Pacifier Sizing as a Prescription for Better Oral Health Outcomes for Infants: A Call to Action
Abstract
1. Introduction
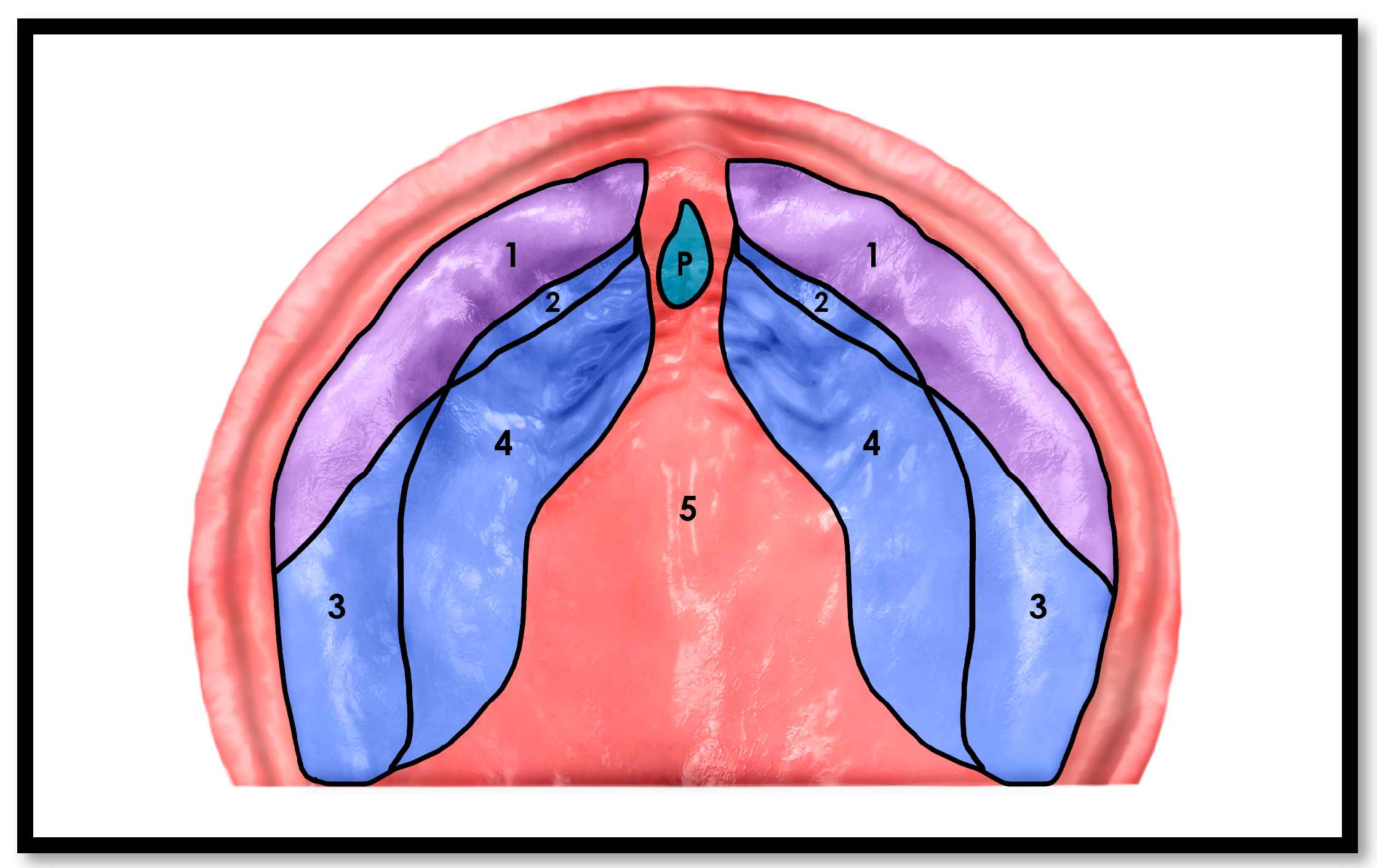
1.1. The Cranio-Facial-Respiratory Complex (CFRC) and Palatal Growth
1.2. Mandibular and TMJ Development
1.3. Malocclusion and Non-Nutritive Sucking
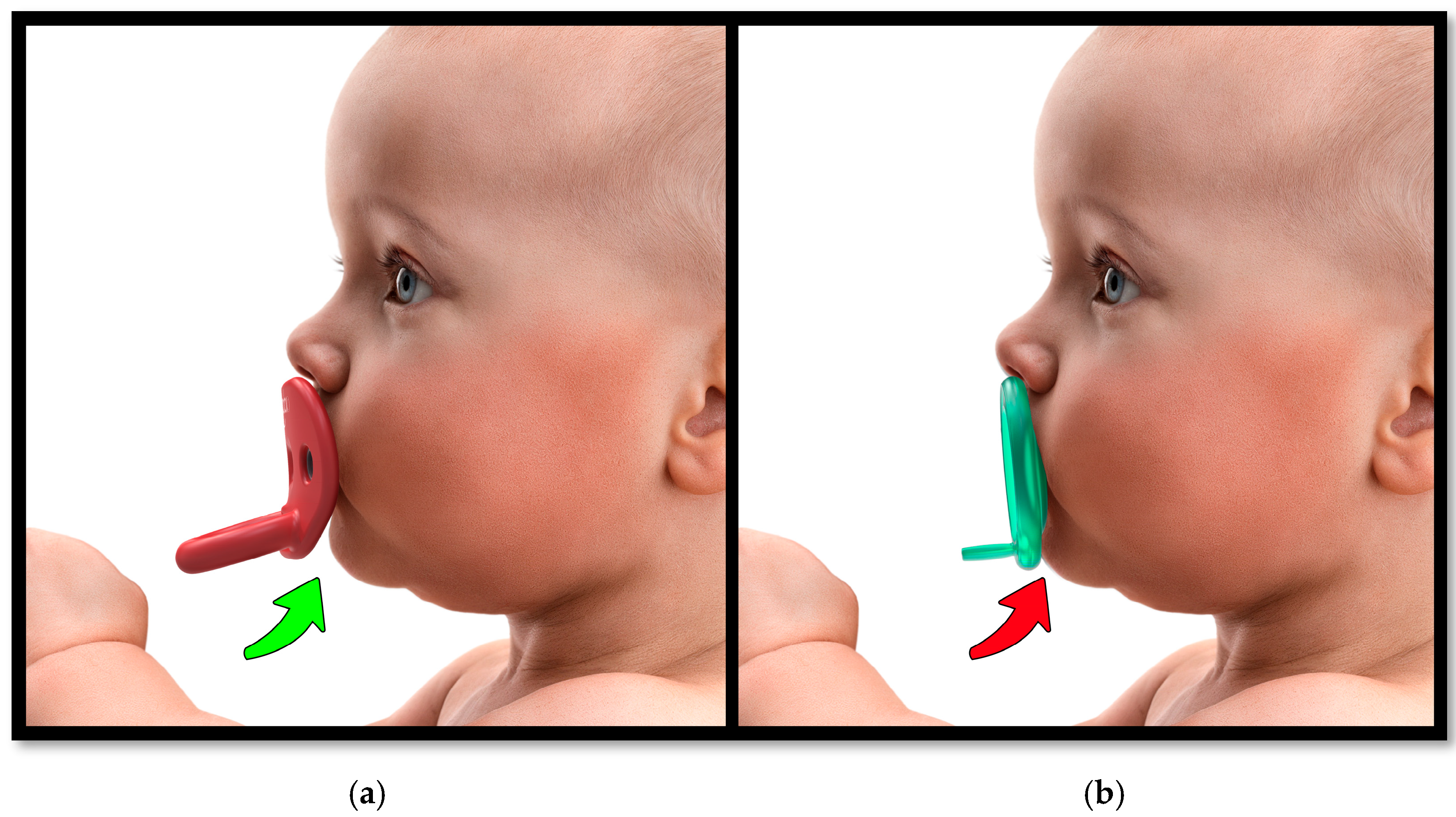
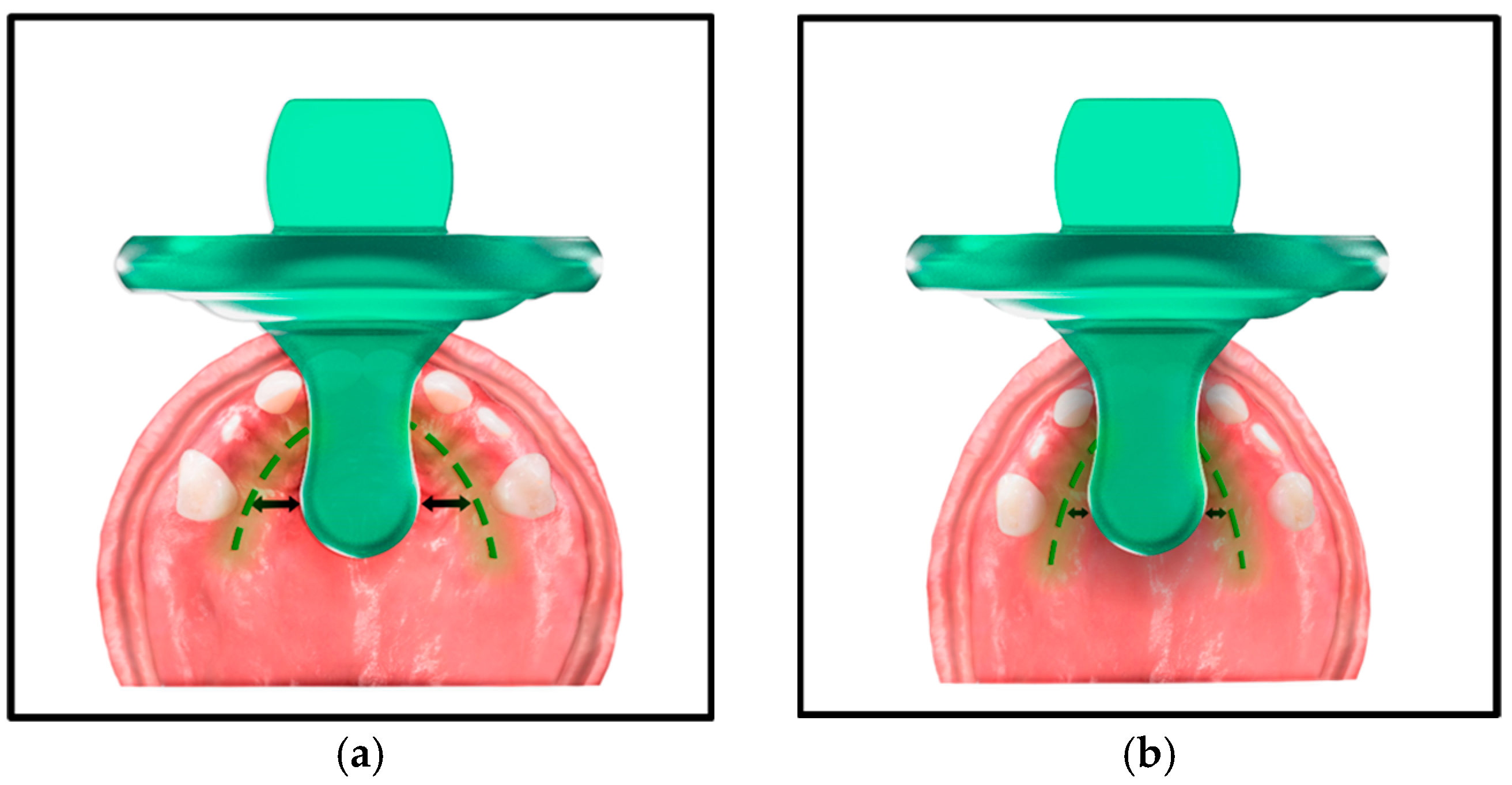
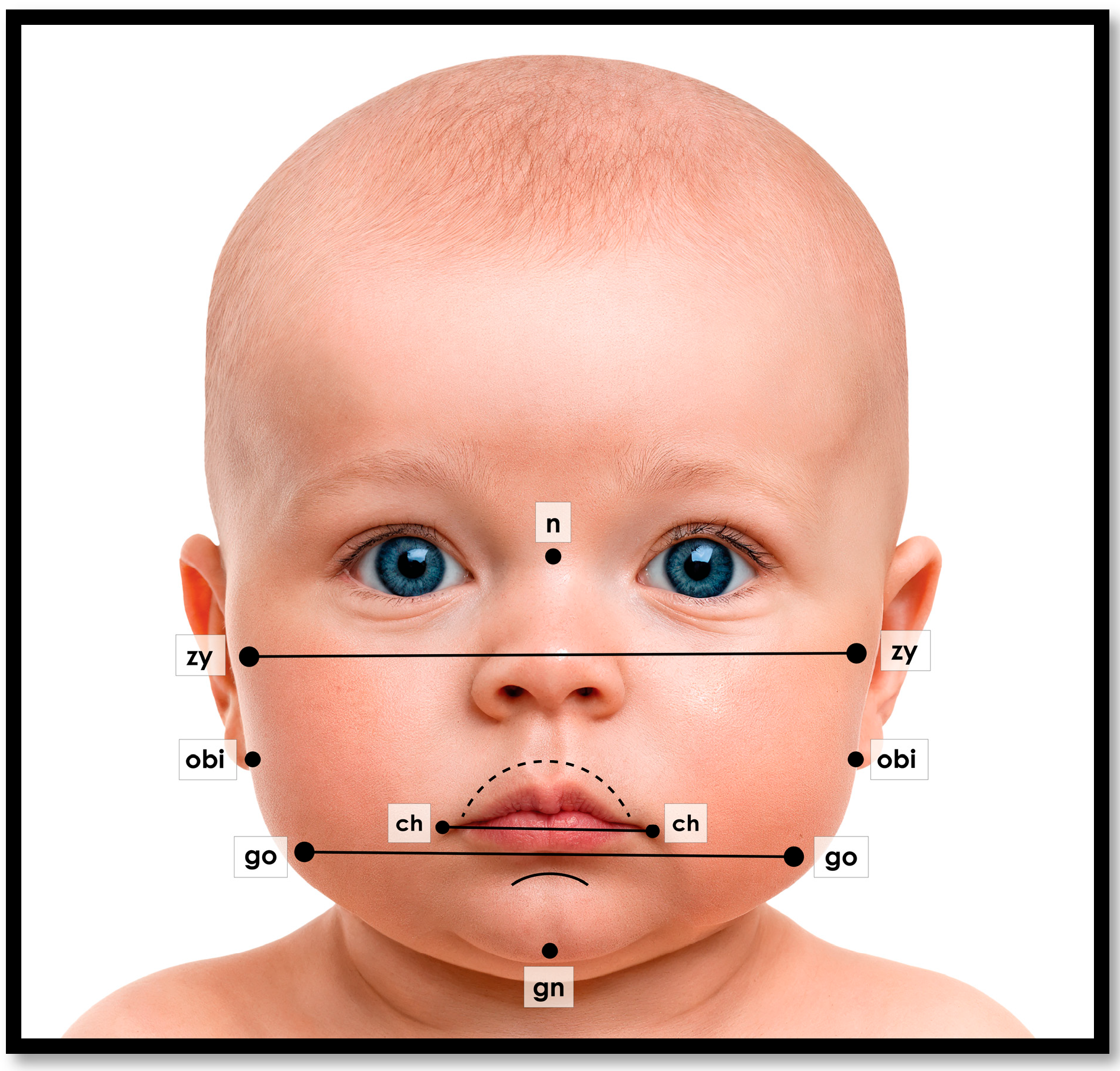
1.4. The Pacifier Paradox: Design, Sizing, and Development
1.5. Evolving Technology for Improving Oral Health and Wellness Outcomes
2. Conclusions
- The harmful effects of pacifier use must be recognized within the context of the development of the CFRC and evaluated by the effects of long-term Oral Health and Wellness Outcomes.
- Modern engineering models should be used by the baby product industry to evaluate the functional aspects of pacifiers.
- Biometric sizing represents a potentially significant development in pediatric oral care. As AI and machine learning continue to evolve, predictive models may soon inform not only pacifier selection but also the optimal timing for upsizing based on projected palatal growth trajectories.
- Advancing technology needs to be leveraged in longitudinal clinical validation studies.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, K.L. Assessment and Therapies for Sleep and Sleep-Related Breathing Disorders Associated with Atopic Disease in Children: A Dental Perspective. In Allergy and Sleep; Fishbein, A., Sheldon, S., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Lindner, A.; Hellsing, E. Cheek and lip pressure against maxillary dental arch during dummy sucking. Eur. J. Orthod. 1991, 13, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.E.; Goodacre, C.J. The temporomandibular joint: A critical review of life-support functions, development, articular surfaces, biomechanics and degeneration. J. Prosthodont. 2020, 29, 772–779. [Google Scholar] [CrossRef]
- Doğramacı, E.J.; Rossi-Fedele, G. Establishing the association between nonnutritive sucking behavior and malocclusions: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2016, 147, 926–934. [Google Scholar] [CrossRef]
- Schmid, K.M.; Kugler, R.; Nalabothu, P.; Bosch, C.; Verna, C. The effect of pacifier sucking on orofacial structures: A systematic literature review. Prog. Orthod. 2018, 19, 8. [Google Scholar] [CrossRef]
- Corrêa, C.D.C.; Bueno, M.D.R.S.; Lauris, J.R.P.; Berretin-Felix, G. Interference of conventional and orthodontic nipples in system stomatognatic: Systematic review 2016. Codas 2016, 28, 182–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hung, M.; Marx, J.; Ward, C.; Schwartz, C. Pacifier Use and Its Influence on Pediatric Malocclusion: A Scoping Review of Emerging Evidence and Developmental Impacts. Dent. J. 2025, 13, 319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kikuchi, Y. Three-dimensional relationship between pharyngeal airway and maxillo-facial morphology. Bull. Tokyo Dent. Coll. 2008, 49, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Tesini, D.A. Design, Sizing, and Ergonomics of Infant Pacifiers: A Biometric Basis for Pacifier Fit. Pediatr. Nurs. 2022, 48, 36–41. [Google Scholar] [CrossRef]
- Ducloyer, M.; Wargny, M.; Medo, C.; Gourraud, P.-A.; Clement, R.; Levieux, K.; Gras-Le Guen, C.; Corre, P.; Rambaud, C. The Ogival Palate: A New Risk Marker of Sudden Unexpected Death in Infancy? Front. Pediatr. 2022, 10, 809725. [Google Scholar] [CrossRef]
- Rambaud, C.; Guilleminault, C. Death, nasomaxillary complex, and sleep in young children. Eur. J. Pediatr. 2012, 171, 1349–1358. [Google Scholar] [CrossRef]
- Caleza-Jiménez, C.; Romero, I.R.; Ribas-Perez, D.; Biedma-Perea, M. Influence of the Physiological Pacifier on the Development of Malocclusions in Children: A Scoping Review. Children 2024, 11, 1353. [Google Scholar] [CrossRef]
- Tesini, D.A.; Hu, L.C.; Usui, B.H.; Lee, C.L. Functional comparison of pacifiers using finite element analysis. BMC Oral Health 2022, 22, 49. [Google Scholar] [CrossRef]
- Solow, B. The pattern of craniofacial associations: A morphological and methodological correlation and factor analysis study on young male adults. Acta Odontol. Scand. 1966, 24, 123–135. [Google Scholar]
- Farkas, L.G. Anthropometry of the Head and Face, 2nd ed.; Raven Press: New York, NY, USA, 1994. [Google Scholar]
- Sillman, J.H. Dimensional changes of the dental arches: Longitudinal study from birth to 25 years. Am. J. Orthod. 1964, 50, 824–842. [Google Scholar] [CrossRef]
- Bishara, S.E.; Ortho, D.; Jakobsen, J.R.; Treder, J.; Nowak, A. Arch width changes from 6 weeks to 45 years of age. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 401–409. [Google Scholar] [CrossRef]
- Bishara, S.E.; Nowak, A.J.; Kohout, F.J.; Heckert, D.A.; Hogan, M.M. Influence of feeding and non-nutritive sucking methods on the development of the dental arches: Longitudinal study of the first 18 months of life. Pediatr. Dent. 1987, 9, 13–21. [Google Scholar]
- Bauer, F.X.; Güll, F.D.; Roth, M.; Ritschl, L.M.; Rau, A.; Gau, D.; Gruber, M.; Eblenkamp, M.; Hilmer, B.; Wolff, K.; et al. A prospective longitudinal study of postnatal dentoalveolar and palatal growth: The anatomical basis for CAD/CAM-assisted production of cleft-lip-palate feeding plates. Clin. Anat. 2017, 30, 846–854. [Google Scholar] [CrossRef]
- Peyton, W.T. The dimensions and growth of the palate in the normal infant and in the infant with gross maldevelopment of the upper lip and palate: A quantitative study. Arch. Surg. 1931, 22, 704–737. [Google Scholar] [CrossRef]
- Bruggink, R.; Baan, F.; Kramer, G.J.C.; van der Molen, A.M. Three-dimensional maxillary growth modeling in newborns. Clin. Oral Investig. 2019, 23, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Kojo, H. Growth and development of the dentition during the first one year of life. Three-dimension measurement on maxillary and mandibular alveolar arch morphological palate. Shoni Shikagaku Zasshi Jpn. J. Pedod. 1988, 26, 112–130. [Google Scholar]
- Ishida, F.; Ukai, Y.; Shimizu, T. Palate and alveolar ridge development in predental infants: A longitudinal study. Eur. J. Paediatr. Dent. 2016, 17, 155–163. [Google Scholar]
- Lindner, A. Measurement of intra-oral negative air pressure during dummy sucking in human newborn. Eur. J. Orthod. 1991, 13, 317–321. [Google Scholar] [CrossRef]
- Øgaard, B.; Larsson, E.; Lindsten, R. The effect of sucking habits, cohort, sex, intercanine arch widths, and breast or bottle feeding on posterior crossbite in Norwegian and Swedish 3-year-old children. Am. J. Orthod. Dentofac. Orthop. 1994, 106, 161–166. [Google Scholar] [CrossRef]
- Zen, I.; Soares, M.; Pinto, L.M.C.P.; Ferelle, A.; Pessan, J.P.; Dezan-Garbelini, C.C. Maxillary arch dimensions in the first 6 months of life and their relationship with pacifier use. Eur. Arch. Paediatr. Dent. 2020, 21, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.S.; Nehra, K.; Sharma, M.; Jayan, B.; Poonia, A.; Bhattal, H. Association between breastfeeding duration, non-nutritive sucking habits and dental arch dimensions in deciduous dentition: A cross-sectional study. Prog. Orthod. 2014, 15, 59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hohoff, A.; Rabe, H.; Ehmer, U.; Harms, E. Palatal development of preterm and low birthweight infants compared to term infants–What do we know? Part 1: The palate of the term newborn. Head Face Med. 2005, 1, 8. [Google Scholar] [CrossRef]
- Horn, M.H.; Kinnamon, D.D.; Ferraro, N.; Curley, M.A. Smaller mandibular size in infants with a history of an apparent life-threatening event. J. Pediatr. 2006, 149, 499–504. [Google Scholar] [CrossRef]
- Hutchinson, E.F.; Kieser, J.A.; Kramer, B. Morphometric growth relationships of the immature human mandible and tongue. Eur. J. Oral Sci. 2014, 122, 181–189. [Google Scholar] [CrossRef]
- Adriano, L.Z.; Derech, C.D.; Massignan, C.; Flores-Mir, C.; Porporatti, A.L.; Canto, G.L.; Bolan, M. Anterior open bite selfcorrection after cessation of non-nutritive sucking habits: A systematic review. Eur. J. Orthod. 2023, 45, 235–243. [Google Scholar] [CrossRef]
- de Oliveira, J.M.L.; Dutra, A.L.T.; Pereira, C.M.; de Toledo, O.A. Etiology and treatment of anterior open bite. Health Sci. Inst. J. 2011, 29, 92–95. [Google Scholar]
- Kutin, G.; Hawes, R.R. Posterior cross-bites in the deciduous and mixed dentitions. Am. J. Orthod. 1969, 56, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Costello, M.; Tesini, D.A. Computational simulation of pacifier deformation and interaction with the palate. Clin. Exp. Dent. Res. 2021, 7, 884–887. [Google Scholar] [CrossRef]
- de Freitas, C.N.; Castelo, P.M.; Noritomi, P.Y.; Scudine, K.G.d.O.; Rontani, R.M.P.; Miziara, T.; Machado, L.M.R. Mechanical stress distribution over the palate by different pacifiers assessed by finite element analysis and clinical data. Clin. Anat. 2024, 37, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Romero, J.; Norton, A.; Nóbrega, J.M. Advancing the assessment of pacifier effects with a novel computational method. BMC Oral Health 2024, 24, 87. [Google Scholar] [CrossRef]
- Levrini, L.; Paracchini, L.; Ricci, L.; Sparaco, M.; Saran, S.; Mulè, G. Biomechanical Analysis of Different Pacifiers and Their Effects on the Upper Jaw and Tongue. Appl. Sci. 2025, 15, 8624. [Google Scholar] [CrossRef]
- Pereira, R.; Romero, J.; Santos, C.P.; Norton, A.; Nóbrega, J.M. Effect of different pacifier designs on orofacial tissues: A computational simulation comparative study. Clin. Oral Investig. 2025, 29, 356. [Google Scholar] [CrossRef]
- Maintz, M.; Nalabothu, P.; Thieringer, F.M.; Verna, C. Influence of pacifier design on pacifier-palate contact: A finite element analysis. Head Face Med. 2025, 21, 50. [Google Scholar] [CrossRef]
- Sistenich, G.; Middelberg, C.; Stamm, T.; Dirksen, D.; Hohoff, A. Conformity between pacifier design and palate shape in preterm and term infants considering age-specific palate size, facial profile and lip thickness. Children 2022, 9, 773. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry Policy on Pacifiers. In the Reference Manual of Pediatric Dentistry. 2024. Available online: https://www.aapd.org/globalassets/media/policies_guidelines/p_pacifiers.pdf (accessed on 19 May 2025).
- Hohoff, A.; Stamm, T.; Meyer, U.; Wiechmann, D.; Ehmer, U. Objective growth monitoring of the maxilla in full term infants. Arch. Oral Biol. 2006, 51, 222–235. [Google Scholar] [CrossRef]
- Kihara, T.; Kaihara, Y.; Iwamae, S.; Niizato, N.; Gion, S.; Taji, T.; Kozai, K.; Nikawa, H. Three-dimensional longitudinal changes of maxilla and mandible morphology during the predental period. Eur. J. Paediatr. Dent. 2017, 18, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Simon, S. A review of: Statistical Rules of Thumb, Second Edition, by G. van Belle. J. Biopharm. Stat. 2009, 19, 752–754. [Google Scholar] [CrossRef]

| Chronological Age | Biometric Width (mm) | Brand/Pacifier Trade Name |
|---|---|---|
| 0–3 mo. | 12.5 | Philips Avent Soothie |
| 3–18 mo. | 12.5 | Philips Avent Super Soothie |
| 0–3 mo. | 18.7 | MAM Original Start |
| 0–3 mo. | 15.4 | MAM Comfort |
| 0–6 mo. | 17.6 | NUK Orthodontic |
| 18–36 mo. | 23.1 | NUK Orthodontic |
| 0–12 mo. | 17.3 | NUK Nature Comfy Duet |
| 18–36 mo. | 17.6 | Tommee Tippee Nighttime Orthodontic |
| 0–6 mo. | 12.9 | Dr Brown Happy Paci |
| 0+ mo. | 19.0 | Ryan & Rose Cuttie Pat Smile |
| 0–6 mo. | 12.1 | Itzy Ritzy Natural Rubber Soother |
| Biometric Staging with Overlap Based on Range Rule ([44]) | ||
|---|---|---|
| Newborn | ≥12.0 mm | ≤14.0 mm |
| Biometric Stage 1 | ≥13.3 mm | ≤16.4 mm |
| Biometric stage 2 | ≥15.7 mm | ≤20.9 mm |
| Biometric stage 3 | ≥18.9 mm | ≤25.0 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesini, D.A.; Friedman, C.; Kethu, A.; Hendricks, K.W. Pacifier Sizing as a Prescription for Better Oral Health Outcomes for Infants: A Call to Action. Children 2025, 12, 1257. https://doi.org/10.3390/children12091257
Tesini DA, Friedman C, Kethu A, Hendricks KW. Pacifier Sizing as a Prescription for Better Oral Health Outcomes for Infants: A Call to Action. Children. 2025; 12(9):1257. https://doi.org/10.3390/children12091257
Chicago/Turabian StyleTesini, David A., Clive Friedman, Adithya Kethu, and Kristin W. Hendricks. 2025. "Pacifier Sizing as a Prescription for Better Oral Health Outcomes for Infants: A Call to Action" Children 12, no. 9: 1257. https://doi.org/10.3390/children12091257
APA StyleTesini, D. A., Friedman, C., Kethu, A., & Hendricks, K. W. (2025). Pacifier Sizing as a Prescription for Better Oral Health Outcomes for Infants: A Call to Action. Children, 12(9), 1257. https://doi.org/10.3390/children12091257






