Outcomes of DISE-Directed Surgery for Obstructive Sleep Apnoea in Children
Abstract
Highlights
- DISE-directed surgery improved PSG outcomes in younger, non-obese children and those with severe pre-operative OSA.
- Tongue-base reduction had the highest rate of improvement among DISE-guided interventions, while children with Trisomy 21 showed limited benefit.
- DISE allows for targeted, individualised surgical planning in complex paediatric OSA cases, with better outcomes in specific subgroups.
- Children with multiple comorbidities, Trisomy 21, or obesity may require alternative or adjunctive approaches beyond DISE-directed surgery.
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Subjects
2.3. Polysomnography
2.4. Drug-Induced Sleep Nasendoscopy
2.5. Data Analysis
3. Results
3.1. Demographics
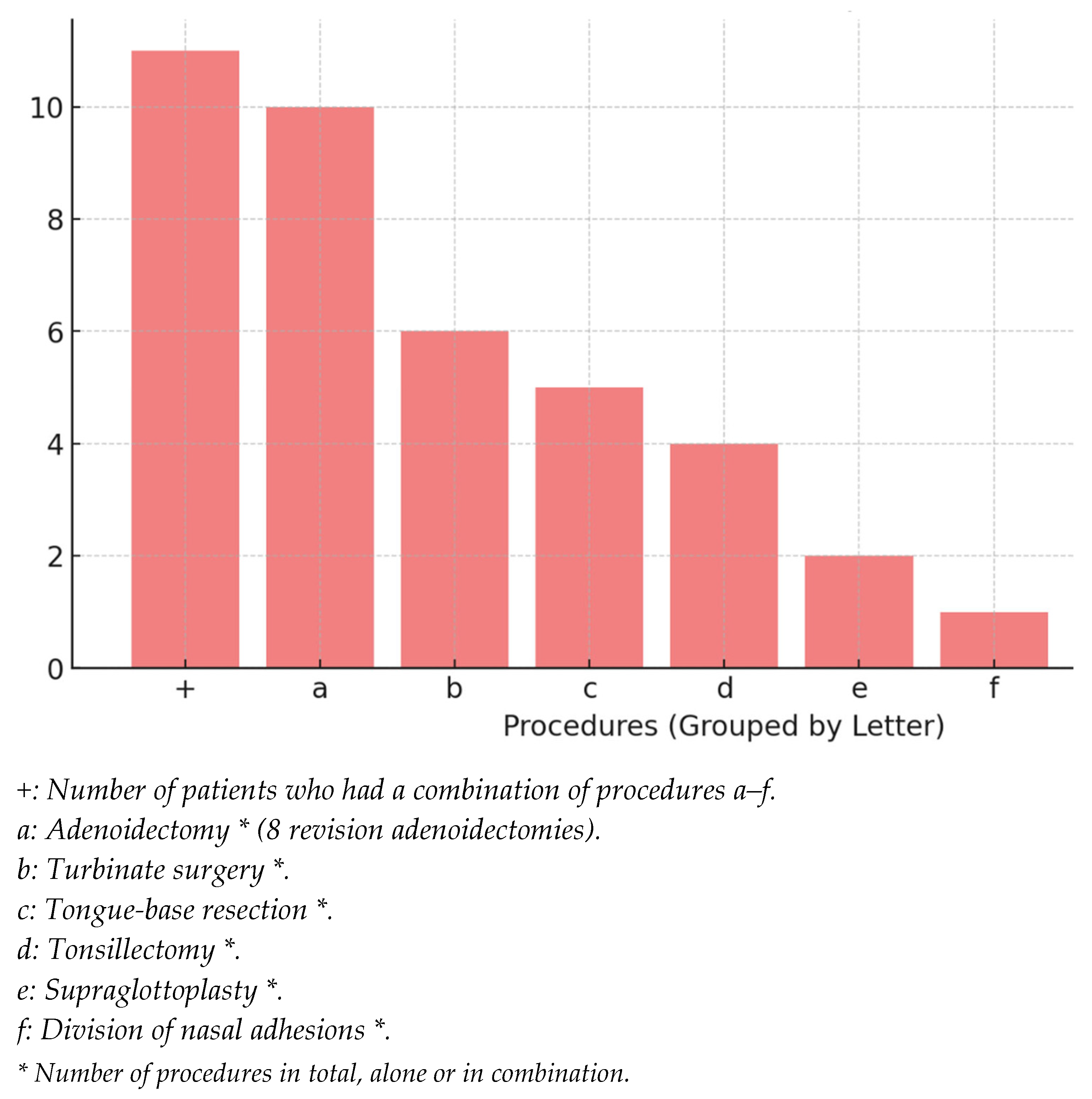
3.2. Surgical Interventions
- Adenoidectomy (N = 10, 52.6%)
- 2.
- Turbinate reduction (N = 6, 31.6%)
- 3.
- Tongue-base reduction (N = 5, 26.3%)
- 4.
- Tonsil surgery (N = 4, 21.1%)
- 5.
- Supraglottoplasty (N = 2, 10.5%)
- 6.
- Division of nasal synechiae (N = 1, 5.3%)
3.3. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASM | American Academy of Sleep Medicine Guidelines |
| AHI | Apnoea–Hypopnoea Index |
| BMI | Body Mass Index |
| CAs | Central Apnoeas |
| CAHS | Children’s and Adolescent Health Service |
| CHs | Central Hypopnoeas |
| CineMRI | Cine Magnetic Resonance Imaging |
| CITs | Cautery of Inferior Turbinates |
| CPAP | Continuous Positive Airway Pressure |
| CT | Computed Tomography |
| DISE | Drug-Induced Sleep Endoscopy |
| ECG | Electrocardiography |
| EEG | Electroencephalography |
| EMG | Electromyography |
| ENT | Ear, Nose, and Throat/Otorhinolaryngology |
| EOG | Electrooculography |
| EQUATOR | Enhancing the QUAlity and Transparency Of Health Research |
| FASD | Foetal Alcohol Syndrome |
| GEKO | Governance Evidence Knowledge Outcome |
| IQR | Interquartile Range |
| IV | Intravenous |
| LSAT | Lowest Oxygen Saturation |
| MAs | Mixed Apnoeas |
| MHs | Mixed Hypopnoeas |
| NATA | National Association of Testing Authorities, Australia |
| NPA | Nasopharyngeal Airway |
| OAs | Obstructive Apnoeas |
| OAHI | Obstructive Apnoea–Hypopnoea Index |
| OHs | Obstructive Hypopnoeas |
| OSA | Obstructive Sleep Apnoea |
| PRS | Pierre Robin Sequence |
| PSG | Polysomnography |
| REM | Rapid Eye Movement |
| SD | Standard Deviation |
| SpO2 | Peripheral Oxygen Saturation |
| TBR | Tongue-Base Resection |
| TCO2 | Transcutaneous Carbon Dioxide Monitoring |
| TCI | Target-Controlled Infusion |
| TOF | Tracheoesophageal Fistula |
| VOTE | Vellum, Oropharynx, Tongue Base, and Epiglottis |
References
- Randel, A. AAO-HNS Guidelines for Tonsillectomy in Children and Adolescents. Am. Fam. Physician 2011, 84, 566–573. [Google Scholar] [PubMed]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A Randomized Trial of Adenotonsillectomy for Childhood Sleep Apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef] [PubMed]
- Garde, A.J.B.; Gibson, N.A.; Samuels, M.P.; Evans, H.J. Recent advances in paediatric sleep disordered breathing. Breathe 2022, 18, 220151. [Google Scholar] [CrossRef]
- Arganbright, J.M.; Lee, J.C.; Weatherly, R.A. Pediatric drug-induced sleep endoscopy: An updated review of the literature. World J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 221–227. [Google Scholar] [CrossRef]
- Akkina, S.R.; Ma, C.C.; Kirkham, E.M.; Horn, D.L.; Chen, M.L.; Parikh, S.R. Does drug induced sleep endoscopy-directed surgery improve polysomnography measures in children with Down Syndrome and obstructive sleep apnea? Acta Otolaryngol. 2018, 138, 1009–1013. [Google Scholar] [CrossRef]
- Croft, C.B.; Pringle, M. Sleep nasendoscopy: A technique of assessment in snoring and obstructive sleep apnoea. Clin. Otolaryngol. Allied Sci. 1991, 16, 504–509. [Google Scholar] [CrossRef]
- Socarras, M.A.; Landau, B.P.; Durr, M.L. Diagnostic techniques and surgical outcomes for persistent pediatric obstructive sleep apnea after adenotonsillectomy: A systematic review and meta-analysis. Int. J. Pediatr. Otorhinolaryngol. 2019, 121, 179–187. [Google Scholar] [CrossRef]
- Iber, C.; American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Pamula, Y.; Campbell, A.; Coussens, S.; Davey, M.; Griffiths, M.; Martin, J.; Maul, J.; Nixon, G.; Sayers, R.; Teng, A.; et al. ASTA/ASA Addendum to the AASM Guidelines for the Recording and Scoring of Paediatric Sleep; Wiley-Blackwell Publishing: Oxford, UK, 2011; Volume 20, p. 4. [Google Scholar]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Ward, S.D.; Sheldon, S.H.; Shiffman, R.N.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, e714–e755. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.E.; Marcus, C.L. Pediatric Polysomnography. Sleep Med. Clin. 2009, 4, 393–406. [Google Scholar] [CrossRef]
- Kushida, C.A.; Littner, M.R.; Morgenthaler, T.; Alessi, C.A.; Bailey, D.; Coleman, J., Jr.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice Parameters for the Indications for Polysomnography and Related Procedures: An Update for 2005. Sleep 2005, 28, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Kales, A.; Rechtschaffen, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects; National Institutes of Health Publication, No. 204; Rechtschaffen, A., Kales, A., Eds.; U. S. National Institute of Neurological Diseases and Blindness, Neurological Information Network: Bethesda, MD, USA, 1968.
- Kezirian, E.J.; Hohenhorst, W.; De Vries, N. Drug-induced sleep endoscopy: The VOTE classification. Eur. Arch. Otorhinolaryngol. 2011, 268, 1233–1236. [Google Scholar] [CrossRef]
- Esteller, E.; Villatoro, J.; Agüero, A.; Matiñó, E.; Lopez, R.; Aristimuño, A.; Nuñez, V.; Díaz-Herrera, M. Outcome of drug-induced sleep endoscopy-directed surgery for persistent obstructive sleep apnea after adenotonsillar surgery. Int. J. Pediatr. Otorhinolaryngol. 2019, 120, 118–122. [Google Scholar] [CrossRef]
- Saniasiaya, J.; Kulasegarah, J. Outcome of drug induced sleep endoscopy directed surgery in paediatrics obstructive sleep apnoea: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110482. [Google Scholar] [CrossRef]
- Wootten, C.T.; Chinnadurai, S.; Goudy, S.L. Beyond adenotonsillectomy: Outcomes of sleep endoscopy-directed treatments in pediatric obstructive sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1158–1162. [Google Scholar] [CrossRef]
- He, S.; Peddireddy, N.S.; Smith, D.F.; Duggins, A.L.; Heubi, C.; Shott, S.R.; Ishman, S.L. Outcomes of Drug-Induced Sleep Endoscopy–Directed Surgery for Pediatric Obstructive Sleep Apnea. Otolaryngol. Neck Surg. 2018, 158, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Sigaard, R.K.; Bertelsen, J.B.; Ovesen, T. Does DISE increase the success rate of surgery for obstructive sleep apnea in children? A systematic review of DISE directed treatment of children with OSAS. Am. J. Otolaryngol. 2023, 44, 103992. [Google Scholar] [CrossRef]
- Kenneth Sims, R.; Leeds, A.; Johnson, G.; Davide, A.; Camacho, M. Drug Induced Sleep Endoscopy-Directed Tongue Surgery to Treat Persistent Pediatric Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Clin. Otolaryngol. 2025, 50, 438–445. [Google Scholar] [CrossRef]
- Imanguli, M.; Ulualp, S.O. Risk factors for residual obstructive sleep apnea after adenotonsillectomy in children. Laryngoscope 2016, 126, 2624–2629. [Google Scholar] [CrossRef]
- Zhen, E.; Locatelli Smith, A.; Herbert, H.; Vijayasekaran, S. Midline posterior glossectomy and lingual tonsillectomy in children with refractory obstructive sleep apnoea: Factors that influence outcomes. Aust. J. Otolaryngol. 2022, 5, 24. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kheirandish-Gozal, L.; Spruyt, K.; Mitchell, R.B.; Promchiarak, J.; Simakajornboon, N.; Kaditis, A.G.; Splaingard, D.; Splaingard, M.; Brooks, L.J.; et al. Adenotonsillectomy Outcomes in Treatment of Obstructive Sleep Apnea in Children: A Multicenter Retrospective Study. Am. J. Respir. Crit. Care Med. 2010, 182, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Van De Perck, E.; Van Hoorenbeeck, K.; Verhulst, S.; Saldien, V.; Vanderveken, O.M.; Boudewyns, A. Effect of body weight on upper airway findings and treatment outcome in children with obstructive sleep apnea. Sleep Med. 2021, 79, 19–28. [Google Scholar] [CrossRef] [PubMed]
| Syndrome/Comorbidity | Number | Other Airway Factors | Surgeries Performed |
|---|---|---|---|
| Trisomy 21 | 6 | 2 patients with TOF * repair, 1 with laryngomalacia | Turbinoplasty 2× TBR $ Revision adenoidectomy and CIT @ Revision adenoidectomy and tonsillotomy Revision adenoidectomy and turbinectomy |
| FASD # | 1 | TBR $ | |
| Achondroplasia | 1 | Revision adenoidectomy, tonsillotomy | |
| Rubinstein–Taybi Syndrome | 1 | Lingual tonsillectomy | |
| Patau Syndrome | 1 | Cleft palate | Tonsillotomy |
| Opitz G/BBB Syndrome | 1 | Tracheobronchomalacia cleft palate | Division of nasal synechiae |
| Prader–Willi Syndrome | 1 | Revision adenoidectomy and TBR $ | |
| Ehlers–Danlos Syndrome | 1 | Laryngeal cleft | Supraglottoplasty and repair of type 1 cleft |
| Pierre Robin Sequence (PRS) | 1 | Cleft palate | Supraglottoplasty and NPA § insertion |
| Asthma | 1 | Revision adenoidectomy and CIT @ | |
| Ex-Preterm | 1 | Micrognathia | Adenoidectomy |
| None | 3 | 2× Revision adenoidectomy and CITs @ Adenotonsillectomy |
| Patients Who Showed Improvement After DISE-Directed Surgery * (n = 10) | Patients Who Had No Improvement After DISE-Directed Surgery * (n = 9) | p-Value (95% CI) | |
|---|---|---|---|
| Age (years ± SD) | 3.6 ± 2.1 | 10.7 ± 4.1 | 0.001 * (−10.5, −3.6) |
| Comorbidities n (%) | |||
| Trisomy 21 n (%) | 1 (10.0) | 5 (55.5) | 0.057 (0.06, 0.76) |
| Prior adenotonsillectomy n (%) | 6 (60.0) | 9 (100) | 0.09 (−0.03, 0.74) |
| Median BMI (IQR #) | 18.0 (5.3) | 16.9 (2.8) | 0.36 (−1.5, 9.5) |
| Pre-operative PSG scores | |||
| Median OAHI (IQR #) | 14.2 (18.0) | 4.0 (8.3) | 0.03 * (1.0, 20.9) |
| Median of mean SpO2 (IQR #) | 97.0 (2.3) | 97.0 (1.0) | 0.27 (−3.0, 0.5) |
| Median SpO2 nadir (IQR #) | 83.5 (14.5) | 90.0 (11.0) | 0.22 (−16.0, 3.0) |
| Median VOTE score (IQR #) | 5.0 (2.5) | 5.0 (2.0) | 0.80 (−2.0, 1.0) |
| Median (IQR) Score Before DISE-Directed Surgery | Median (IQR) Score After DISE-Directed Surgery | p-Value (95% CI) | |
|---|---|---|---|
| OAHI (events/h) | 10.1 (13.8) | 2.2 (6.4) | 0.018 * (1.65, 18.15) |
| Mean SpO2 (%) | 97.0 (1.0) | 97.5 (2.0) | 0.69 (−3.5, 1.0) |
| SpO2 nadir (%) | 85.0 (13.0) | 86.0 (17.0) | 0.80 (−10.0, 10.0) |
| Median (IQR) Score Before DISE-Directed Surgery | Median (IQR) Score After DISE-Directed Surgery | p-Value (95% CI) | |
|---|---|---|---|
| OAHI (events/h) | 18.5 (10.0) | 7.0 (10.0) | 0.039 * (3.9, 30.0) |
| Mean SpO2 (%) | 97.0 (1.3) | 98.0 (1.9) | 0.35 (−7.0, 1.5) |
| SpO2 nadir (%) | 74.5 (18.3) | 81.0 (11.0) | 0.74 (−24.5, 13) |
| Pre-Operative Median (IQR) | Post-Operative Median (IQR) | p-Value (95% CI) | |
|---|---|---|---|
| OAHI (events/h) | 8.2 (12.3) | 2.6 (8.7) | 0.02 * (−15.3, −0.9) |
| Mean SpO2 (%) | 97.0 (1.3) | 97.0 (2.0) | 0.82 (−1.5, 3.3) |
| SpO2 nadir (%) | 86.0 (21.3) | 88.0 (13.3) | 0.61 (−7.0, 10.0) |
| OA (events/h) | 7.5 (34.3) | 5.0 (13.3) | 0.14 (−53.5, 6.0) |
| MA (events/h) | 0.0 (1.0) | 0.5 (2.0) | 0.73 (−3.0, 3.0) |
| CA (events/h) | 9.0 (8.5) | 5.0 (19.8) | 1.0 (−4.0, 7.0) |
| OH (events/h) | 44.0 (73.5) | 17.0 (38.5) | 0.045 * (−61.0, −1.5) |
| MH (events/h) | 0.0 (0.0) | 0.0 (0.0) | - |
| CH (events/h) | 0.0 (0.0) | 0.0 (1.5) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blokland, R.; Friedland, Y.; Kalra, A.; Withers, A.; Vijayasekaran, S. Outcomes of DISE-Directed Surgery for Obstructive Sleep Apnoea in Children. Children 2025, 12, 1185. https://doi.org/10.3390/children12091185
Blokland R, Friedland Y, Kalra A, Withers A, Vijayasekaran S. Outcomes of DISE-Directed Surgery for Obstructive Sleep Apnoea in Children. Children. 2025; 12(9):1185. https://doi.org/10.3390/children12091185
Chicago/Turabian StyleBlokland, Rachel, Yael Friedland, Aryan Kalra, Adelaide Withers, and Shyan Vijayasekaran. 2025. "Outcomes of DISE-Directed Surgery for Obstructive Sleep Apnoea in Children" Children 12, no. 9: 1185. https://doi.org/10.3390/children12091185
APA StyleBlokland, R., Friedland, Y., Kalra, A., Withers, A., & Vijayasekaran, S. (2025). Outcomes of DISE-Directed Surgery for Obstructive Sleep Apnoea in Children. Children, 12(9), 1185. https://doi.org/10.3390/children12091185







