration Study. Biochimie 2016, 128–129, 201–208. [Google Scholar] [CrossRef]
- Centers for Disease Control. Recommendations for the Use of Folic Acid to Reduce the Number of Cases of Spina Bifida and Other Neural Tube Defects. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 1992, 41, 1–7. [Google Scholar]
- Nguyen, P.T.H.; Pham, N.M.; Chu, K.T.; Van Duong, D.; Van Do, D. Gestational Diabetes and Breastfeeding Outcomes: A Systematic Review. Asia Pac. J. Public Health 2019, 31, 183–198. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Breastfeeding and the Maternal Risk of Type 2 Diabetes: A Systematic Review and Dose–Response Meta-Analysis of Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 107–115. [Google Scholar] [CrossRef]
- Michael Weindling, A. Offspring of Diabetic Pregnancy: Short-Term Outcomes. Semin. Fetal. Neonatal Med. 2009, 14, 111–118. [Google Scholar] [CrossRef]
- Dabelea, D.; Mayer-Davis, E.J.; Lamichhane, A.P.; D’Agostino, R.B.; Liese, A.D.; Vehik, K.S.; Narayan, K.M.V.; Zeitler, P.; Hamman, R.F. Association of Intrauterine Exposure to Maternal Diabetes and Obesity with Type 2 Diabetes in Youth. Diabetes Care 2008, 31, 1422–1426. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Cavalli, P.; Copp, A.J.; D’Anna, R.; Kandaraki, E.; Greene, N.D.E.; Unfer, V.; Experts Group on Inositol in Basic and Clinical Research. An Update on the Use of Inositols in Preventing Gestational Diabetes Mellitus (GDM) and Neural Tube Defects (NTDs). Expert Opin. Drug Metab. Toxicol. 2020, 16, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.; Moore, C.J.; Fusch, G.; Teo, K.K.; Atkinson, S.A. Factors Associated with Serum 25-Hydroxyvitamin D Concentration in Two Cohorts of Pregnant Women in Southern Ontario, Canada. Nutrients 2019, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sheng, C.; Xie, R.; Sun, W.; Asztalos, E.; Moddemann, D.; Zwaigenbaum, L.; Walker, M.; Wen, S.W. New Perspective on Impact of Folic Acid Supplementation during Pregnancy on Neurodevelopment/Autism in the Offspring Children—A Systematic Review. PLoS ONE 2016, 11, e0165626. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Valadan, M.; Bahramnezhad, Z.; Golshahi, F.; Feizabad, E. The Role of First-Trimester HbA1c in the Early Detection of Gestational Diabetes. BMC Pregnancy Childbirth 2022, 22, 71. [Google Scholar] [CrossRef] [PubMed]
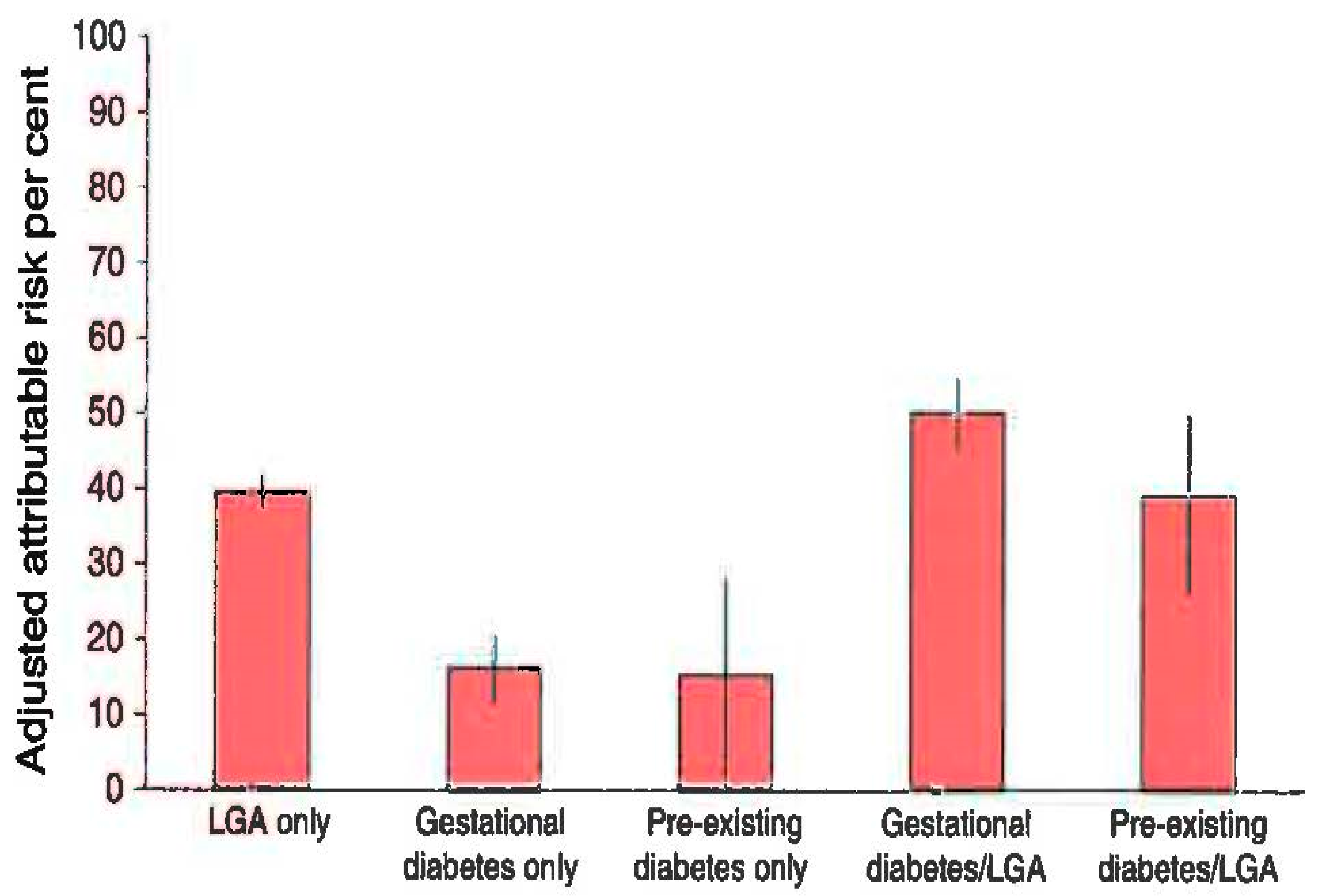
A. GDM epidemiology and oxidative stress. |
B. Obesity in pregnancy and effects on the mother. |
C. GDM prevention complications for the mother and offspring. |
 |
| During the First Visit of the Pregnant Woman |
|
| Glucose–Insulin Curve (with Administration of 75 g Glucose) |
|
| Affected System | Congenital Malformations |
|---|---|
| Congenital heart diseases | Truncus arteriosus Ventricular septal defect Transposition of great arteries Tricuspid atresia Single ventricle complex |
| Musculoskeletal anomalies | Sacral agenesis Meningocele Longitudinal limb deficiencies |
| Other congenital malformations | Anal agenesis Hypoplastic kidneys Renal agenesis Cleft palate |
| Central nervous system defects | Holoprosencephaly Anencephaly Hydrocephalus Anotia/microtia Craniorachischisis |
| Neurodevelopmental disorders | Attention deficit-hyperactivity disorder (ADHD) Autism Fine and gross motor disorders Psychiatric diseases (schizophrenia and anxiety disorder) |
| Maternal complications | Hypertension Postpartum hemorrhage |
| GDM | |
| Preeclampsia | |
| Obstructive sleep apnea Preterm premature rupture of membranes Postpartum venous thromboembolism Labor induction Caesarian section | |
| Fetal/neonatal complications | Macrosomia Shoulder dystocia |
| Large and small for gestational age (SGA and LGA) Neural tube defects and cerebral palsy Hydrocephaly Anorectal atresia Cardiac defects Cleft lip/palate | |
| Stillbirth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaza, M.; Paltoglou, G.; Rodolaki, K.; Kakleas, K.; Karanasios, S.; Karavanaki, K. Gestational Diabetes and Obesity: Immediate and Late Sequelae for Offspring. Children 2025, 12, 1263. https://doi.org/10.3390/children12091263
Kaza M, Paltoglou G, Rodolaki K, Kakleas K, Karanasios S, Karavanaki K. Gestational Diabetes and Obesity: Immediate and Late Sequelae for Offspring. Children. 2025; 12(9):1263. https://doi.org/10.3390/children12091263
Chicago/Turabian StyleKaza, Maria, George Paltoglou, Kalliopi Rodolaki, Konstantinos Kakleas, Spyridon Karanasios, and Kyriaki Karavanaki. 2025. "Gestational Diabetes and Obesity: Immediate and Late Sequelae for Offspring" Children 12, no. 9: 1263. https://doi.org/10.3390/children12091263
APA StyleKaza, M., Paltoglou, G., Rodolaki, K., Kakleas, K., Karanasios, S., & Karavanaki, K. (2025). Gestational Diabetes and Obesity: Immediate and Late Sequelae for Offspring. Children, 12(9), 1263. https://doi.org/10.3390/children12091263







