Epidemiological Trends in Pediatric Osteoarticular Infections—Results from a Single-Center Retrospective Study Covering 2015–2023
Abstract
Highlights
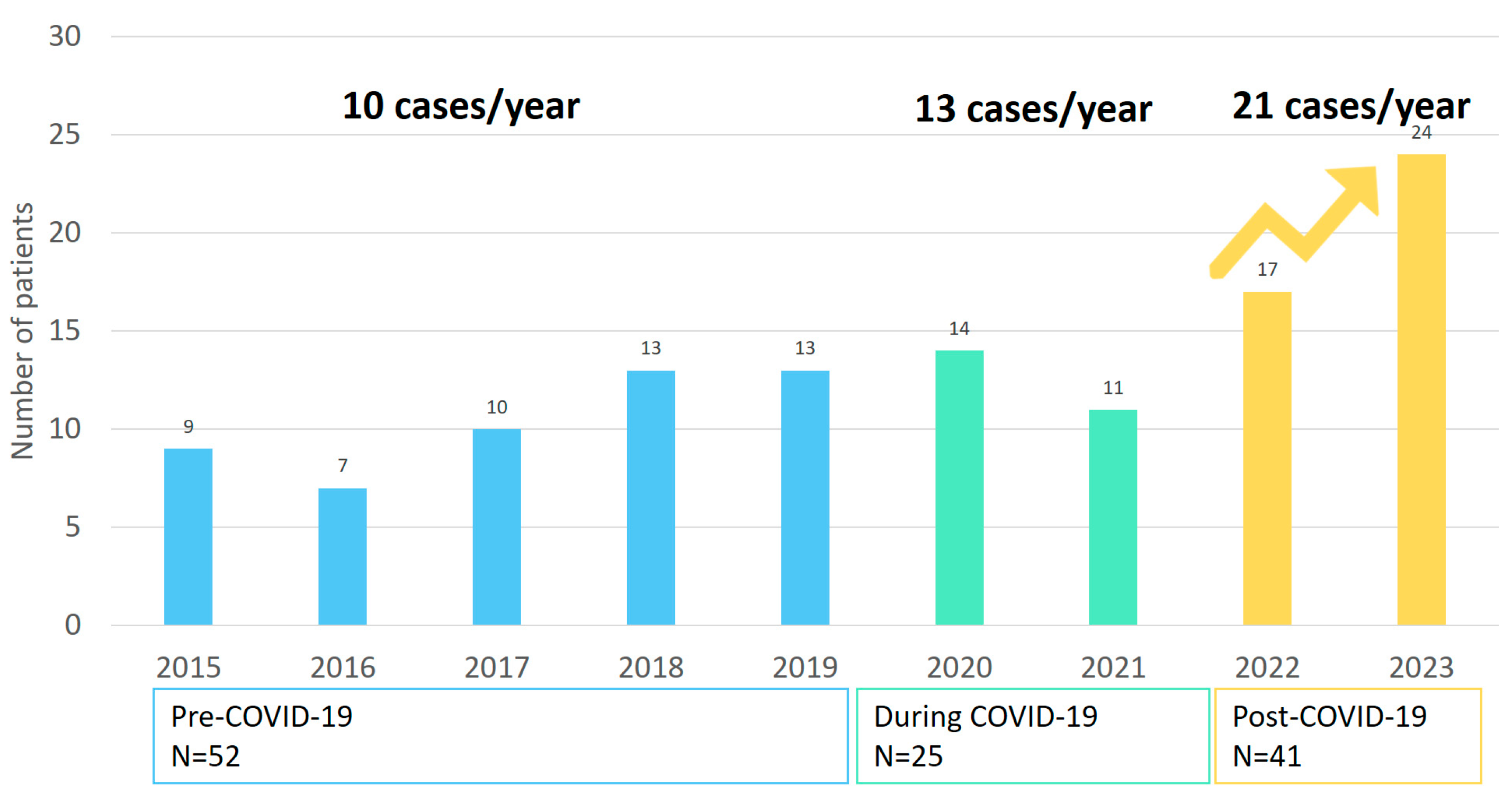
- In our observational single-center study, pediatric osteoarticular infection increased from 10/year between 2015 and 2019 to 21/year between 2022 and 2023.
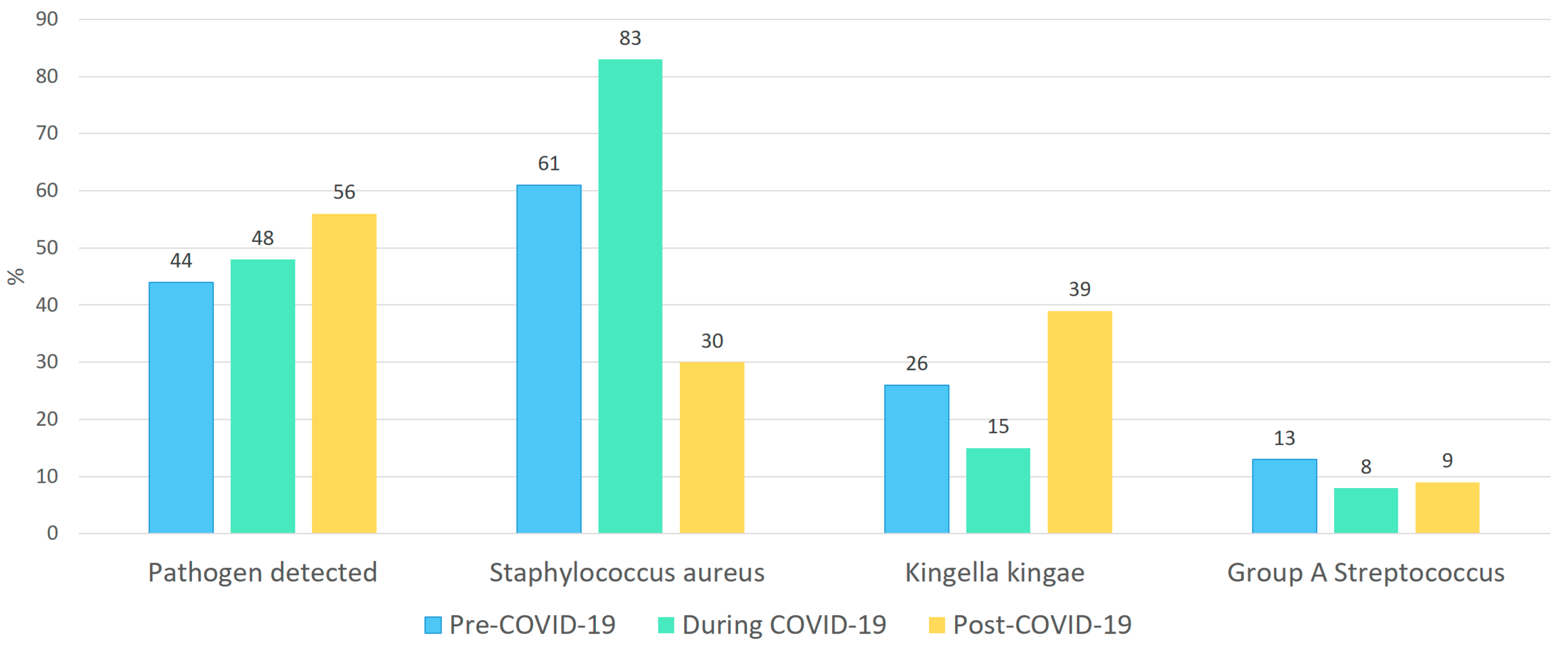
- During the pandemic period, a non-significant decrease in Kingella kingae and Group A streptococcus was observed, accompanied by a relative increase in Staphylococcus aureus. In contrast, the post-pandemic period showed a proportional rise in Kingella kingae detections, while the contribution of Staphylococcus aureus declined.
- Post-pandemic, there seems to be an increase in pediatric osteoarticular infections. This highlights the need for further multicenter research to assess whether this reflects a broader epidemiological change and to guide future diagnostic and therapeutic strategies.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Analysis
3. Results
3.1. Basic Characteristics Regarding Pre-, Mid-, and Post-COVID-19 Pandemic Periods
3.2. Diagnostic Findings
3.3. Pathogen Distribution
4. Discussion
4.1. Implications
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | septic or infectious arthritis |
| GAS | Group A streptococcus |
| NPI | non-pharmaceutical interventions |
| OA | osteomyelitis combined with arthritis |
| OM | osteomyelitis |
References
- Levy, C.; Cohen, R. Infectious diseases in the COVID-19 era: Gaps between countries. Lancet Infect. Dis. 2023, 23, 987–988. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Holm, M.; Rabie, H.; Rytter, M. The pattern of childhood infections during and after the COVID-19 pandemic. Lancet Child. Adolesc. Health 2024, 8, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Shmueli, M.; Lendner, I.; Ben-Shimol, S. Effect of the COVID-19 pandemic on the pediatric infectious disease landscape. Eur. J. Pediatr. 2024, 183, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Trobisch, A.; Schweintzger, N.A.; Kohlfürst, D.S.; Sagmeister, M.G.; Sperl, M.; Grisold, A.J.; Feierl, G.; Herberg, J.A.; Carrol, E.D.; Paulus, S.C.; et al. EUCLIDS consortium. Osteoarticular Infections in Pediatric Hospitals in Europe: A Prospective Cohort Study From the EUCLIDS Consortium. Front Pediatr. 2022, 10, 744182. [Google Scholar] [CrossRef] [PubMed]
- DeRonde, K.J.; Girotto, J.E.; Nicolau, D.P. Management of Pediatric Acute Hematogenous Osteomyelitis, Part I: Antimicrobial Stewardship Approach and Review of Therapies for Methicillin-Susceptible Staphylococcus aureus, Streptococcus pyogenes, and Kingella kingae. Pharmacotherapy 2018, 38, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.B.; Holm, M.; Lindhard, M.S.; Glenthøj, J.P.; Borch, L.; Hartling, U.; Schmidt, L.S.; Rytten, M.J.H.; Rasmussen, A.H.; Damkjær, M.; et al. Oral versus intravenous empirical antibiotics in children and adolescents with uncomplicated bone and joint infections: A nationwide, randomised, controlled, non-inferiority trial in Denmark. Lancet Child. Adolesc. Health. 2024, 8, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Lenglart, L.; Titomanlio, L.; Bognar, Z.; Bressan, S.; Buonsenso, D.; De, T.; Farrugia, R.; Honeyford, K.; Maconochie, I.K.; Moll, H.A.; et al. Surge of Pediatric Respiratory Tract Infections after the COVID-19 Pandemic and the Concept of “Immune Debt”. J. Pediatr. 2024, 284, 114420. [Google Scholar] [CrossRef] [PubMed]
- van Kempen, E.B.; Bruijning-Verhagen, P.C.J.; Borensztajn, D.; Vermont, C.L.; Quaak, M.S.W.; Janson, J.-A.; Maat, I.; Stol, K.; Vlaminckx, B.J.M.; Wieringa, J.W.; et al. Increase in Invasive Group a Streptococcal Infections in Children in the Netherlands, A Survey Among 7 Hospitals in 2022. Pediatr. Infect. Dis. J. 2023, 42, e122–e124. [Google Scholar] [CrossRef] [PubMed]
- de Gier, B.; Marchal, N.; de Beer-Schuurman, I.; Wierik, M.T.; Hooiveld, M.; ISIS-AR Study Group; GAS Study group; de Melker, H.E.; van Sorge, N.M. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in the Netherlands, 2022. Eurosurveillance 2022, 28, 2200941. [Google Scholar] [CrossRef]
- Nygaard, U.; Hartling, U.B.; Munkstrup, C.; Nielsen, A.B.; Dungu, K.H.S.; Schmidt, L.S.; Glenthøj, J.; Matthesen, A.T.; Rytter, M.J.H.; Holm, M. Invasive group A streptococcal infections in children and adolescents in Denmark during 2022–23 compared with 2016–17 to 2021–22: A nationwide, multicentre, population-based cohort study. Lancet Child. Adolesc. Health 2024, 8, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Rijksinstituut voor Volksgezondheid en Milieu (RIVM). Tijdlijn Maatregelen COVID-19 2022 [Internet]; RIVM: Bilthoven, The Netherlands, 2022. Available online: https://www.rivm.nl/gedragsonderzoek/tijdlijn-maatregelen-covid-2022 (accessed on 17 July 2025).
- Pigeolet, M.; Haumont, E.; Rubinsztajn, R.; Pannier, S.; Gaumé, M. Increase in paediatric group A streptococcal infections. Lancet Infect. Dis. 2023, 23, 282. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E. Can group A streptococcus infections be influenced by viruses in the respiratory tract? Lancet Infect. Dis. 2023, 23, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Dokal, K.; Channon-Wells, S.; Davis, C.; Dokal, K.; Channon-Wells, S.; Davis, C.; Estrada-Rivadeneyra, D.; Huse, K.K.; Lias, A.; Hamilton, S.; et al. Immunity to Streptococcus pyogenes and Common Respiratory Viruses at Age 0–4 Years after COVID-19 restrictions: A Cross-Sectional Study. medRxiv 2025, 10, 25325549. [Google Scholar] [CrossRef]
- McNeil, J.C.; Joseph, M.; Sommer, L.M.; Flores, A.R. Staphylococcus aureus Colonization in Healthy Children during the First Year of the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic. J. Pediatr. 2022, 249, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, C.; Duarte, M.; Norte, S.; Arcangelo, J.; Pinto, M.; Correia, C.; Simões, M.J.; Canhão, H.; Tavares, D. Kingella kingae Displaced S. aureus as the Most Common Cause of Acute Septic Arthritis in Children of All Ages. Pediatr. Infect. Dis. J. 2021, 40, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Coulin, B.; Demarco, G.; Spyropoulou, V.; Juchler, C.; Vendeuvre, T.; Habre, C.; Tabard-Fougère, A.; Dayer, R.; Steiger, C.; Ceroni, D. Osteoarticular infection in children. Bone Joint J. 2021, 103–B, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.J.; Murray-Krezan, C.; Dehority, W. Suppurative complications of acute hematogenous osteomyelitis in children. J. Pediatr. Orthop. B 2017, 26, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.S.; McNeil, J.C.; Hultén, K.G.; Vallejo, J.G.; E Scheurer, M.; Kaplan, S.L. Assessing Risk for Complications in Acute Hematogenous Osteomyelitis in Children: Validation of 2 Predictive Scores. J. Pediatr. Infect. Dis. Soc. 2023, 12, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Alcobendas Rueda, M.A.; Núñez, E.; Martín, L.; Hernández, M.B.; Saavedra-Lozano, J.; Udaondo, C.; Murias, S.; Remesal, A.; Calvo, C.; Rioped Group. Oral Versus Intravenous Antibiotics for Pediatric Osteoarticular Infection: When and to Whom? Pediatr. Infect. Dis. J. 2022, 41, e351–e357. [Google Scholar] [CrossRef] [PubMed]
- Font, A.; Agüera, M.; Ríos-Barnés, M.; Gamell, A.; Moreno-Romo, D.; López-Ramos, M.G.; Monsonís, M.; Fumadó, V.; Simó-Nebot, S.; Fontecha, C.G.; et al. Impact of paediatric antimicrobial stewardship program in haematogenous bone and joint infections. Eur. J. Pediatr. 2025, 184, 426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Osteomyelitis | Arthritis | |
|---|---|---|
| Confirmed cases | Bacteriologic evidence (positive blood and/or bone culture) plus at least one of the following: (1) localized pain or tenderness with other typical features such as warmth or swelling of the affected region; (2) imaging findings consistent with osteomyelitis (e.g., MRI or bone scan); or (3) histopathological confirmation (intraoperative specimen) | Isolation of a microorganism from blood or synovial fluid and imaging consistent with arthritis (MRI and/or bone scan). |
| Suspected cases | Localized pain or tenderness and other typical clinical features of osteomyelitis, such as warmth and/or swelling of the affected region; and imaging that excludes other diagnoses (X-ray and/or ultrasound); and followed an osteomyelitis treatment plan | Presence of localized pain/tenderness and other typical features of arthritis, such as warmth and/or swelling of the affected region; and image findings that are consistent with arthritis (ultrasound and/or X-ray); and followed an arthritis treatment plan |
| Rejected cases | No current presentation; or did not visit a pediatric or orthopedic doctor; or was not treated with an osteomyelitis treatment plan; or diagnosis or treatment was performed outside the Juliana Children’s Hospital; or image findings were not consistent with or exclude osteomyelitis (MRI, bone culture, X-ray, and/or ultrasound); or was autoimmune-related osteomyelitis (e.g., chronic recurrent multifocal osteomyelitis) | No current presentation; or did not visit a pediatric or orthopedic doctor; or was not treated with an arthritis treatment plan; or diagnosis or treatment was performed outside the Juliana Children’s Hospital; or image findings were not consistent with or exclude arthritis (MRI, bone culture, X-ray, and/or ultrasound); or was autoimmune-related arthritis (e.g., reactive arthritis or rheumatoid arthritis, including juvenile idiopathic arthritis and Henoch–Schönlein purpura arthritis) |
|
Total
N = 118 (%) |
Pre-COVID-19
P1 (2015–2019) N = 52 (%) |
Mid-COVID-19
P2 (2020–2021) N = 25 (%) | Post-COVID-19 P3 (2022–2023) N = 41 (%) | |
|---|---|---|---|---|
| General characteristics | ||||
| Gender, female | 46 (39) | 18 (35) | 10 (40) | 18 (44) |
| Median age, in months | 24 (IQR 12–96) | 24 (IQR 10–87) | 48 (IQR 24–96) | 30 (IQR 12–96) |
| Arthritis | 50 (42) | 20 (39) | 11 (44) | 19 (46) |
| Osteomyelitis | 59 (50) | 28 (54) | 13 (52) | 18 (44) |
| Osteomyelitis and arthritis | 9 (8) | 4 (8) | 1 (4) | 4 (10) |
| Pathogen | ||||
| Pathogen detected | 58 (49) | 23 (44) | 13 (48) | 22 (54) |
| Staphylococcus aureus | 30 (51) | 14 (61) | 8 (83) | 8 (36) |
| Group A Streptococcus | 6 (10) | 3 (13) | 1 (8) | 2(9) |
| Kingella kingae | 16 (27) | 6 (26) | 2 (15) | 9 (40) |
| Other * | 6 (10) | |||
| Diagnostic findings at presentation | ||||
| CRP level determined at first presentation | 117 (99) | |||
| Median CRP level (mg/L) | 60.5 (IQR 28.5–121) | 61.5 (IQR 37–39) | 41 (IQR 5–98) | 75 (IQR 25–135.5) |
| ESR level determined at first presentation | 93 (79) | |||
| Median ESR level in mm | 38 (IQR 19–60) | 39 (IQR 24–52) | 37 (IQR 15.5–67) | 31 (IQR 19–70) |
| Abscess | 23 | 5/51 (10) | 7/25 (28) | 11/41 (27) |
| Outcome | ||||
| Pediatric intensive care unit admission | 0 | |||
| Mortality | 0 |
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of joint and/or bone punctures per year | 4 | 3 | 8 | 6 | 5 | 10 | 6 | 12 | 17 | 71 |
| Number and % of PCR performed on Kingella kingae in bone and/or joint puncture | 0 | 0 | 1 (12.5) | 1 (16.6) | 3 (60) | 2 (20) | 4 (66) | 8 (66) | 12 (70) | 30 (42) |
| Number and percentage of positive PCR on Kingella kingae | 0 | 0 | 1 (12.5%) | 1 (16.6%) | 3 (60%) | 1 (10%) | 1 (16.6%) | 5 (41.6%) | 3 (17.6%) | 15 (21.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Kempen, E.B.; Scholma, A.; Cheung, N.N.; van Veen, M.; van Linge, J.H. Epidemiological Trends in Pediatric Osteoarticular Infections—Results from a Single-Center Retrospective Study Covering 2015–2023. Children 2025, 12, 1210. https://doi.org/10.3390/children12091210
van Kempen EB, Scholma A, Cheung NN, van Veen M, van Linge JH. Epidemiological Trends in Pediatric Osteoarticular Infections—Results from a Single-Center Retrospective Study Covering 2015–2023. Children. 2025; 12(9):1210. https://doi.org/10.3390/children12091210
Chicago/Turabian Stylevan Kempen, Evelien B., Ayla Scholma, Nam Nam Cheung, Mirjam van Veen, and Joost H. van Linge. 2025. "Epidemiological Trends in Pediatric Osteoarticular Infections—Results from a Single-Center Retrospective Study Covering 2015–2023" Children 12, no. 9: 1210. https://doi.org/10.3390/children12091210
APA Stylevan Kempen, E. B., Scholma, A., Cheung, N. N., van Veen, M., & van Linge, J. H. (2025). Epidemiological Trends in Pediatric Osteoarticular Infections—Results from a Single-Center Retrospective Study Covering 2015–2023. Children, 12(9), 1210. https://doi.org/10.3390/children12091210





