Expanding Access to Presurgical Cleft Care: Digital Nasoalveolar Molding with Clear Aligners in a Rural Low-Income Population †
Abstract
Highlights
- Digital NAM using clear aligners significantly reduced anterior (–5.38 mm) and posterior (–3.39 mm) cleft widths.
- Intermolar width increased (+1.23 mm) and intercanine width remained stable, indicating preserved maxillary arch form during treatment.
- A digitally planned, low-contact NAM clear aligner protocol can achieve effective presurgical molding with minimal in-office visits.
- This model supports broader access to cleft care in underserved and remote populations by enabling caregiver participation and digital workflow scalability.
Abstract
1. Introduction
2. Materials and Methods
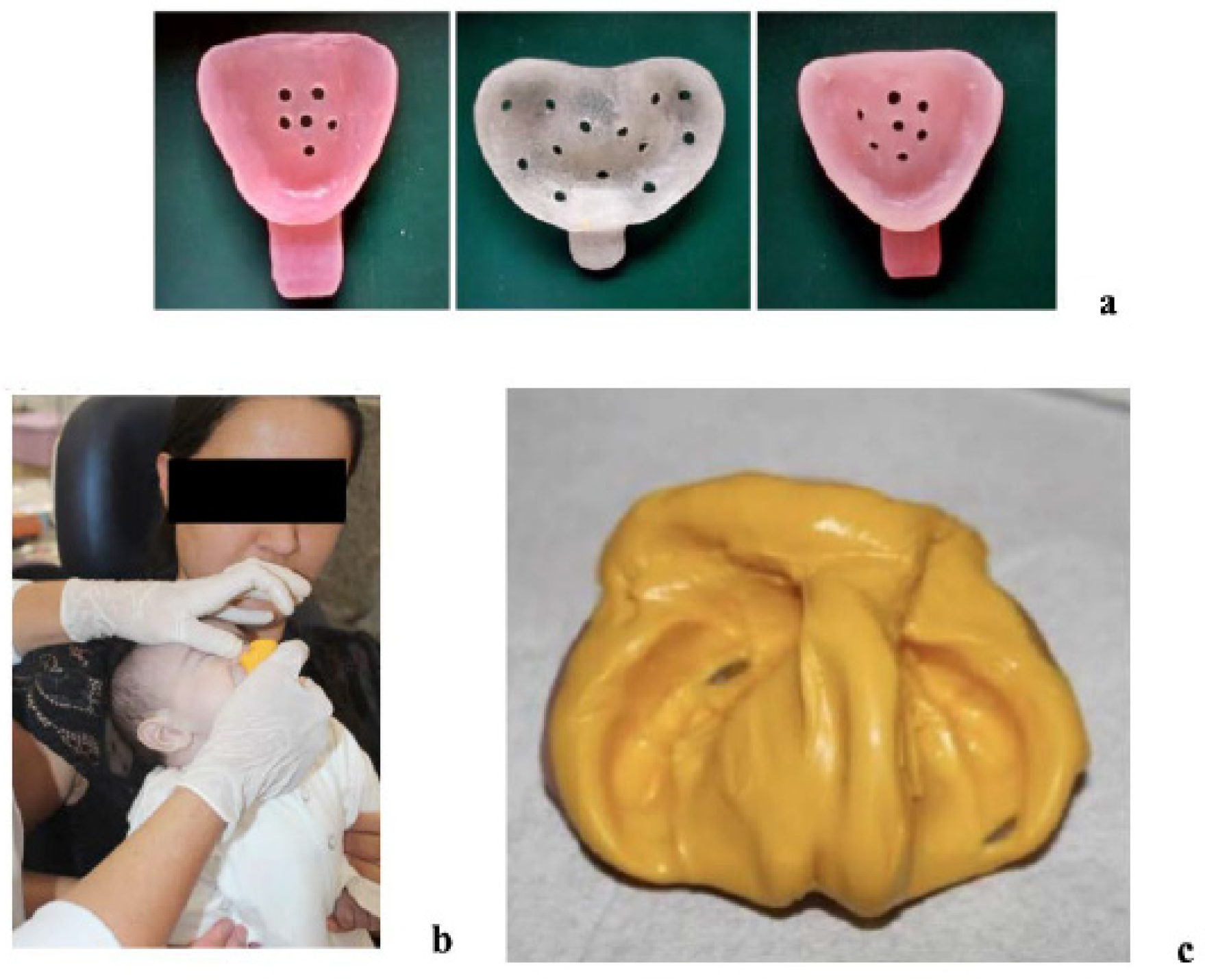
2.1. Clinical Protocol
Initial Evaluation and Impressions
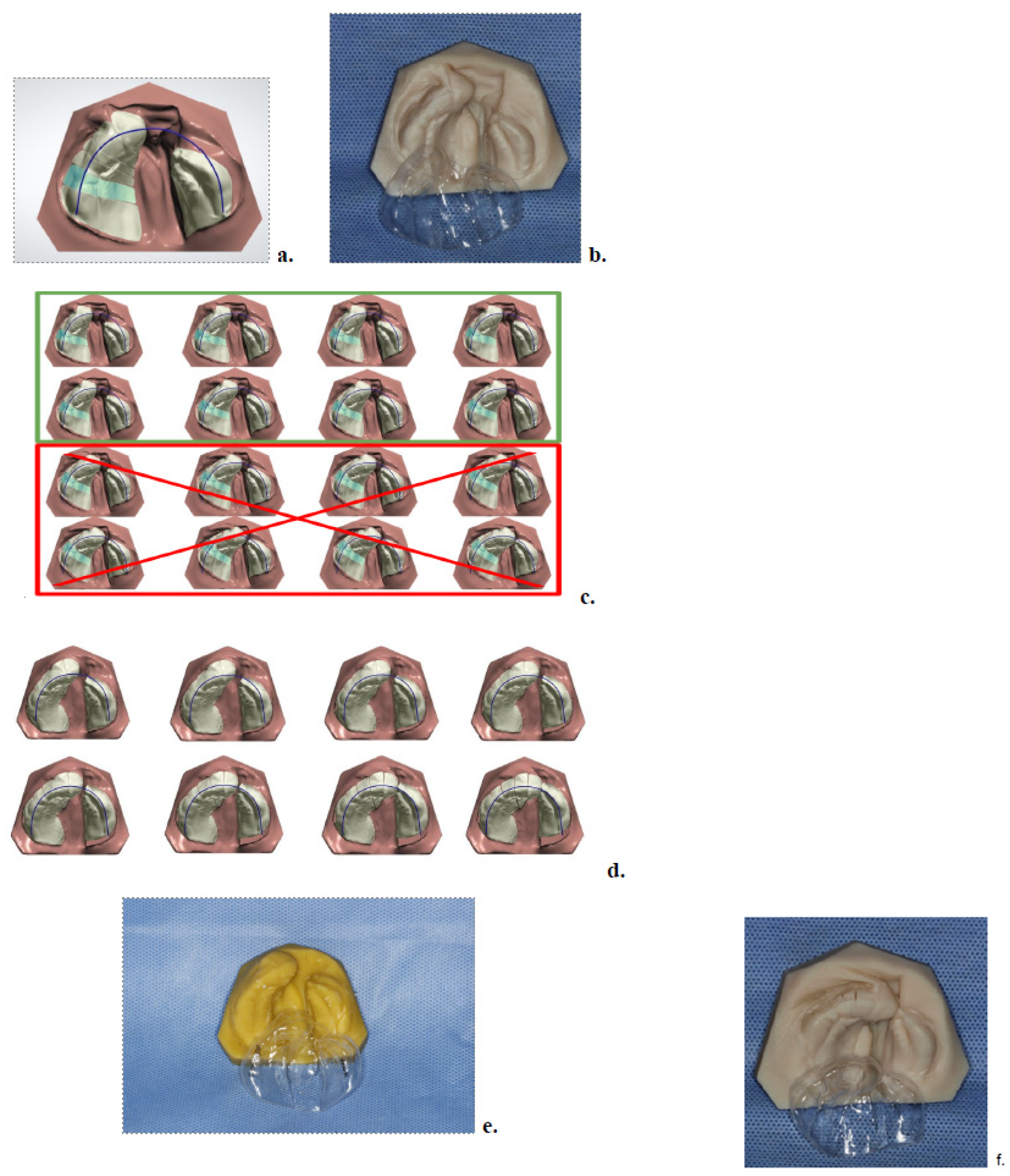
2.2. Digital Scanning and Treatment Planning
2.3. Model Printing and Aligner Fabrication
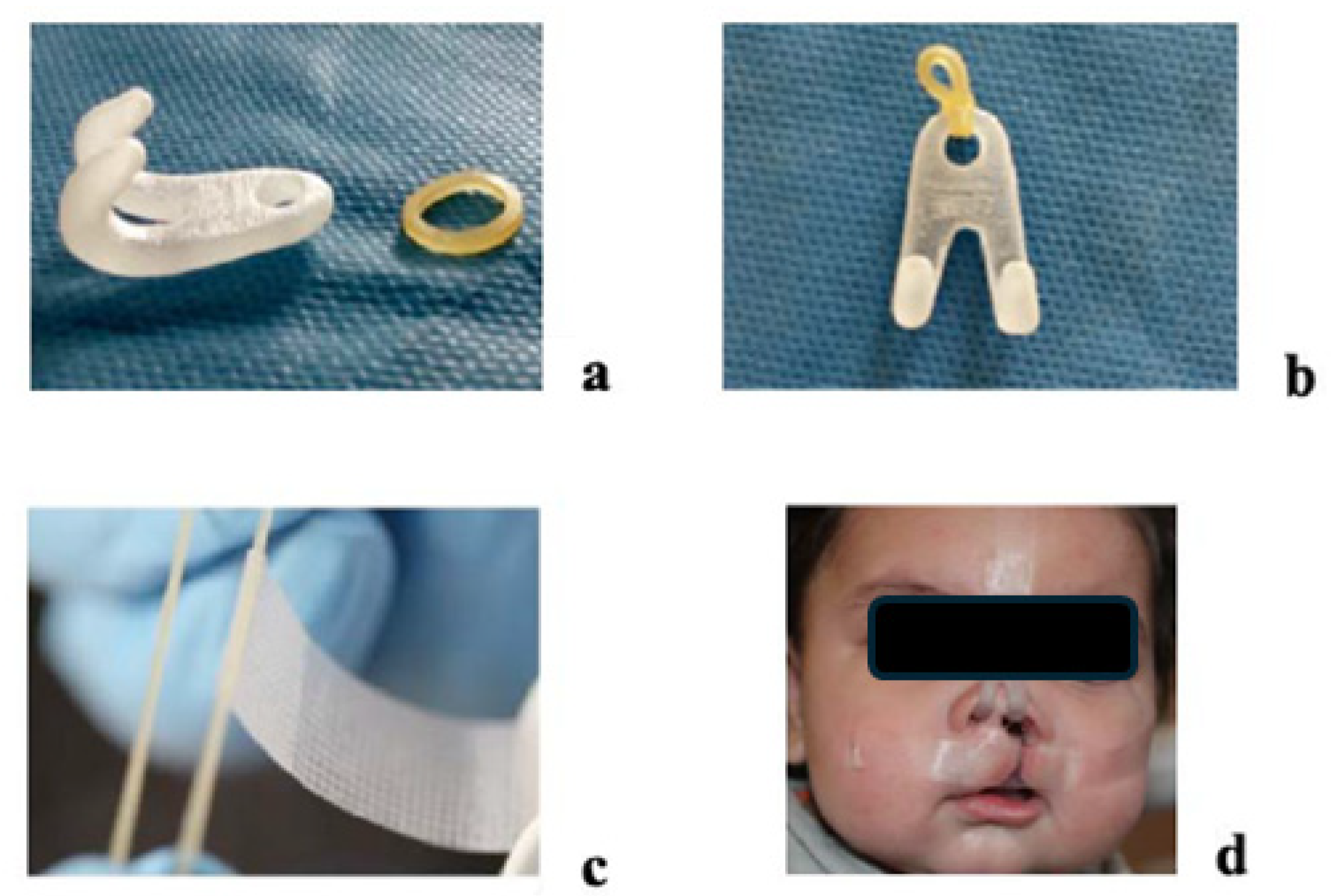
2.4. Appliance Delivery and At-Home Protocol
2.5. Midpoint Evaluation and Phase II
2.6. Final Visit and Post-Treatment Records
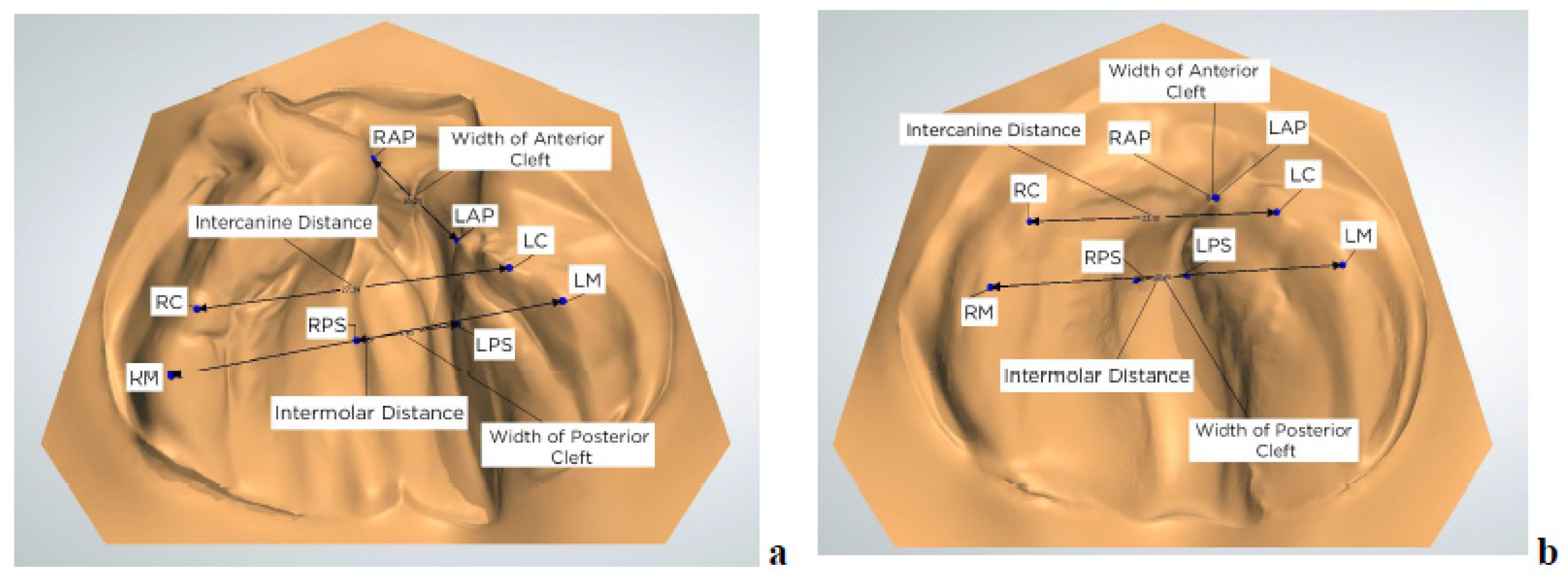
2.7. Measurements and Statistical Analysis
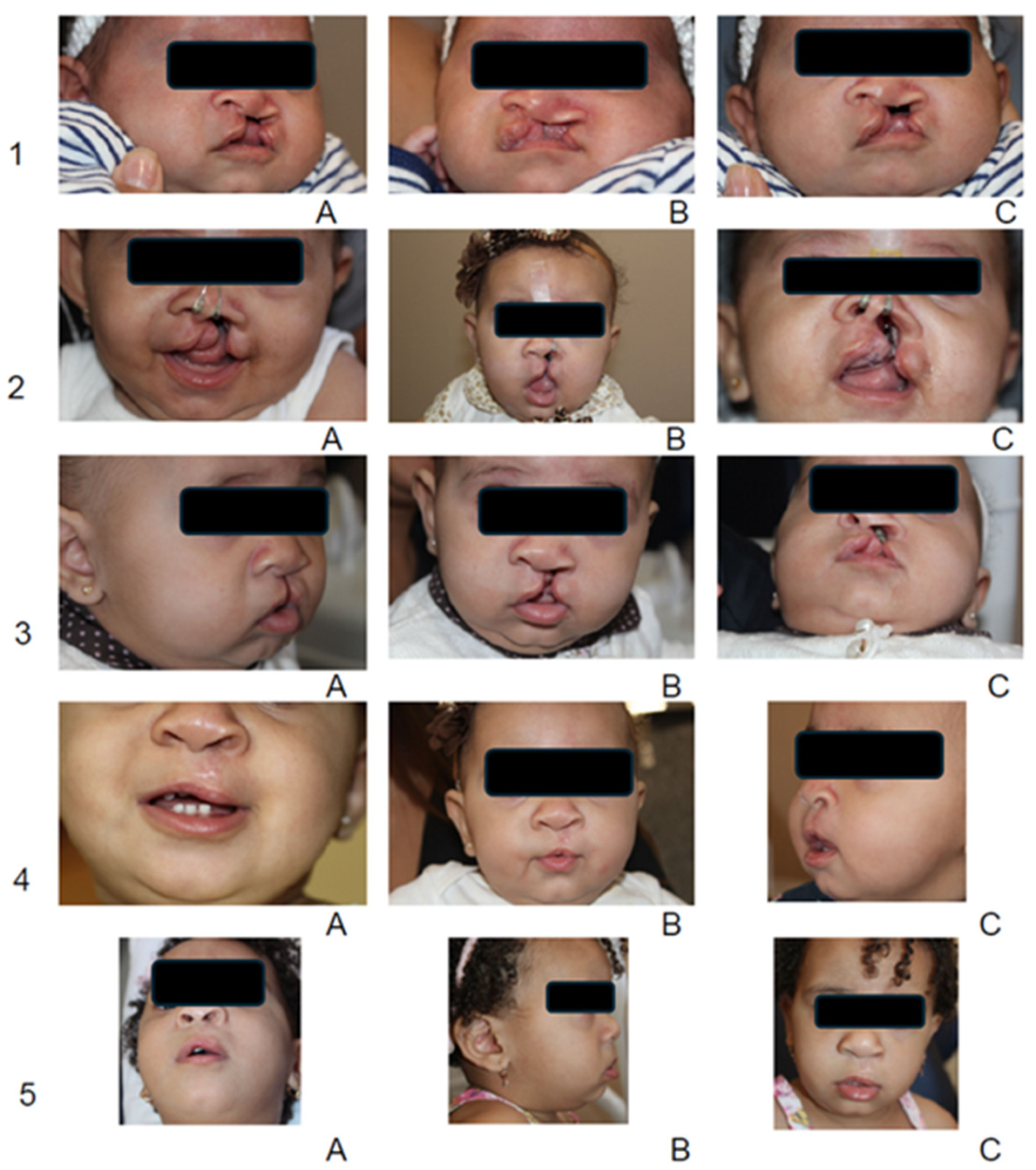
3. Results
3.1. Quantitative Outcomes
3.2. Qualitative Observations
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raposo do Amaral, C.E.; Kuczynski, E.; Alonso, N. Qualidade de vida de crianças com fissura labiopalatina: Análise crítica dos instrumentos de mensuração. Rev. Bras. Cir. Plástica 2011, 26, 639–644. [Google Scholar] [CrossRef]
- Grayson, B.H.; Santiago, P.E.; Brecht, L.E.; Cutting, C.B. Presurgical nasoalveolar molding in infants with cleft lip and palate. Cleft Palate Craniofac. J. 1999, 36, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.D.; Levy-Bercowski, D.; Santiago, P.E. 3-D effects of nasoalveolar molding in patients with orofacial clefts: Geometric morphometrics. Cleft Palate Craniofac. J. 2005, 42, 403–409. [Google Scholar] [CrossRef]
- Singh, G.D.; Levy-Bercowski, D.; Yañez, M.A.; Santiago, P.E. Three-dimensional facial morphology following surgical repair of unilateral cleft lip and palate in patients after nasoalveolar molding. Orthod. Craniofac. Res. 2007, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Grayson, B.H.; Maull, D. Nasoalveolar molding for infants born with clefts of the lip, alveolus, and palate. Clin. Plast. Surg. 2004, 31, 149–158. [Google Scholar] [CrossRef]
- Grayson, B.H.; Cutting, C.B. Presurgical nasoalveolar orthopaedic molding in primary correction of the nose, lip and alveolus of infants born with unilateral and bilateral clefts. Cleft Palate Craniofac. J. 2001, 38, 193–198. [Google Scholar] [CrossRef]
- Kasper, F.K.; Ghivizzani, M.M.; Chiquet, B.T. Emerging applications of 3D printing in nasoalveolar molding therapy: A narrative review. J. 3D Print. Med. 2019, 3, 195–208. [Google Scholar] [CrossRef]
- Abd El-Ghafour, M.; Aboulhassan, M.A.; El-Beialy, A.R.; Fayed, M.M.S.; Eid, F.H.K.; El-Gendi, M.; Emara, D. Is taping alone an efficient presurgical infant orthopedic approach in infants with unilateral cleft lip and palate? A randomized controlled trial. Cleft Palate Craniofac. J. 2020, 57, 1382–1391. [Google Scholar] [CrossRef]
- Shetty, V.; Agrawal, R.K.; Sailer, H.F. Long-term effect of presurgical nasoalveolar molding on growth of maxillary arch in unilateral cleft lip and palate: Randomized controlled trial. Int. J. Oral. Maxillofac. Surg. 2017, 46, 977–987. [Google Scholar] [CrossRef]
- Gong, X.; Yu, Q. Correction of maxillary deformity in infants with bilateral cleft lip and palate using computer-assisted design. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2012, 114, 74–78. [Google Scholar] [CrossRef]
- Koya, S.; Shetty, S.; Husain, A.; Khader, M. Presurgical nasoalveolar molding therapy using figueroa’s NAM technique in unilateral cleft lip and palate patients: A preliminary study. J. Clin. Pediatr. Dent. 2016, 40, 410–416. [Google Scholar] [CrossRef]
- Alperovich, M.; Brecht, L.E.; Warren, S.M. Discussion on: Nasoalveolar molding therapy for the treatment of unilateral cleft lip and palate improves nasal symmetry and maxillary alveolar dimensions. J. Craniofac Surg. 2016, 27, 1983–1984. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Liao, Y.F. A modified nasoalveolar molding technique for correction of unilateral cleft nose deformity. J. Craniomaxillofac. Surg. 2015, 43, 2100–2105. [Google Scholar] [CrossRef]
- Chou, P.Y.; Hallac, R.R.; Ajiwe, T.; Xie, X.J.; Liao, Y.F.; Kane, A.A.; Park, Y.J. The role of nasoalveolar molding: A 3D prospective analysis. Sci. Rep. 2017, 7, 9901. [Google Scholar] [CrossRef]
- Maillard, S.; Retrouvey, J.M.; Ahmed, M.K.; Taub, P.J. Correlation between nasoalveolar molding and surgical, aesthetic, functional and socioeconomic outcomes following primary repair surgery: A systematic review. J. Oral. Maxillofac. Res. 2017, 8, e2. [Google Scholar] [CrossRef]
- Shen, C.; Yao, C.A.; Magee, W., 3rd; Chai, G.; Zhang, Y. Presurgical nasoalveolar molding for cleft lip and palate: The application of digitally designed molds. Plast. Reconstr. Surg. 2015, 135, 1007e–1015e. [Google Scholar] [CrossRef] [PubMed]
- Sischo, L.; Chan, J.W.; Stein, M.; Smith, C.; van Aalst, J.; Broder, H.L. Nasoalveolar molding: Prevalence of cleft centers offering NAM and who seeks it. Cleft Palate Craniofac. J. 2012, 49, 270–275. [Google Scholar] [CrossRef]
- Frazao, D.C. Evaluation of Presurgical Nasoalveolar Molding in Infants with Cleft Lip and Palate. Ph.D. Dissertation, Universidade Estadual Paulista (UNESP), São Paulo, Brazil, 2022. Available online: https://repositorio.unesp.br/server/api/core/bitstreams/138b10a2-d8b7-4dda-8620-c0c43d8b4165/content (accessed on 1 February 2022).
- Isik Aslan, B.; Gülşen, A.; Findikçioğlu, K.; Uzuner, D.; Üçüncü, N. Effects of nasoalveolar molding therapy on alveolar and palatal cleft deformities in unilateral and bilateral cleft lip and palate. J. Craniofac. Surg. 2018, 29, e179–e184. [Google Scholar] [CrossRef] [PubMed]
- Thiruvenkatachari, B.; Bonanthaya, K.; Kuijpers Jagtman, A.M.; Sandler, J.; Powar, R.S.; Hussain, S.A.; Subramaniyan, B.; Bhola, N.; Bhat, H.K.; Ramachandra, V.; et al. A multi-centric, single-blinded, randomized, parallel-group study to evaluate the effectiveness of nasoalveolar moulding treatment in non-syndromic patients with complete unilateral cleft lip, alveolus and palate (NAMUC study): A study protocol for a randomized controlled trial. Trials 2024, 25, 453. [Google Scholar] [CrossRef]
- Parhofer, R.; Rau, A.; Strobel, K.; Gölz, L.; Stark, R.; Ritschl, L.M.; Wolff, K.D.; Kesting, M.R.; Grill, F.D.; Seidel, C.L. The impact of passive alveolar molding vs. nasoalveolar molding on cleft width and other parameters of maxillary growth in unilateral cleft lip palate. Clin. Oral. Investig. 2023, 27, 5001–5009. [Google Scholar] [CrossRef]
- Maull, D.; Grayson, B.H.; Cutting, C.B.; Brecht, L.E.; Bookstein, F.L.; Khorrambadi, D.; McCarthy, J.G. Long-term effects of nasoalveolar molding on feeding in infants with cleft lip and palate. Cleft Palate Craniofac. J. 1999, 36, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Vyas, H.J.; Sharma, S.M.; Sailer, H.F. A comparison of results using nasoalveolar moulding in cleft infants treated within 1 month of life versus those treated after this period: Development of a new protocol. Int. J. Oral Maxillofac. Surg. 2012, 41, 28–36. [Google Scholar] [CrossRef]
- Masarei, A.L.; Sell, D.; Habel, A.; Mars, M.; Sommerlad, B.C.; Wade, A. The nature of feeding in infants with cleft lip and palate compared with healthy non-cleft infants. Cleft Palate Craniofac. J. 2007, 44, 321–328. [Google Scholar] [CrossRef]
- Lee, C.T.H.; Garfinkle, J.S.; Warren, S.M.; Brecht, L.E.; Cutting, C.B.; Grayson, B.H. Nasoalveolar Molding Improves Appearance of Children with Bilateral Cleft Lip–Cleft Palate. Plast. Reconstr. Surg. 2008, 122, 1131–1137. [Google Scholar] [CrossRef]
- Abd El-Ghafour, M.; Aboulhassan, M.A.; Fayed, M.M.S.; El-Beialy, A.R.; Eid, F.H.K.; Hegab, S.E.D.; El-Gendi, M.; Emara, D. Effectiveness of a Novel 3D-Printed Nasoalveolar Molding Appliance (D-NAM) on Improving the Maxillary Arch Dimensions in Unilateral Cleft Lip and Palate Infants: A Randomized Controlled Trial. Cleft Palate-Craniofacial J. 2020, 57, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Al-Khateeb, K.A.; Aboulfotouh, M.H.; Abdelsayed, F.; Mohamed, W.; Abd-El-Ghafour, M. Three-dimensional assessment of maxillary arch changes in infants with bilateral cleft lip and palate using vacuum-formed nasoalveolar molding with active screw versus conventional nasoalveolar molding appliances: A randomized clinical trial. BMC Oral Health 2025, 25, 1339. [Google Scholar] [CrossRef] [PubMed]
| Measurement | Average at T1 | Average at T2 | Difference (T2–T1) | 95% CI | t-Value | p-Value |
|---|---|---|---|---|---|---|
| Anterior Cleft Width (mm) | 10.02 | 4.64 | −5.38 | (−7.58, −3.18) | 5.06 | <0.001 * |
| Intercanine Distance (mm) | 27.01 | 27.5 | 0.49 | (0.05, 2.04) | −0.66 | 0.515 |
| Posterior Cleft Width (mm) | 11.6 | 8.2 | −3.39 | (−4.79, −1.99) | 5.01 | <0.001 * |
| Intermolar Distance (mm) | 35.34 | 36.57 | 1.23 | (0.44, 2.02) | −2.21 | 0.036 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazao, D.C.; Salgado, M.A.C.; Cody, R.J.; Kay, E.M.; Pretti, H.; Singh, G.D.; Pimenta, L.A. Expanding Access to Presurgical Cleft Care: Digital Nasoalveolar Molding with Clear Aligners in a Rural Low-Income Population. Children 2025, 12, 1231. https://doi.org/10.3390/children12091231
Frazao DC, Salgado MAC, Cody RJ, Kay EM, Pretti H, Singh GD, Pimenta LA. Expanding Access to Presurgical Cleft Care: Digital Nasoalveolar Molding with Clear Aligners in a Rural Low-Income Population. Children. 2025; 12(9):1231. https://doi.org/10.3390/children12091231
Chicago/Turabian StyleFrazao, Diogo C., Miguel A. C. Salgado, Ryan J. Cody, Elizabeth M. Kay, Henrique Pretti, G. Dave Singh, and Luiz A. Pimenta. 2025. "Expanding Access to Presurgical Cleft Care: Digital Nasoalveolar Molding with Clear Aligners in a Rural Low-Income Population" Children 12, no. 9: 1231. https://doi.org/10.3390/children12091231
APA StyleFrazao, D. C., Salgado, M. A. C., Cody, R. J., Kay, E. M., Pretti, H., Singh, G. D., & Pimenta, L. A. (2025). Expanding Access to Presurgical Cleft Care: Digital Nasoalveolar Molding with Clear Aligners in a Rural Low-Income Population. Children, 12(9), 1231. https://doi.org/10.3390/children12091231







