Biofeedback in Pediatric, Adolescent, and Young Adult Cancer Care: A Systematic Review
Abstract
1. Introduction
1.1. What Is Biofeedback?
1.2. Biofeedback Modalities
- Cardiovascular Biofeedback: This modality measures heart rate (HR) and heart rate variability (HRV). HRV, the variation in time between consecutive heartbeats, is a crucial indicator of autonomic nervous system balance and overall health [9]. It has been widely studied in relation to cardiovascular health, mental well-being, stress regulation, and performance optimization. Research has demonstrated that low HRV is associated with a range of psychophysiological conditions, including anxiety, depression, post-traumatic stress disorder (PTSD), chronic pain, gastrointestinal disorders, and high blood pressure [10,11,12,13,14,15]. In adult cancer populations, HRV has been identified as a prognostic marker, with lower HRV predicting poorer treatment outcomes [16,17].
1.3. Efficacy of Biofeedback in Adult Populations
1.4. Biofeedback in Adult Oncology
1.5. Biofeedback in Pediatric and AYA Populations
1.6. The Need for Research in Pediatric and AYA Oncology
2. Materials and Methods
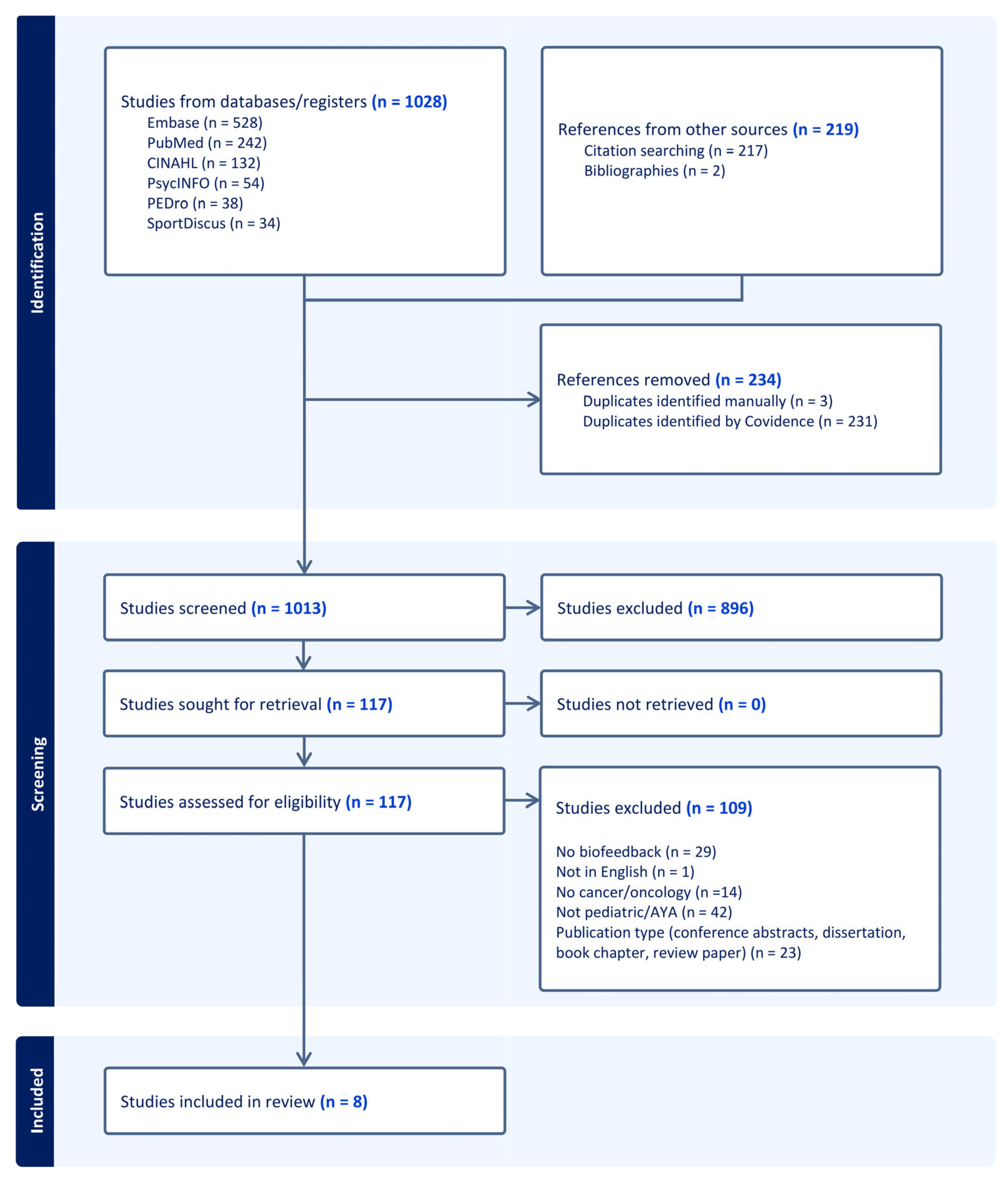
2.1. Literature Search
2.2. Study Inclusion Criteria
2.3. Study Selection and Characteristics
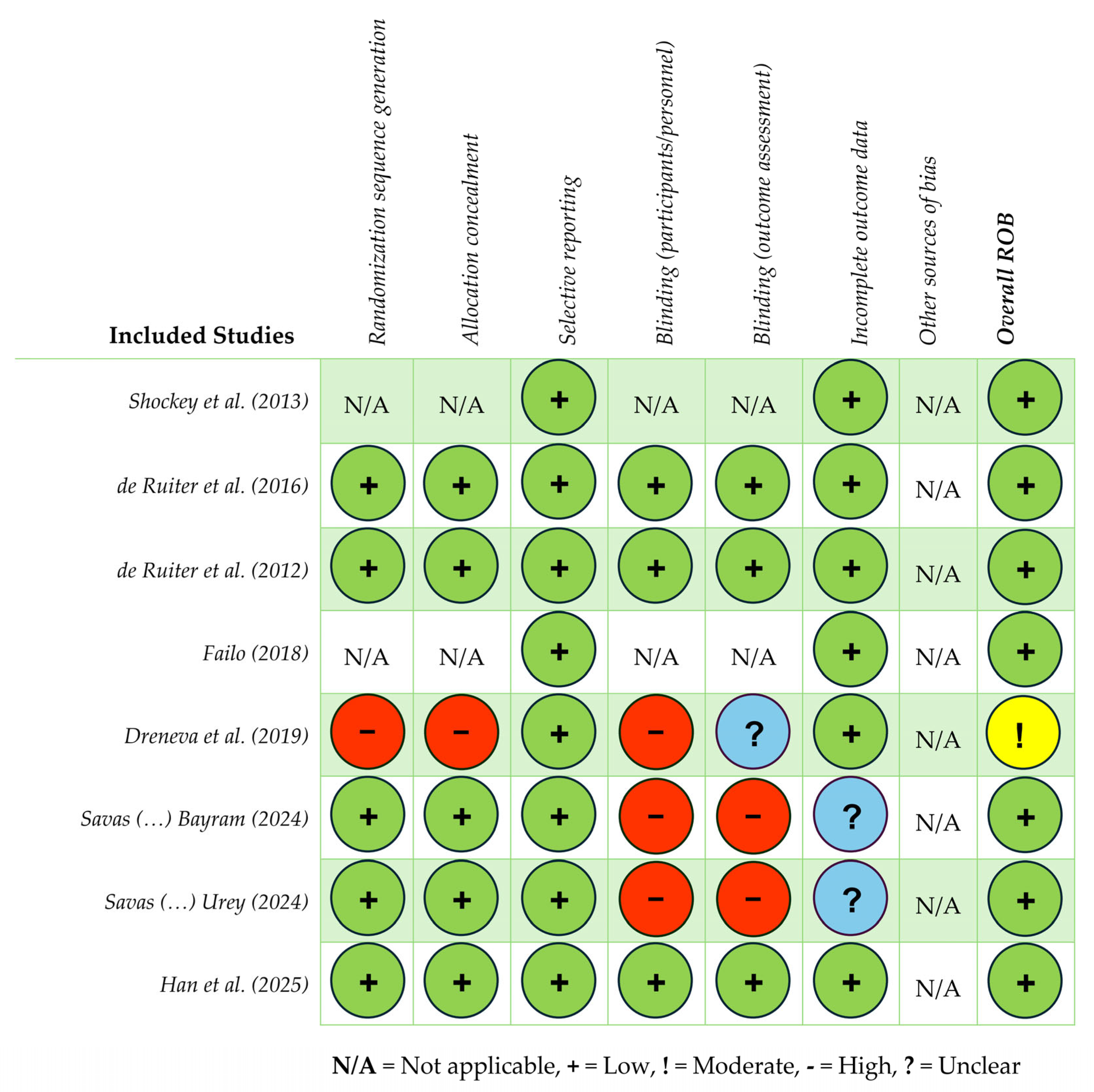
3. Results
| Authors (Year), Country | Study Design | Eligibility Criteria | Sample Characteristics | Aims | Biofeedback (BF) Modalities and Measurements | Results | Limitations | Clinical Implications/ Future Research |
|---|---|---|---|---|---|---|---|---|
| de Ruiter et al. (2016) [65] The Netherlands | Double-blind parallel placebo-controlled design. Groups: (1) NF, (2) PF. Time Points: (T0) Pre-training, (T1) post-training, (T2) 6 mos post-training. | Patients 8–18 yrs old, treated for brain tumor > 2 yrs prior. Experiencing caregiver-reported neurocognitive complaints. | N = 82 (M age = 13.9 yrs, SD = 3.2; 49% male). Groups: • NF: n = 40. • PF: n = 40. • Refused qEEG: n = 2. • Completed training and included in analysis: n = 71. | (1) Assess the efficacy of NF in improving cognitive function among PBTSs. (2) Evaluate the impact of NF on neurocognitive outcomes (e.g., attention, memory, and executive functioning). (3) Compare outcomes of NF group with PF group to determine the specific effects of NF. (4) Provide evidence-based recommendations for the use of NF among PBTSs. | NF EEG | There were no specific treatment effects of NF on neurocognitive functioning. Both groups: PBTSs improved over time on the majority of primary and some secondary outcome measures, with small-to-medium effects. NF does not have favorable effects on neurocognitive and psychosocial functioning in PBTSs as compared with PF. | Participants selected based on parent-reported concerns, not objective measures. | Implications: Healthcare providers may need to focus on individualized care plans for PBTSs, given the lack of NF effectiveness in the study. Research: Explore alternate, more effective interventions or therapies for PBTSs for improving specific outcomes. Develop and test interventions to improve neurocognitive functioning in PBTSs that minimize the risk of medication side effects, using double-blind, randomized, placebo-controlled trials, including a healthy control group and/or other disease groups. Examine the impact of more targeted BF modalities (e.g., HRV) on PBTS neurocognitive outcomes. |
| De Ruiter et al. (2012) [66] The Netherlands | Randomized placebo-controlled double-blind trial. Groups: (1a) Experimental group, receiving NF, (1b) placebo group, receiving placebo training, (2) control group (healthy siblings). Time Points: (T0) Pre-training, (T1) post-training, (T2) 6 mos post-training. | Patients 8–18 yrs old, treated for brain tumor >2 yrs prior. Experiencing caregiver-reported neurocognitive complaints. Control: Siblings, 8–18 yrs old. | Total sample: N = 70. Groups: • NF group: n = 35. • Placebo group: n = 35. • Matched control group: n = 35. | (1) Describe the protocol of the PRISMA study, an RCT to investigate the efficacy of NF to improve neurocognitive functioning among PBTSs. | NF qEEG Assessments: Neurocognitive testing, patient/caregiver/teacher reports, qEEG, portable Brainquiry PET NF device. | The study reports the PRISMA intervention methods (study design, participants, intervention, randomization) and procedure (randomization, assessment, power calculation, statistical analyses). | Small sample size. Heterogeneous brain tumor patients (tumor diagnosis, tumor location, age at diagnosis, treatment, time since diagnosis, time since treatment ended). 5 yr survival rates of approximately 65%, some patients may relapse or discontinue participation. | Implications: If proven effective, NF could be a valuable addition to current interventions for PBTSs to address common deficits. Automatic threshold adjustment makes NF more feasible in clinical settings and training without active brain monitoring. Research: Future studies should test NF feasibility in home/school settings and investigate automated systems further. Explore ways to integrate automated-threshold NF protocols in routine clinical or school-based interventions, and compare outcomes with traditional monitoring protocols. |
| Dreneva et al. (2020) [68] Russia | Prospective quasi-RCT. Groups: (1) Intervention (BF training and usual activities), (2) waitlist (usual activities). Time Points: (1) Baseline: Days 1–3 after arrival, pre-training. (2) Wk 2: Group A post training, Group B midpoint. (3) Wk 4: Group A 2 wk follow-up, Group B post-training. | Patients <18 yrs old; survivors of PFT and healthy controls (siblings). | Total sample: N = 60. Groups: Intervention: n = 35 (M age = 11.4 yrs, SD = 3.5; 23 female). Completed intervention: n = 28. Control: n = 25 (M age = 12.6 yrs, SD = 2.1; 14 female). | (1) Quantify the postural control state in PFT survivors. (2) Compare PFT survivor stabilometric measurements (postural balance) with healthy controls. (3) Evaluate BF training effects on postural balance enhancement. | Intervention group: BF 6-session training program; used visual feedback about their center-of-pressure location. Stabilometric test measures: Ellipse area, Ellipse Square, mean velocity of CoP, mean root square oscillations in frontal and sagittal planes. | Intervention group: Some change was observed in stability parameters in the 2nd test compared to baseline. Ellipse Square parameter results: there was a substantial decrease in the open-eye condition, indicating improvement in postural stability (positive medium-sized effect). Mean root square oscillation results: this parameter decreased dramatically in the frontal and sagittal planes post-training (medium-sized effects). Both open- and closed-eye conditions were similar. Control group: No significant positive change was observed with open and closed eyes. | Not pure control group. Small sample size. Large age variation in participants. Short duration of training program. Limited and small recruitment pool from one rehabilitation center. Potential influence of disease and treatment on postural impairments, making it difficult to isolate specific contributing factors to balance. | Implications: Short-term (2 wk) rehab is insufficient for balance issues in PFT survivors; extended programs (8–12 wks) with BF can enhance postural control strategies. Visual sensory system interference must be considered in therapy plans for balance. Apply the stabilometric method in clinical practice for both diagnostics and rehabilitation to track postural improvements; explore its use for monitoring subtle changes in stability over time. Research: Refine and validate the stabilometric method, explore proprioceptive and visual sensory system roles in balance, and ensure reliability and utility in clinical diagnostics and rehabilitation. Validate and refine the BF rehabilitation program for broader application and assess longitudinal outcomes. |
| Shockey et al. (2013) [64] U.S.A. | Feasibility study. 1-group, non-randomized, repeat-measures design. Time Points: (Sessions 1–4): Pre/post-assessment at each session (60 min). | Children and AYAs undergoing active cancer treatment. | Total sample: N = 12 (M age = 11.0 yrs; ages 8–14; 42% female). Treatment status: • Recently diagnosed: 75%. • Relapsed: 25%. | (1) Assess the feasibility and potential benefits of a 4-session combined relaxation and BF intervention that aims to alleviate procedural distress and increase self-regulation during medical procedures. | HRV via HeartMath emWave System (30 min) for computer with finger sensor in 3/4 sessions; baseline HR and RR in 4/4 sessions. HRV measured pre/post each intervention session using HeartMath emWave System (during sessions 2–4); pre/post-intervention RR and HR measurements for all 4 sessions. | Responses to the parameters and requirements of the study were positive, reported to be well received. State anxiety scores evidenced decreased state anxiety across all sessions, specifically when comparing Session 1 to Session 4. There was improvement in coherence (HRV) scores to a significant degree in Sessions 3- 4. The combination of belly breathing and BF techniques allowed participants to feel in charge of their bodies before their procedures (81% of participants). | Small sample size. No control group. Investigator present at each session providing extra attention to both child and parent during interaction. Reporting discrepancy identified between FACES scale modification and pre-intervention coherence scores. Poor follow-through with homework. | Implications: A combined BF and relaxation protocol is feasible for integration into pediatric oncology care and may reduce pre-procedural distress and enhance coping skills and emotional regulation. Research: More robust evidence is needed to support the protocol’s efficacy and generalizability, including comparing to a control group and assessing the dose and timing of sessions. Replicate with a control group, expand the sample size, lengthen the intervention (6 vs. 4 sessions), and include follow-up sessions to assess maintenance through coherence measurements. Test with other pediatric illness-specific populations (e.g., sickle cell disease), or add caregiver sessions to increase the utilization of tools and homework engagement. |
| Failo et al. (2018) [67] Italy | Single-case report. Time Points: (Sessions 1–4): Baseline/post. (Sessions 1, 4): Interviews. | Adolescent patient, undergoing active cancer treatment. | N = 1. Adolescent male diagnosed with ALL. | (1) Evaluate and illustrate a BF protocol (utilizing BF-Assisted Relaxation Training (BART)) integrated into multidisciplinary care, in a format that is acceptable and within a specific treatment time frame. (2) Session Aims: Increase awareness and control of physiological self-regulation to improve pain-related anxiety during oncological invasive procedures and treatments. | Combining relaxation training and practice (deep breathing and mini-PMR) with psychoeducation about pain mechanisms. Modalities: Abdominal (diaphragmatic) breathing exercises, resonance frequency HR and breathing synchronization, breathing app, sEMG training, PMR, HRV, sEMG. BF device: ProComp5 Infiniti System w/Bio Graph Infiniti Software-T7525 (no software version number provided). | The study suggests that BF training may improve emotional regulation due to one’s ability to manage and adapt physiological arousal in response to situational demands during oncological treatment. Outcomes: significant differences were observed between sEMG and HRV measures; RSA was increased; there was an overall increased balance between sympathetic and parasympathetic systems; and there was an increase in awareness about muscle tension and the ability to stimulate blood flow. These results could effectively reflect a decrease in negative psychological symptoms related to pain, suggesting an ability to reframe personal pain perception. | Single-case study. Limited generalizability in outcomes and oncology treatment types. Not manualized. Conducted in Italy with potentially different medical system and treatment model than other countries. | Implications: BF may enhance emotional regulation and pain reappraisal due to an increased ability to manage and adapt physiological arousal in response to situational demands during treatment. Knowledge gained from even one BF session can be used to reduce negative physiological arousal. Caregivers may also benefit from increased physiological awareness to support children during treatment (e.g., through procedures and physical symptoms including pain, anxiety). Research: Evaluate short-term BF impacts on arousal and pain perception; assess transferability to caregivers. Manualize protocol for greater generalizability and dissemination. Assess the efficacy of BF tools in anxiety- and pain-inducing oncology procedures. Educate providers on session modules and exercise, and the use of targeted BF techniques (e.g., breathing, specific muscle-relaxation, HRV tools), oncology-specific situations (e.g., procedures, needles, scans), and physical symptoms (e.g., pain, anxiety). Conduct additional assessments of the outcomes of simple, accessible, and engaging BF tools as a guide and/or coach in the setting of pediatric/AYA anxiety-provoking and painful medical procedures. Utilize a mixed-methods approach to assessing the reconceptualization of anxiety and pain perception after BF utilization and education. |
| Hanzade Savas et al. (2024) [70] Turkey | Feasibility study. 1 group, non-randomized. Time Points: (1) Pre-procedure (1 h pre). (2) Procedure: During procedure, reported pain level. (3) Post-procedure (1 h post). | Patients 6–12 yrs old, treated with chemotherapy. No prior experience with respiratory BF in VR. | Total sample: N = 15 pediatric oncology patients and their parents. Intervention: • M age = 9.66 yrs (SD = 2.10). • Gender: 53.3% male. • M duration of port catheter usage: 4.25 mos (SD = 1.81). | (1) Evaluate the feasibility, safety, acceptability, and preliminary effectiveness of a respiratory-based BF VR game, BioVirtualPed, in reducing procedural pain, anxiety, and fear in pediatric oncology patients undergoing port catheter insertions. | Using VR headset to engage in game that guided slow, deep, diaphragmatic breathing. Aimed to promote emotional regulation and physiological calming during medical procedure. Oculus Quest 2 headset; respiratory data captured using ADXL354 accelerometer. Data integrated with Arduino IDE software (no software version number provided). | The intervention group showed significantly lower post-procedure pain, fear, and anxiety scores compared to the control group (p < 0.001). There were no significant differences in pre-procedure scores (p > 0.05). Mothers reported lower pain and fear levels for their children with higher satisfaction scores (p < 0.001). The intervention group exhibited a significantly lower mean RR during procedures (p < 0.001). Agreement between child and mother ratings on pain, fear, and anxiety was strong across both groups (p < 0.001). | Small sample size and single-site study. No long-term follow-up to assess durability of effects. Lack of physiological measurements beyond RR. Limited diversity in diagnosis and procedure types. Game currently compatible only with specific computer systems and Oculus VR glasses, which are expensive and limit accessibility. Absence of mobile-compatible version restricts daily use and continuous engagement, reducing potential therapeutic impact. | Implications: BioVirtualPed may reduce procedural pain, anxiety, and fear in pediatric oncology patients by promoting emotional regulation through respiratory BF. Intervention is feasible and acceptable for use during needle-related procedures and requires minimal provider effort, making it a scalable tool in routine care. Research: Evaluate effectiveness across larger and more diverse populations. Explore integration into other pediatric procedures. Assess caregiver involvement to support family-centered care. Conduct RCTs with broader samples, develop and assess mobile-compatible versions for increased accessibility, expand applicability to other medical procedures, and investigate long-term outcomes. |
| Hanzade Savas et al. (2024) [69] Turkey | Randomized controlled study. Groups: Intervention group wore VR headset and respiratory sensor during needle insertion. Control: Standard of care. Time Points: (1) Pre-procedure (1 h before), (2) during procedure, (3) post-procedure (~3 min after port insertion). | Patients 6–12 yrs old and mothers; with experience inserting port catheter needle ≥1 time. | Total sample: N = 62. Groups: Child group: • Intervention: n = 31 (M age = 9.33 yrs, SD = 2.08). • Control: n = 31 (M age = 9.74 yrs, SD = 1.76). Mother group: • Intervention: M age = 39.22 yrs (SD = 4.47). • Control group: M age = 39.29 yrs (SD = 3.39). | (1) Does using the BioVirtualPed during port catheter needle insertion reduce the level of procedure-related pain, anxiety, and fear in children? (2) Does using BioVirtualPed during port catheter needle insertion affect RR during the procedure? (3) Does using the BioVirtualPed during port catheter needle insertion increase procedure-related satisfaction? | RR obtained using ADXL354 sensor during breathing exercises on BioVirtualPed VR game. Sensor features: Low noise density, high sensitivity, programmable digital high- and low-pass filters. In BioVirtualPed, participation in game realized through feedback provided by breathing. ADXL354 sensor used in intervention group to obtain respiratory data that would enable interaction with game. Cardiorespiratory signal from ADXL354 sensor: Mean RR per min during needle insertion. | The intervention group showed lower mean pain scores than the control group (p < 0.001). Intervention-group mothers reported significantly lower mean pain scores for their child’s needle insertion compared to the control group. There was no difference in pre-procedure fear and anxiety scores between groups (p > 0.05 and p > 0.05, respectively). Post-procedure fear and anxiety scores were lower in the intervention group (p < 0.001 and p < 0.001, respectively). Mothers’ post-procedure fear and anxiety scores were lower in the intervention group with a large effect size (p < 0.001, d = 1.714 and p < 0.001, d = 1.907, respectively). The intervention-group mean RR was lower (p < 0.001) and satisfaction scores were higher (p < 0.001) during needle insertion. Agreement between children and mothers on pain, fear, and anxiety scores was good and excellent across groups (p < 0.001). | No blinding of participant and researcher. Interaction between patients themselves between groups not controllable. Participants with varied experiences (e.g., number of prior needle insertions, healthcare professional performing insertion). | Implications: BioVirtualPed showed promising results. Respiratory BF using a VR game format may be feasible and effective and shows potential for reducing pain, anxiety, and fear during procedures in pediatric patients, while improving physiological outcomes by regulating respiration (respiratory rate). It also improves caregiver satisfaction, making it a holistic tool for procedural support. Research: Further validation is needed across treatment centers to determine generalizability. Explore the mechanisms underlying the emotional and physiological benefits of the VR-based respiratory feedback system. Assess feasibility and outcomes across diverse treatment settings. Expand dissemination and provider education on the use of respiratory BF for other oncology-related procedures. |
| Han et al. (2025) [71] China | Two-group, randomized controlled study. Groups: Both groups: Received health education. Intervention: Received 4-unit BF intervention. Time points: (T0) Baseline, (T1) post-intervention, (T2) 4 wk follow-up. | Children: Aged 6–18 yrs, diagnosed with ALL, currently receiving chemotherapy, completed ≥1 invasive procedures per chemotherapy cycle. Able to communicate and express their emotions. Caregiver: ≥18 yrs old, primary caregiver, access to mobile phone/internet, possessing basic reading and writing skills. | Total sample: N = 80 child–caregiver dyads (40 per group). Groups: Intervention (n = 40): • M age children = 9.20 yrs (SD = 3.04). • M age caregivers = 38.63 yrs (SD = 8.60). Control (n = 40): • M age children = 9.25 yrs (SD = 3.25). • M age caregivers = 39.08 yrs (SD = 6.17). | (1) Assess the effectiveness of a structured BF intervention in alleviating pain, fear, depression, and anxiety related to invasive procedures in children and caregivers with ALL. (2) Improve sleep quality in both children and caregivers. | Intelligent Body and Mind training system 4.0, which integrates HR, respiration, and galvanic skin response. Intervention: 4 structured units: music relaxation, deep breathing training, imaginative relaxation, computer game activity. | The intervention group reported significantly lower post-procedure pain, fear, and worry scores compared to the control group (p < 0.001). There were no significant differences in pre-procedure pain, fear, or worry between groups (p > 0.05). Caregivers in the intervention showed significantly lower post-intervention anxiety and improved sleep quality compared to controls (p < 0.05). No significant differences were found in caregiver depression scores or children’s sleep quality between groups (p > 0.05). | Small sample size, limited generalizability due to majority of caregivers being parents, exclusion of younger children (0–6 yrs) and other leukemia types. | Implications: BF provides a non-pharmacological approach to managing pain, fear, and negative emotions triggered by invasive procedures in pediatric oncology. Incorporating BF into routine care can improve children’s procedural compliance and emotional regulation. For caregivers, reduced anxiety and improved sleep quality enhance overall well-being and caregiving ability, indirectly benefiting the child’s experience and recovery. Research: Explore the optimization of the intervention, including ideal session frequency and duration, and assess long-term outcomes in both children and caregivers. Broader studies could also evaluate generalizability across pediatric populations (e.g., different diseases) and settings. Conduct larger, multicenter RCTs with longer-term follow-up. Use objective sleep and emotional regulation measures to enhance the validity of findings. |
4. Discussion
4.1. Future Research
4.2. Clinical Implications
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AYA | Adolescent and young adult |
| ALL | Acute lymphocytic leukemia |
| AAPB | Association for Applied Psychophysiology and Biofeedback |
| BF | Biofeedback |
| BCIA | Biofeedback Certification International Alliance |
| EEG | Electroencephalography |
| EMG | Electromyography |
| HR | Heart rate |
| HRV | Heart rate variability |
| ISNR | International Society for Neurofeedback and Research |
| mos | month |
| NF | Neurofeedback |
| PBTS | Pediatric brain tumor survivor |
| PF | Placebo feedback |
| PFT | Posterior fossa tumor |
| PMR | Progressive muscle relaxation |
| PTSD | Post-traumatic stress disorder |
| ROB | Risk of bias |
| sEMG | Surface electromyography |
| VR | Virtual reality |
| wk | week |
| yr | year |
References
- Hooke, M.C.; Linder, L.A. Symptoms in Children Receiving Treatment for Cancer-Part I: Fatigue, Sleep Disturbance, and Nausea/Vomiting. J. Pediatr. Oncol. Nurs. 2019, 36, 244–261. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.A.; Salley, C.G.; Barnett, M.; DeRosa, A.P.; Werk, R.S.; Hourani, A.; Hoekstra, A.B.; Ford, J.S. Anxiety Among Adolescent Survivors of Pediatric Cancer. J. Adolesc. Health 2017, 61, 409–423. [Google Scholar] [CrossRef] [PubMed]
- van Warmerdam, J.; Zabih, V.; Kurdyak, P.; Sutradhar, R.; Nathan, P.C.; Gupta, S. Prevalence of anxiety, depression, and posttraumatic stress disorder in parents of children with cancer: A meta-analysis. Pediatr. Blood Cancer 2019, 66, e27677. [Google Scholar] [CrossRef] [PubMed]
- Fournié, C.; Chouchou, F.; Dalleau, G.; Caderby, T.; Cabrera, Q.; Verkindt, C. Heart rate variability biofeedback in chronic disease management: A systematic review. Complement. Ther. Med. 2021, 60, 102750. [Google Scholar] [CrossRef]
- Lehrer, P.; Kaur, K.; Sharma, A.; Shah, K.; Huseby, R.; Bhavsar, J.; Sgobba, P.; Zhang, Y. Heart Rate Variability Biofeedback Improves Emotional and Physical Health and Performance: A Systematic Review and Meta Analysis. Appl. Psychophysiol. Biofeedback 2020, 45, 109–129. [Google Scholar] [CrossRef]
- Darling, K.E.; Benore, E.R.; Webster, E.E. Biofeedback in pediatric populations: A systematic review and meta-analysis of treatment outcomes. Transl. Behav. Med. 2020, 10, 1436–1449. [Google Scholar] [CrossRef]
- Taylor, M.R.; Knight, J.M.; Rosenberg, A.R. The biology of stress in cancer: Applying the biobehavioral framework to adolescent and young adult oncology research. Brain Behav. Immun. Health 2021, 17, 100321. [Google Scholar] [CrossRef]
- Schwartz, M.S.; Andrasik, F. Biofeedback: A Practitioner’s Guide, 4th ed.; The Guilford Press: New York, NY, USA, 2016. [Google Scholar]
- Billman, G.E.; Huikuri, H.V.; Sacha, J.; Trimmel, K. An introduction to heart rate variability: Methodological considerations and clinical applications. Front. Physiol. 2015, 6, 55. [Google Scholar] [CrossRef]
- Ali, M.K.; Chen, J.D.Z. Roles of Heart Rate Variability in Assessing Autonomic Nervous System in Functional Gastrointestinal Disorders: A Systematic Review. Diagnostics 2023, 13, 293. [Google Scholar] [CrossRef]
- Ge, F.; Yuan, M.; Li, Y.; Zhang, W. Posttraumatic Stress Disorder and Alterations in Resting Heart Rate Variability: A Systematic Review and Meta-Analysis. Psychiatry Investig. 2020, 17, 9–20. [Google Scholar] [CrossRef]
- Koch, C.; Wilhelm, M.; Salzmann, S.; Rief, W.; Euteneuer, F. A meta-analysis of heart rate variability in major depression. Psychol. Med. 2019, 49, 1948–1957. [Google Scholar] [CrossRef]
- Sharma, M.; Rajput, J.S.; Tan, R.S.; Acharya, U.R. Automated Detection of Hypertension Using Physiological Signals: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5838. [Google Scholar] [CrossRef]
- Tracy, L.M.; Ioannou, L.; Baker, K.S.; Gibson, S.J.; Georgiou-Karistianis, N.; Giummarra, M.J. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 2016, 157, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, Y.; Zhang, Y.; Chen, L.; Zou, Y.; Xiao, J.; Min, W.; Yuan, C.; Ye, Y.; Li, M.; et al. Heart rate variability in generalized anxiety disorder, major depressive disorder and panic disorder: A network meta-analysis and systematic review. J. Affect. Disord. 2023, 330, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kloter, E.; Barrueto, K.; Klein, S.D.; Scholkmann, F.; Wolf, U. Heart Rate Variability as a Prognostic Factor for Cancer Survival—A Systematic Review. Front. Physiol. 2018, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ma, Z.; Zhang, L.; Zhou, S.; Wang, J.; Wang, B.; Fu, W. Heart rate variability in the prediction of survival in patients with cancer: A systematic review and meta-analysis. J. Psychosom. Res. 2016, 89, 20–25. [Google Scholar] [CrossRef]
- Madhusudhan, D.K.; Glied, K.N.; Nguyen, E.; Rose, J.; Bravata, D.M. Real-world evaluation of a novel technology-enabled capnometry-assisted breathing therapy for panic disorder. J. Ment. Health Clin. Psychol. 2020, 4, 39–46. [Google Scholar] [CrossRef]
- Meuret, A.E.; Rosenfield, D.; Millard, M.M.; Ritz, T. Biofeedback Training to Increase P co2 in Asthma With Elevated Anxiety: A One-Stop Treatment of Both Conditions? Psychosom. Med. 2023, 85, 440–448. [Google Scholar] [CrossRef]
- Tolin, D.F.; McGrath, P.B.; Hale, L.R.; Weiner, D.N.; Gueorguieva, R. A Multisite Benchmarking Trial of Capnometry Guided Respiratory Intervention for Panic Disorder in Naturalistic Treatment Settings. Appl. Psychophysiol. Biofeedback 2017, 42, 51–58. [Google Scholar] [CrossRef]
- Martino Cinnera, A.; Morone, G.; Bisirri, A.; Lucenti, T.; Rotundo, M.; Monaci, S.; Berton, C.; Paoluzzi, M.; Iosa, M.; Ciancarelli, I. Headaches treatment with EMG biofeedback: A focused systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2023, 59, 697–705. [Google Scholar] [CrossRef]
- Nestoriuc, Y.; Rief, W.; Martin, A. Meta-analysis of biofeedback for tension-type headache: Efficacy, specificity, and treatment moderators. J. Consult. Clin. Psychol. 2008, 76, 379–396. [Google Scholar] [CrossRef]
- Sielski, R.; Rief, W.; Glombiewski, J.A. Efficacy of Biofeedback in Chronic back Pain: A Meta-Analysis. Int. J. Behav. Med. 2017, 24, 25–41. [Google Scholar] [CrossRef]
- Novak, P. Electrochemical skin conductance: A systematic review. Clin. Auton. Res. 2019, 29, 17–29. [Google Scholar] [CrossRef]
- Yu, B.; Funk, M.; Hu, J.; Wang, Q.; Feijs, L. Biofeedback for everyday stress management: A systematic review. Front. ICT 2018, 5, 23. [Google Scholar] [CrossRef]
- Freedman, R.R. Physiological mechanisms of temperature biofeedback. Biofeedback Self Regul. 1991, 16, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Nestoriuc, Y.; Martin, A. Efficacy of biofeedback for migraine: A meta-analysis. Pain 2007, 128, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Nigro, S.E. The Efficacy of Neurofeedback for Pediatric Epilepsy. Appl. Psychophysiol. Biofeedback 2019, 44, 285–290. [Google Scholar] [CrossRef]
- Van Doren, J.; Arns, M.; Heinrich, H.; Vollebregt, M.A.; Strehl, U.; Loo, S.K. Sustained effects of neurofeedback in ADHD: A systematic review and meta-analysis. Eur. Child. Adolesc. Psychiatry 2019, 28, 293–305. [Google Scholar] [CrossRef]
- Calderone, A.; Mazzurco Masi, V.M.; De Luca, R.; Gangemi, A.; Bonanno, M.; Floridia, D.; Corallo, F.; Morone, G.; Quartarone, A.; Maggio, M.G.; et al. The impact of biofeedback in enhancing chronic pain rehabilitation: A systematic review of mechanisms and outcomes. Heliyon 2025, 11, e41917. [Google Scholar] [CrossRef]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef]
- Jenkins, S.; Cross, A.; Osman, H.; Salim, F.; Lane, D.; Bernieh, D.; Khunti, K.; Gupta, P. Effectiveness of biofeedback on blood pressure in patients with hypertension: Systematic review and meta-analysis. J. Hum. Hypertens. 2024, 38, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kenemore, J.; Benham, G.; Charak, R.; Hernandez Rodriguez, J. Heart Rate Variability Biofeedback as a Treatment for Military PTSD: A Meta-Analysis. Mil. Med. 2024, 189, e1903–e1909. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Cho, E.Y.; Kim, S.M.; Yoon, S. Efficacy of psychological treatment for headache disorder: A systematic review and meta-analysis. J. Headache Pain. 2019, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Jones, C.I.; Sen, A. Galvanic Skin Response (GSR)/Electrodermal/Skin Conductance Biofeedback on Epilepsy: A Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Sah, A. Efficacy of biofeedback for migraine: A systematic review and meta-analysis. Complement. Ther. Med. 2025, 90, 103153. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fu, L.; Xu, W.; Gharibans, A.A.; O’Grady, G. The effects of heart rate variability biofeedback on functional gastrointestinal disorders: A scoping review. Front. Physiol. 2025, 16, 1511391. [Google Scholar] [CrossRef]
- Pizzoli, S.F.M.; Marzorati, C.; Gatti, D.; Monzani, D.; Mazzocco, K.; Pravettoni, G. A meta-analysis on heart rate variability biofeedback and depressive symptoms. Sci. Rep. 2021, 11, 6650. [Google Scholar] [CrossRef]
- Caldwell, Y.T.; Steffen, P.R. Adding HRV biofeedback to psychotherapy increases heart rate variability and improves the treatment of major depressive disorder. Int. J. Psychophysiol. 2018, 131, 96–101. [Google Scholar] [CrossRef]
- Malik, K.; Dua, A. Advancing Patient Care With Biofeedback. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Reneau, M. Heart Rate Variability Biofeedback to Treat Fibromyalgia: An Integrative Literature Review. Pain. Manag. Nurs. 2020, 21, 225–232. [Google Scholar] [CrossRef]
- Windthorst, P.; Mazurak, N.; Kuske, M.; Hipp, A.; Giel, K.E.; Enck, P.; Nieß, A.; Zipfel, S.; Teufel, M. Heart rate variability biofeedback therapy and graded exercise training in management of chronic fatigue syndrome: An exploratory pilot study. J. Psychosom. Res. 2017, 93, 6–13. [Google Scholar] [CrossRef]
- Addleman, J.S.; Lackey, N.S.; Tobin, M.A.; Lara, G.A.; Sinha, S.; Morse, R.M.; Hajduczok, A.G.; Gharbo, R.S.; Gevirtz, R.N. Heart Rate Variability Applications in Medical Specialties: A Narrative Review. Appl. Psychophysiol. Biofeedback 2025. [Google Scholar] [CrossRef]
- Burch, J.B.; Ginsberg, J.P.; McLain, A.C.; Franco, R.; Stokes, S.; Susko, K.; Hendry, W.; Crowley, E.; Christ, A.; Hanna, J.; et al. Symptom Management Among Cancer Survivors: Randomized Pilot Intervention Trial of Heart Rate Variability Biofeedback. Appl. Psychophysiol. Biofeedback 2020, 45, 99–108. [Google Scholar] [CrossRef]
- Hasuo, H.; Kanbara, K.; Shizuma, H.; Morita, Y.; Fukunaga, M. Short-term efficacy of home-based heart rate variability biofeedback on sleep disturbance in patients with incurable cancer: A randomised open-label study. BMJ Support. Palliat. Care 2023, 13, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Spada, G.E.; Masiero, M.; Pizzoli, S.F.M.; Pravettoni, G. Heart Rate Variability Biofeedback in Cancer Patients: A Scoping Review. Behav. Sci. 2022, 12, 389. [Google Scholar] [CrossRef]
- Greenberg, B.R.; Grossman, E.F.; Bolwell, G.; Reynard, A.K.; Pennell, N.A.; Moravec, C.S.; McKee, M.G. Biofeedback Assisted Stress Management in Patients with Lung Cancer: A Feasibility Study. Appl. Psychophysiol. Biofeedback 2015, 40, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Makvand-Hosseini, S.; Nazemi, H.; Hoseini, S. Biofeedback-Assisted Relaxation Therapy on Women with Breast Cancer after Mastectomy: Effects of EMG-HRV Biofeedback on Psychological Symptoms. Int. J. Behav. Sci. 2020, 13, 147–152. [Google Scholar]
- Sumneangsanor, T.; Ruchiwit, M.; Weglicki, L. The effects of a biofeedback and music training programme in reducing stress in Thai patients living with cancer receiving palliative care. Int. J. Palliat. Nurs. 2022, 28, 453–463. [Google Scholar] [CrossRef]
- Tsai, P.S.; Chen, P.L.; Lai, Y.L.; Lee, M.B.; Lin, C.C. Effects of electromyography biofeedback-assisted relaxation on pain in patients with advanced cancer in a palliative care unit. Cancer Nurs. 2007, 30, 347–353. [Google Scholar] [CrossRef]
- Masafi, S.; Rezaei, O.; Ahadi, H. Efficacy of Biofeedback Associated with Relaxation in Decreasing Anxiety in Women with Breast Cancer During Chemotherapy. Procedia—Soc. Behav. Sci. 2011, 30, 143–148. [Google Scholar] [CrossRef]
- Zhao, E.; Li, Z.; Zhang, J.; Li, B.; He, J.; Liu, H.; Wang, J. The Effect of Electroencephalographic Biofeedback Therapy on Anxiety and Overall Well-being in Patients with Rectal Cancer. Appl. Psychophysiol. Biofeedback 2025. [Google Scholar] [CrossRef]
- Bhatia, S.; Pappo, A.S.; Acquazzino, M.; Allen-Rhoades, W.A.; Barnett, M.; Borinstein, S.C.; Casey, R.; Choo, S.; Chugh, R.; Dinner, S.; et al. Adolescent and Young Adult (AYA) Oncology, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2023, 21, 851–880. [Google Scholar] [CrossRef]
- McKenna, K.; Gallagher, K.A.; Forbes, P.W.; Ibeziako, P. Ready, set, relax: Biofeedback-assisted relaxation training (BART) in a pediatric psychiatry consultation service. Psychosomatics 2015, 56, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, I.; Cano-Crespo, A.; Rivera, F. Results of Neurofeedback in Treatment of Children with ADHD: A Systematic Review of Randomized Controlled Trials. Appl. Psychophysiol. Biofeedback 2022, 47, 145–181. [Google Scholar] [CrossRef] [PubMed]
- Stubberud, A.; Varkey, E.; McCrory, D.C.; Pedersen, S.A.; Linde, M. Biofeedback as Prophylaxis for Pediatric Migraine: A Meta-analysis. Pediatrics 2016, 138, e20160675. [Google Scholar] [CrossRef] [PubMed]
- Yetwin, A.K.; Mahrer, N.E.; Bell, T.S.; Gold, J.I. Heart Rate Variability biofeedback therapy for children and adolescents with chronic pain: A pilot study. J. Pediatr. Nurs. 2022, 66, 151–159. [Google Scholar] [CrossRef]
- Dormal, V.; Vermeulen, N.; Mejias, S. Is heart rate variability biofeedback useful in children and adolescents? A systematic review. J. Child. Psychol. Psychiatry 2021, 62, 1379–1390. [Google Scholar] [CrossRef]
- Thabrew, H.; Ruppeldt, P.; Sollers, J.J., 3rd. Systematic Review of Biofeedback Interventions for Addressing Anxiety and Depression in Children and Adolescents with Long-Term Physical Conditions. Appl. Psychophysiol. Biofeedback 2018, 43, 179–192. [Google Scholar] [CrossRef]
- Sonne, T.; Merritt, T.; Marshall, P.; Lomholt, J.J.; Müller, J.; Grønbæk, K. Calming children when drawing blood using breath-based biofeedback. In DIS 2017, Proceedings of the 2017 ACM Conference on Designing Interactive Systems, Edinburgh, United Kingdom, 10–14 June 2017; ACM: New York, NY, USA, 2017; pp. 725–737. [Google Scholar] [CrossRef]
- Leclercq, E.; Leeflang, M.M.; van Dalen, E.C.; Kremer, L.C. Validation of search filters for identifying pediatric studies in PubMed. J. Pediatr. 2013, 162, 629–634.e622. [Google Scholar] [CrossRef]
- Tessier, V.; Lacourse, M.; Canadian Health Libraries Association. Adolescents and Young Adults (Search Filter, Age Specific). Available online: https://extranet.santecom.qc.ca/wiki/!biblio3s/doku.php?id=concepts:adolescents-et-jeunes-adultes (accessed on 6 May 2025).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 2011, 343, d5928. [Google Scholar] [CrossRef]
- Shockey, D.P.; Menzies, V.; Glick, D.F.; Taylor, A.G.; Boitnott, A.; Rovnyak, V. Preprocedural distress in children with cancer: An intervention using biofeedback and relaxation. J. Pediatr. Oncol. Nurs. 2013, 30, 129–138. [Google Scholar] [CrossRef]
- de Ruiter, M.A.; Oosterlaan, J.; Schouten-van Meeteren, A.Y.; Maurice-Stam, H.; van Vuurden, D.G.; Gidding, C.; Beek, L.R.; Granzen, B.; Caron, H.N.; Grootenhuis, M.A. Neurofeedback ineffective in paediatric brain tumour survivors: Results of a double-blind randomised placebo-controlled trial. Eur. J. Cancer 2016, 64, 62–73. [Google Scholar] [CrossRef]
- de Ruiter, M.A.; Schouten-Van Meeteren, A.Y.; van Mourik, R.; Janssen, T.W.; Greidanus, J.E.; Oosterlaan, J.; Grootenhuis, M.A. Neurofeedback to improve neurocognitive functioning of children treated for a brain tumor: Design of a randomized controlled double-blind trial. BMC Cancer 2012, 12, 581. [Google Scholar] [CrossRef]
- Failo, A. A single case study of biofeedback training in an adolescent with cancer-related pain. J. Pediatr. Neonatal Care 2018, 8, 287–290. [Google Scholar] [CrossRef]
- Dreneva, A.A.; Skvortsov, D.V. Postural balance in pediatric posterior fossa tumor survivors: Through impairments to rehabilitation possibilities. Clin. Biomech. 2020, 71, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Savas, E.H.; Semerci, R.; Bayram, C. The effect of a biofeedback-based virtual reality game on pain, fear and anxiety levels during port catheter needle insertion in pediatric oncology patients: A randomized controlled study. Eur. J. Oncol. Nurs. 2024, 70, 102621. [Google Scholar] [CrossRef] [PubMed]
- Savaş, E.H.; Semerci, R.; Sayın, A.; Dinçer, B.; Semiz, B.; Ürey, H. A Biofeedback Based Virtual Reality Game for Pediatric Population (BioVirtualPed): A Feasibility Trial. Semin. Oncol. Nurs. 2024, 40, 151615. [Google Scholar] [CrossRef]
- Han, J.; Song, H.; Wang, L.; Bi, L.; Yang, F. The Effects of Biofeedback Intervention on Negative Emotions and Sleep Quality in Children With Leukemia Receiving Invasive Procedures and Their Caregivers: A Randomized Controlled Trial. Psychooncology 2025, 34, e70134. [Google Scholar] [CrossRef]
- Ward, Z.J.; Yeh, J.M.; Bhakta, N.; Frazier, A.L.; Atun, R. Estimating the total incidence of global childhood cancer: A simulation-based analysis. Lancet Oncol. 2019, 20, 483–493. [Google Scholar] [CrossRef]
- World Health Organization. Childhood Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer-in-children (accessed on 6 May 2025).
- Li, W.; Liang, H.; Wang, W.; Liu, J.; Liu, X.; Lao, S.; Liang, W.; He, J. Global cancer statistics for adolescents and young adults: Population based study. J. Hematol. Oncol. 2024, 17, 99. [Google Scholar] [CrossRef]
- Linder, L.A.; Hooke, M.C. Symptoms in Children Receiving Treatment for Cancer-Part II: Pain, Sadness, and Symptom Clusters. J. Pediatr. Oncol. Nurs. 2019, 36, 262–279. [Google Scholar] [CrossRef]
- PDQ® Pediatric Treatment Editorial Board. Late Effects of Treatment for Childhood Cancer (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- White, G.E.; Caterini, J.E.; McCann, V.; Rendall, K.; Nathan, P.C.; Rhind, S.G.; Jones, H.; Wells, G.D. The Psychoneuroimmunology of Stress Regulation in Pediatric Cancer Patients. Cancers 2021, 13, 4684. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Seer*Stat Database (SEER 1975–2022): April 2025 Release, Based on the November 2024 Submission. Surveillance Research Program, National Cancer Institute, Washington, DC, USA. Available online: www.seer.cancer.gov (accessed on 24 July 2025).
- Acker, L.; Xu, K.; Ginsberg, J.P. The brain-heart-immune axis: A vago-centric framework for predicting and enhancing resilient recovery in older surgery patients. Bioelectron. Med. 2024, 10, 21. [Google Scholar] [CrossRef]
- Ramakumar, N.; Sama, S. Exploring Heart Rate Variability Biofeedback as a Nonpharmacological Intervention for Enhancing Perioperative Care: A Narrative Review. Turk. J. Anaesthesiol. Reanim. 2024, 52, 125–133. [Google Scholar] [CrossRef]
- Yucha, C.; Montgomery, D.; Association for Applied Psychophysiology Biofeedback (AAPB). Evidence-Based Practice in Biofeedback and Neurofeedback; AAPB: Wheat Ridge, CO, USA, 2008. [Google Scholar]
- Addab, S.; Hamdy, R.; Le May, S.; Thorstad, K.; Tsimicalis, A. The use of virtual reality during medical procedures in a pediatric orthopedic setting: A mixed-methods pilot feasibility study. Paediatr. Neonatal Pain 2024, 6, 45–59. [Google Scholar] [CrossRef]
- Rockstroh, C.; Blum, J.; Göritz, A.S. Virtual reality in the application of heart rate variability biofeedback. Int. J. Hum.-Comput. Stud. 2019, 130, 209–220. [Google Scholar] [CrossRef]
- Samnakay, S.; Bell, E.; Evans, D.; Sommerfield, D.; Sommerfield, A.; Hauser, N.; von Ungern-Sternberg, B.S. Assessing the Use and Acceptability of Virtual Reality to Assist Coping in Children Undergoing Clinical Procedures. J. Spec. Pediatr. Nurs. 2025, 30, e70002. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnett, M.; Langer, S.A.; Matsoukas, K.; Dugad, S.; Mdleleni, A.; Khazan, I. Biofeedback in Pediatric, Adolescent, and Young Adult Cancer Care: A Systematic Review. Children 2025, 12, 998. https://doi.org/10.3390/children12080998
Barnett M, Langer SA, Matsoukas K, Dugad S, Mdleleni A, Khazan I. Biofeedback in Pediatric, Adolescent, and Young Adult Cancer Care: A Systematic Review. Children. 2025; 12(8):998. https://doi.org/10.3390/children12080998
Chicago/Turabian StyleBarnett, Marie, Shari A. Langer, Konstantina Matsoukas, Sanjana Dugad, Anelisa Mdleleni, and Inna Khazan. 2025. "Biofeedback in Pediatric, Adolescent, and Young Adult Cancer Care: A Systematic Review" Children 12, no. 8: 998. https://doi.org/10.3390/children12080998
APA StyleBarnett, M., Langer, S. A., Matsoukas, K., Dugad, S., Mdleleni, A., & Khazan, I. (2025). Biofeedback in Pediatric, Adolescent, and Young Adult Cancer Care: A Systematic Review. Children, 12(8), 998. https://doi.org/10.3390/children12080998