Spatiotemporal Gait Variables and Step-to-Step Variability in Preschool-Aged Children Born Very Preterm at Risk for Developmental Coordination Disorder: A Cohort Study
Abstract
1. Introduction
2. Materials and Methods
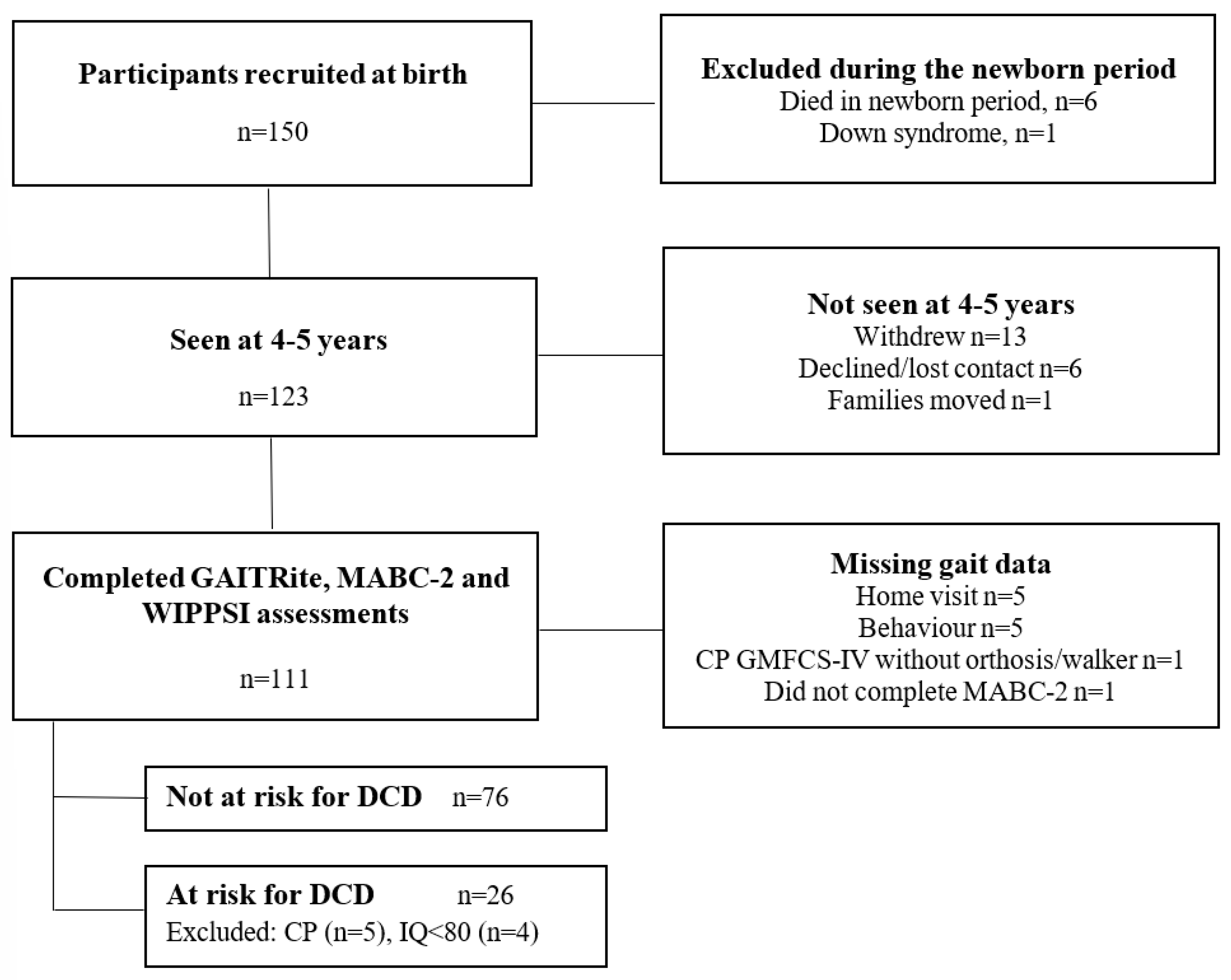
2.1. Participants
2.2. Predictor
2.2.1. Risk for Developmental Coordination Disorder
- Movement Assessment Battery for Children, Second Edition
- Wechsler Preschool and Primary Scale of Intelligence, 4th Edition
2.2.2. Gait Assessment
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spittle, A.J.; Cameron, K.; Doyle, L.W.; Cheong, J.L.; Victorian Infant Collaborative Study Group. Motor impairment trends in extremely preterm children: 1991–2005. Pediatrics 2018, 141, e20173410. [Google Scholar] [CrossRef]
- Williams, J.; Lee, K.J.; Anderson, P.J. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2010, 52, 232–237. [Google Scholar] [CrossRef]
- Albesher, R.A.; Spittle, A.J.; McGinley, J.L.; Dobson, F.L. Gait Characteristics of Children Born Preterm. Neoreviews 2019, 20, e397–e408. [Google Scholar] [CrossRef]
- Albesher, R.A.; Spittle, A.J.; Dobson, F.L.; Mentiplay, B.F.; FitzGerald, T.L.; Cameron, K.L.; Zannino, D.; Josev, E.K.; Doyle, L.W.; Cheong, J.L.Y.; et al. Spatiotemporal gait variables and step-to-step variability in preschool-aged children born< 30 weeks’ gestation and at term in preferred speed, dual-task paradigm, and tandem walking. Gait Posture 2022, 92, 236–242. [Google Scholar] [PubMed]
- Ferrari, F.; Gallo, C.; Pugliese, M.; Guidotti, I.; Gavioli, S.; Coccolini, E.; Zagni, P.; Casa, E.D.; Rossi, C.; Lugli, L.; et al. Preterm birth and developmental problems in the preschool age. Part I: Minor motor problems. J. Matern.-Fetal Neonatal Med. 2012, 25, 2154–2159. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Berube, M.; Erlandson, K.; Haug, S.; Johnstone, H.; Meagher, M.; Sarkodee-Adoo, S.; Zwicker, J. Developmental Coordination Disorder in School-Aged Children Born Very Preterm and/or at Very Low Birth Weight: A Systematic Review. J. Dev. Behav. Pediatr. 2011, 32, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Polatajko, H.J.; Cantin, N. Developmental coordination disorder (dyspraxia): An overview of the state of the art. In Seminars in Pediatric Neurology; Polatajko, H.J., Cantin, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Smith, M.; Ward, E.; Williams, C.M.; Banwell, H.A. Differences in walking and running gait in children with and without developmental coordination disorder: A systematic review and meta-analysis. Gait Posture 2020, 83, 177–184. [Google Scholar] [CrossRef]
- Goetschalckx, M.; Moumdjian, L.; Feys, P.; Rameckers, E. Interlimb coordination and spatiotemporal variability during walking and running in children with developmental coordination disorder and typically developing children. Hum. Mov. Sci. 2024, 96, 103252. [Google Scholar] [CrossRef] [PubMed]
- Cherng, R.-J.; Liang, L.-Y.; Chen, Y.-J.; Chen, J.-Y. The effects of a motor and a cognitive concurrent task on walking in children with developmental coordination disorder. Gait Posture 2009, 29, 204–207. [Google Scholar] [CrossRef]
- Wilmut, K.; Du, W.; Barnett, A.L. Gait patterns in children with Developmental Coordination Disorder. Hum. Mov. Sci. 2016, 48, 22–33. [Google Scholar] [CrossRef]
- Blank, R.; Smits-Engelsman, B.; Polatajko, H.; Wilson, P. European Academy for Childhood Disability (EACD): Recommendations on the definition, diagnosis and intervention of developmental coordination disorder (long version). Dev. Med. Child Neurol. 2012, 54, 54. [Google Scholar] [CrossRef]
- De Roubaix, A.; Van de Velde, D.; Roeyers, H.; Van Waelvelde, H. Standardized motor assessments before the age of five predicting school-aged motor outcome including DCD: A systematic review. Eur. J. Paediatr. Neurol. 2021, 30, 29–57. [Google Scholar] [CrossRef]
- Lee, E.J.; Zwicker, J.G. Early identification of children with/at risk of developmental coordination disorder: A scoping review. Dev. Med. Child Neurol. 2021, 63, 649–658. [Google Scholar] [CrossRef]
- Stodden, D.F.; Goodway, J.D.; Langendorfer, S.J.; Roberton, M.A.; Rudisill, M.E.; Garcia, C.; Garcia, L.E. A developmental perspective on the role of motor skill competence in physical activity: An emergent relationship. Quest 2008, 60, 290–306. [Google Scholar] [CrossRef]
- Cinar, E.; Saxena, S.; Gagnon, I. Differential effects of concurrent tasks on gait in typically developing children: A meta-analysis. J. Mot. Behav. 2020, 53, 509–522. [Google Scholar] [CrossRef]
- Spittle, A.J.; McGinley, J.L.; Thompson, D.; Clark, R.; FitzGerald, T.L.; Mentiplay, B.F.; Lee, K.J.; Olsen, J.E.; Burnett, A.; Treyvaud, K.; et al. Motor trajectories from birth to 5 years of children born at less than 30 weeks’ gestation: Early predictors and functional implications. Protocol for a prospective cohort study. J. Physiother. 2016, 62, 222–223. [Google Scholar] [CrossRef]
- Wilson-Ching, M.; Pascoe, L.; Doyle, L.W.; Anderson, P.J. Effects of correcting for prematurity on cognitive test scores in childhood. J. Paediatr. Child Health 2014, 50, 182–188. [Google Scholar] [CrossRef]
- Dewey, D.; Thompson, D.K.; Kelly, C.E.; Spittle, A.J.; Cheong, J.L.Y.; Doyle, L.W.; Anderson, P.J. Very preterm children at risk for developmental coordination disorder have brain alterations in motor areas. Acta Paediatr. 2019, 108, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.E.; Sugden, D.A.; Barnett, A.L. Movement Assessment Battery for Children; Harcourt Assessment: London, UK, 2007. [Google Scholar]
- Schoemaker, M.M.; Niemeijer, A.S.; Flapper, B.C.; Smits-Engelsman, B.C. Validity and reliability of the movement assessment battery for children-2 checklist for children with and without motor impairments. Dev. Med. Child Neurol. 2012, 54, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence—Fourth Edition Australian and New Zealand Standardised Edition (WPPSI-IV A&NZ); Pearson: Melbourne, Australia, 2014. [Google Scholar]
- Thorpe, D.E.; Dusing, S.C.; Moore, C.G. Repeatability of temporospatial gait measures in children using the GAITRite electronic walkway. Arch. Phys. Med. Rehabil. 2005, 86, 2342–2346. [Google Scholar] [CrossRef]
- Wondra, V.C.; Pitetti, K.H.; Beets, M.W. Gait parameters in children with motor disabilities using an electronic walkway system: Assessment of reliability. Pediatr. Phys. Ther. 2007, 19, 326–331. [Google Scholar] [CrossRef]
- Bjornson, K.; Song, K.; Lisle, J.; Robinson, S.; Killien, E.; Barrett, T.; Zhou, C. Measurement of walking activity throughout childhood: Influence of leg length. Pediatr. Exerc. Sci. 2010, 22, 581–595. [Google Scholar] [CrossRef]
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of Changes in Health-related Quality of Life: The Remarkable Universality of Half a Standard Deviation. Med. Care 2003, 41, 582–592. [Google Scholar] [CrossRef]
- Sloan, J.A.; Cella, D.; Hays, R.D. Clinical significance of patient-reported questionnaire data: Another step toward consensus. J. Clin. Epidemiol. 2005, 58, 1217–1219. [Google Scholar] [CrossRef]
- Griffiths, A.; Toovey, R.; Morgan, P.E.; Spittle, A.J. Psychometric properties of gross motor assessment tools for children: A systematic review. BMJ Open 2018, 8, e021734. [Google Scholar] [CrossRef]
- Hagmann-von Arx, P.; Manicolo, O.; Perkinson-Gloor, N.; Weber, P.; Grob, A.; Lemola, S. Gait in Very Preterm School-Aged Children in Dual-Task Paradigms. PLoS ONE 2015, 10, e0144363. [Google Scholar] [CrossRef]
- Kuijpers, R.; Smulders, E.; Groen, B.E.; Smits-Engelsman, B.C.M.; Nijhuis-van der Sanden, M.W.G.; Weerdesteyn, V. The effects of a visuo-motor and cognitive dual task on walking adaptability in children with and without Developmental Coordination Disorder. Gait Posture 2022, 95, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Wickens, C.D. Multiple resources and performance prediction. Theor. Issues Ergon. Sci. 2002, 3, 159–177. [Google Scholar] [CrossRef]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Yogev, G.; Springer, S.; Simon, E.S.; Giladi, N. Walking is more like catching than tapping: Gait in the elderly as a complex cognitive task. Exp. Brain Res. 2005, 164, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Zemany, L.; Peng, C.; Goldberger, A.L. Maturation of gait dynamics: Stride-to-stride variability and its temporal organization in children. J. Appl. Physiol. 1999, 86, 1040–1047. [Google Scholar] [CrossRef]
- Hagmann-von Arx, P.; Manicolo, O.; Lemola, S.; Grob, A. Walking in School-Aged Children in a Dual-Task Paradigm Is Related to Age But Not to Cognition, Motor Behavior, Injuries, or Psychosocial Functioning. Front. Psychol. 2016, 7, 352. [Google Scholar] [CrossRef]
- Katz-Leurer, M.; Rotem, H.; Keren, O.; Meyer, S. Balance abilities and gait characteristics in post-traumatic brain injury, cerebral palsy and typically developed children. Dev. Neurorehabilit. 2009, 12, 100–105. [Google Scholar] [CrossRef]
- Lum, J.A.; Shandley, K.; Albein-Urios, N.; Kirkovski, M.; Papadopoulos, N.; Wilson, R.B.; Rinehart, N.J. Meta-analysis reveals gait anomalies in autism. Autism Res. 2020, 14, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Manicolo, O.; Brotzmann, M.; Hagmann-von Arx, P.; Grob, A.; Weber, P. Gait in children with infantile/atypical autism: Age-dependent decrease in gait variability and associations with motor skills. Eur. J. Paediatr. Neurol. 2019, 23, 117–125. [Google Scholar] [CrossRef]
- Manicolo, O.; Grob, A.; Hagmann-von Arx, P. Gait in children with attention-deficit hyperactivity disorder in a dual-task paradigm. Front. Psychol. 2017, 8, 34. [Google Scholar] [CrossRef][Green Version]
- Manicolo, O.; Grob, A.; Lemola, S.; Hagmann-von Arx, P. Age-related decline of gait variability in children with attention-deficit/hyperactivity disorder: Support for the maturational delay hypothesis in gait. Gait Posture 2016, 44, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Stergiou, N.; Ulrich, B.D. Patterns of gait variability across the lifespan in persons with and without down syndrome. J. Neurol. Phys. Ther. 2011, 5, 170–177. [Google Scholar] [CrossRef]
- Zwicker, J.G.; Lee, E.J. Early intervention for children with/at risk of developmental coordination disorder: A scoping review. Dev. Med. Child Neurol. 2021, 63, 659–667. [Google Scholar] [CrossRef]
- Spittle, A.; Orton, J.; Anderson, P.J.; Boyd, R.; Doyle, L.W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 2015, 11, CD005495. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | At Risk for DCD n = 26 | Not at Risk for DCD n = 76 |
|---|---|---|
| PERINATAL CHARACTERISTICS | ||
| Gestational age (weeks), mean (SD) | 27.74 (1.57) | 27.87 (1.33) |
| Birth weight (g), mean (SD) | 1044.12 (264.93) | 1045.55 (243.45) |
| Female sex, n (%) | 11 (42.31%) | 41 (53.95%) |
| Multiple birth, n (%) | 9 (34.61) | 33 (43.42%) |
| 4–5 YEARS FOLLOW-UP | ||
| CA at assessment (years), mean (SD) | 4.63 (0.12) | 4.67 (0.14) |
| Attending preschool, n (%) | 25 (96.15%) | 71 (93.42%) |
| Weight at assessment (kg), mean (SD) | 17.45 (3.98) | 17.44 (2.33) |
| Height at assessment (cm), mean (SD) | 105.64 (5.25) | 106.45 (4.40) |
| Leg length at assessment (cm), mean (SD) | 55.04 (4.39) | 55.37 (3.55) |
| MABC-2—Total standard score, mean (SD) | 5.35 (1.45) | 10.62 (2.05) |
| ≤5th percentile on MABC-2, n (%) | 12 (46%) | — |
| WPPSI—FSCIQ score, mean (SD) | 96.35 (8.67) | 105.33 (12.07) |
| Preferred speed condition, # n (%) | 26 (100%) | 75 (98.68%) |
| Cognitive dual-task condition, # n (%) | 24 (92.31%) | 68 (89.47%) |
| Motor dual-task condition, # n (%) | 25 (96.15%) | 72 (94.74%) |
| Tandem condition, # n (%) | 24 (92.31%) | 74 (97.39%) |
| Gait Variable | Walking Condition | At Risk for DCD Mean (SD) | Not at Risk for DCD Mean (SD) | Mean Difference (95% CI) | p Value |
|---|---|---|---|---|---|
| Speed (cm/sec) | Preferred speed | 99.43 (17.79) | 100.65 (18.07) | −0.65 (−10.46, 9.15) | 0.896 |
| Dual-task-Cognitive | 70.35 (21.13) | 72.14 (19.27) | 0.20 (−11.40, 11.80) | 0.973 | |
| Dual-task-Motor | 55.12 (25.04) | 60.33 (18.33) | −5.40 (−14.74, 3.95) | 0.258 | |
| Tandem walk | 69.22 (20.09) | 65.67 (16.30) | 4.40 (−4.80, 13.60) | 0.348 | |
| Cadence (steps/min) | Preferred speed | 145.17 (18.84) | 144.86 (15.53) | −0.44 (−7.63, 6.74) | 0.904 |
| Dual-task-Cognitive | 122.84 (26.92) | 122.89 (19.54) | 1.08 (−10.51, 12.67) | 0.856 | |
| Dual-task-Motor | 112.36 (27.73) | 115.89 (20.12) | −3.13 (−12.95, 6.68) | 0.532 | |
| Tandem walk | 128.12 (23.76) | 117.71 (20.87) | 10.93 (1.63, 20.23) | 0.021 *^ | |
| Step time (sec) | Preferred speed | 0.42 (0.05) | 0.42 (0.05) | 0.00 (−0.02, 0.02) | 0.859 |
| Dual-task-Cognitive | 0.51 (0.11) | 0.50 (0.08) | 0.00 (−0.05, 0.05) | 0.869 | |
| Dual-task-Motor | 0.57 (0.15) | 0.53 (0.10) | 0.03 (−0.03, 0.08) | 0.317 | |
| Tandem walk | 0.48 (0.09) | 0.53 (0.11) | −0.05 (−0.09, −0.01) | 0.023 *^ | |
| Step length (cm) | Preferred speed | 40.97 (4.15) | 41.53 (4.98) | 0.09 (−2.92, 3.11) | 0.952 |
| Dual-task-Cognitive | 34.00 (5.47) | 34.76 (4.96) | −0.24 (−3.66, 3.18) | 0.892 | |
| Dual-task-Motor | 28.16 (6.82) | 30.70 (5.23) | −2.41 (−5.51, 0.68) | 0.127 | |
| Tandem walk | 31.97 (5.21) | 33.20 (4.06) | −1.17 (−3.55, 1.22) | 0.338 | |
| Base of support (cm) | Preferred speed | 8.24 (1.38) | 7.94 (1.86) | 0.32 (−0.43, 1.08) | 0.401 |
| Dual-task-Cognitive | 9.73 (1.60) | 9.52 (2.27) | 0.41 (−0.60, 1.42) | 0.423 | |
| Dual-task-Motor | 9.73 (1.37) | 8.71 (1.69) | 0.86 (0.10, 1.61) | 0.027 *^ | |
| Tandem walk | 4.17 (1.57) | 3.12 (0.94) | 0.87 (0.18, 1.56) | 0.013 *^ | |
| Single limb support (%) | Preferred speed | 40.94 (1.85) | 40.96 (1.60) | −0.01 (−0.69, 0.68) | 0.985 |
| Dual-task-Cognitive | 38.02 (2.35) | 38.64 (2.48) | −0.38 (−1.48, 0.73) | 0.505 | |
| Dual-task-Motor | 34.14 (4.27) | 35.87 (3.42) | −1.77 (−3.36, −0.19) | 0.028 *^ | |
| Tandem walk | 38.47 (2.68) | 38.28 (2.75) | 0.03 (−1.00, 1.06) | 0.957 | |
| Double limb support (%) | Preferred speed | 18.27 (3.17) | 17.98 (2.78) | 0.07 (−1.47, 1.61) | 0.928 |
| Dual-task-Cognitive | 24.33 (4.66) | 23.03 (4.47) | 0.48 (−1.85, 2.81) | 0.686 | |
| Dual-task-Motor | 31.27 (8.26) | 27.91 (6.27) | 1.11 (1.01, 1.22) # | 0.036 * | |
| Tandem walk | 22.72 (4.79) | 22.94 (4.28) | 0.07 (−1.99, 2.14) | 0.944 |
| Gait Variable | Walking Condition | At Risk for DCD Mean (SD) | Not at Risk for DCD Mean (SD) | Mean Difference (95% CI) | p Value |
|---|---|---|---|---|---|
| Step time variability (sec) | Preferred speed | 0.04 (0.03) | 0.04 (0.02) | 1.04 (0.81, 1.33) # | 0.766 |
| Dual-task-Cognitive | 0.08 (0.05) | 0.07 (0.04) | 1.01 (0.79, 1.52) # | 0.564 | |
| Dual-task-Motor | 0.10 (0.06) | 0.08 (0.06) | 1.33 (1.03, 1.71) # | 0.029 *^ | |
| Tandem walk | 0.09 (0.05) | 0.10 (0.06) | −0.01 (−0.03, 0.01) | 0.500 | |
| Step length variability (cm) | Preferred speed | 4.17 (1.36) | 3.75 (1.18) | 0.31 (−0.20, 0.83) | 0.229 |
| Dual-task-Cognitive | 5.33 (1.36) | 4.22 (1.32) | 0.99 (0.21, 1.77) | 0.013 *^ | |
| Dual-task-Motor | 4.79 (1.16) | 4.39 (1.43) | 0.63 (0.07, 1.20) | 0.028 *^ | |
| Tandem walk | 5.72 (1.49) | 5.18 (1.24) | 0.53 (−0.146, 1.21) | 0.125 | |
| Base of support variability (cm) | Preferred speed | 2.93 (0.64) | 2.41 (0.50) | 0.51 (0.27, 0.75) | <0.001 *^ |
| Dual-task-Cognitive | 2.98 (0.62) | 2.55 (0.65) | 0.32 (0.02, 0.63) | 0.039 *^ | |
| Dual-task-Motor | 2.39 (0.43) | 2.27 (0.50) | 0.24 (−0.05, 0.53) | 0.106 | |
| Tandem walk | 2.64 (0.70) | 2.02 (0.53) | 0.65 (0.12, 1.19) | 0.017 * | |
| Single limb support time variability (sec) | Preferred speed | 0.04 (0.03) | 0.03 (0.01) | 1.08 (0.85, 1.37) # | 0.536 |
| Dual-task-Cognitive | 0.06 (0.03) | 0.05 (0.02) | 0.01 (−0.00, 0.03) | 0.197 | |
| Dual-task-Motor | 0.06 (0.03) | 0.05 (0.02) | 0.01 (0.00, 0.02) | 0.005 *^ | |
| Tandem walk | 0.07 (0.03) | 0.07 (0.03) | 0.00 (−0.02, 0.014) | 0.809 | |
| Double limb support time variability (sec) | Preferred speed | 0.04 (0.02) | 0.03 (0.01) | 1.14 (1.09, 1.42) # | 0.234 |
| Dual-task-Cognitive | 0.07 (0.04) | 0.06 (0.06) | 1.08 (0.78, 1.51) # | 0.633 | |
| Dual-task-Motor | 0.12 (0.09) | 0.09 (0.09) | 1.36 (0.96, 1.90) # | 0.079 | |
| Tandem walk | 0.08 (0.05) | 0.09 (0.08) | 0.83 (0.61,1.14) # | 0.250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albesher, R.A.; McGinley, J.L.; Dobson, F.L.; Mentiplay, B.F.; FitzGerald, T.L.; Cameron, K.L.; Cheong, J.L.Y.; Spittle, A.J. Spatiotemporal Gait Variables and Step-to-Step Variability in Preschool-Aged Children Born Very Preterm at Risk for Developmental Coordination Disorder: A Cohort Study. Children 2025, 12, 1261. https://doi.org/10.3390/children12091261
Albesher RA, McGinley JL, Dobson FL, Mentiplay BF, FitzGerald TL, Cameron KL, Cheong JLY, Spittle AJ. Spatiotemporal Gait Variables and Step-to-Step Variability in Preschool-Aged Children Born Very Preterm at Risk for Developmental Coordination Disorder: A Cohort Study. Children. 2025; 12(9):1261. https://doi.org/10.3390/children12091261
Chicago/Turabian StyleAlbesher, Reem A., Jennifer L. McGinley, Fiona L. Dobson, Benjamin F. Mentiplay, Tara L. FitzGerald, Kate L. Cameron, Jeanie L. Y. Cheong, and Alicia J. Spittle. 2025. "Spatiotemporal Gait Variables and Step-to-Step Variability in Preschool-Aged Children Born Very Preterm at Risk for Developmental Coordination Disorder: A Cohort Study" Children 12, no. 9: 1261. https://doi.org/10.3390/children12091261
APA StyleAlbesher, R. A., McGinley, J. L., Dobson, F. L., Mentiplay, B. F., FitzGerald, T. L., Cameron, K. L., Cheong, J. L. Y., & Spittle, A. J. (2025). Spatiotemporal Gait Variables and Step-to-Step Variability in Preschool-Aged Children Born Very Preterm at Risk for Developmental Coordination Disorder: A Cohort Study. Children, 12(9), 1261. https://doi.org/10.3390/children12091261







