Local Guidance on the Management of Nephropathic Cystinosis in the Gulf Cooperation Council (GCC) Region
Abstract
1. Context
2. Methods
3. Nephropathic Cystinosis in the Gulf Cooperation Council
4. Clinical Management of Nephropathic Cystinosis
4.1. Diagnostic Tests
4.1.1. WBC Cystine Level
4.1.2. Slit-Lamp Examination
4.1.3. CTNS Mutation Assessment
4.2. Cystine-Depleting Therapy
4.2.1. Immediate-Release (IR) Cysteamine
4.2.2. Delayed-Release (DR)-Cysteamine
| KERRYPNX | IR-Cysteamine | DR-Cysteamine |
|---|---|---|
| Composition | Hard capsule containing cysteamine (50 or 150 mg) [31] | Capsule containing individually enteric-coated, pH-sensitive micro-granules of cysteamine (25 or 75 mg) [25,32,33] |
| Mode of delivery | Released into the stomach causing a 3-fold increase in gastric acid production (ulcerogenic) | Released into the small intestine: bypassing stomach [32,33], potentially reducing gastric acid production |
| Dosage | Dosing every 6 h, day and night [31] | Dosing every 12 h |
| Up to 12 years old: 1.3 g/m2/day in four divided doses | Target maintenance dose: 1.3 g/m2/day in two divided doses [32,33,34] | |
| Over 12 years old and >50 kg: 2 g/day in four divided doses | Over 12 years old and >50 kg: 2 g/day in two divided doses | |
| Administration with food | Digestive tolerance improved when taken with or just after food [30] | Taken without food (ideally fast 1 h before and 1 h after dosing) or with a small amount of food (preferably carbohydrate) [34] |
| In children below 6 years, the content of the capsules should be sprinkled on food [31] | In children below 6 years, capsules should be opened, and the content sprinkled on recommended food or drink [34] | |
| Foods such as milk, potatoes, and other starch-based products seem to be appropriate for mixing with the powder [31] | ~100 g of carbohydrates (applesauce/fruit jam) may be mixed with DR-cysteamine granules but frozen, dairy, high-fat, or high protein foods should be avoided [34] | |
| Acidic drinks (e.g., orange juice) should be avoided, as the powder tends not to mix well and may precipitate out [31] | DR-cysteamine may be mixed with 100–150 mL of acidic fruit juice (e.g., orange juice) or water [34] | |
| Adherence | Poor adherence (adolescents and adults) | Better adherence [29] and quality of life [27] |
| Adverse effects | Gastrointestinal complaints, halitosis, and body odor | Less gastrointestinal complaints, halitosis, and body odor Less proton pump inhibitor therapy required [35] |
| Less proton pump inhibitor therapy required [35] |
5. Local Practice in the GCC Region
5.1. Challenges in Nephropathic Cystinosis Management
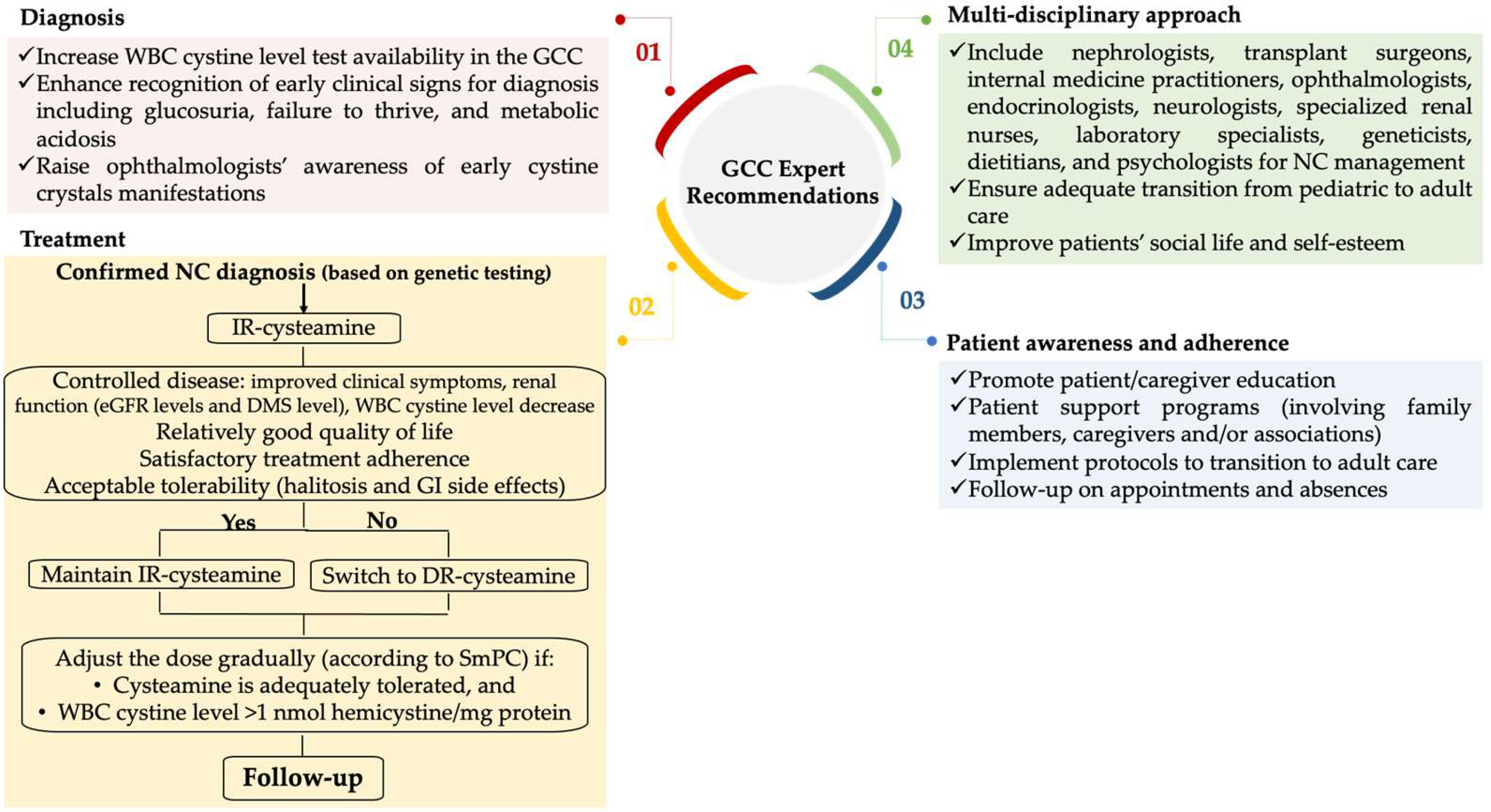
5.2. Expert Recommendations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nesterova, G.; Gahl, W.A. Cystinosis: The evolution of a treatable disease. Pediatr. Nephrol. 2013, 28, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gahl, W.A.; Thoene, J.G.; Schneider, J.A. Cystinosis. N. Engl. J. Med. 2002, 347, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bäumner, S.; Weber, L.T. Nephropathic Cystinosis: Symptoms, Treatment, and Perspectives of a Systemic Disease. Front. Pediatr. 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Emma, F.; Nesterova, G.; Langman, C.; Labbé, A.; Cherqui, S.; Goodyer, P.; Janssen, M.C.; Greco, M.; Topaloglu, R.; Elenberg, E.; et al. Nephropathic cystinosis: An international consensus document. Nephrol. Dial. Transpl. 2014, 29, iv87–iv94. [Google Scholar] [CrossRef]
- Elmonem, M.A.; Veys, K.R.; Soliman, N.A.; van Dyck, M.; van den Heuvel, L.P.; Levtchenko, E. Cystinosis: A review. Orphanet J. Rare Dis. 2016, 11, 47. [Google Scholar] [CrossRef]
- Gahl, W.A.; Kuehl, E.M.; Iwata, F.; Lindblad, A.; Kaiser-Kupfer, M.I. Corneal crystals in nephropathic cystinosis: Natural history and treatment with cysteamine eyedrops. Mol. Genet. Metab. 2000, 71, 100–120. [Google Scholar] [CrossRef]
- Hohenfellner, K.; Rauch, F.; Ariceta, G.; Awan, A.; Bacchetta, J.; Bergmann, C.; Bechtold, S.; Cassidy, N.; Deschenes, G.; Elenberg, E.; et al. Management of bone disease in cystinosis: Statement from an international conference. J. Inherit. Metab. Dis. 2019, 42, 1019–1029. [Google Scholar] [CrossRef]
- Al-Ghanim, K.A. Consanguineous marriage in the Arab societies. J. Psychol. Clin. Psychiatry 2020, 11, 166–168. [Google Scholar] [CrossRef]
- Van Buren, F.; Van Gordon, W. Emirati Women’s Experiences of Consanguineous Marriage: A Qualitative Exploration of Attitudes, Health Challenges, and Coping Styles. Int. J. Ment. Health Addict. 2020, 18, 1113–1127. [Google Scholar] [CrossRef]
- Abdu, Y.; Ahmed, K.; Ibrahim, M.I.M.; Abdou, M.; Ali, A.; Alsiddig, H.; Selim, N.A.; Yassin, M.A. Perception of consanguineous marriage among the qatari population. Front. Public Health 2023, 11, 1228010. [Google Scholar] [CrossRef]
- Besouw, M.T.; Levtchenko, E.N. Improving the prognosis of nephropathic cystinosis. Int. J. Nephrol. Renov. Dis. 2014, 7, 297–302. [Google Scholar] [CrossRef]
- Langman, C.B.; Barshop, B.A.; Deschênes, G.; Emma, F.; Goodyer, P.; Lipkin, G.; Midgley, J.P.; Ottolenghi, C.; Servais, A.; Soliman, N.A.; et al. Controversies and research agenda in nephropathic cystinosis: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016, 89, 1192–1203. [Google Scholar] [CrossRef]
- Wilmer, M.J.; Schoeber, J.P.; Heuvel, L.P.v.D.; Levtchenko, E.N. Cystinosis: Practical tools for diagnosis and treatment. Pediatr. Nephrol. 2011, 26, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, G.; Gahl, W. Nephropathic cystinosis: Late complications of a multisystemic disease. Pediatr. Nephrol. 2008, 23, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.C.; Gangoiti, J.A.; Schneider, J.A.; Barshop, B.A. Time before isolating cystinotic leukocytes affects reliability of cystine determination. Pediatr. Nephrol. 2009, 24, 2465–2466. [Google Scholar] [CrossRef] [PubMed]
- Emma, F.; Hoff, W.V.; Hohenfellner, K.; Topaloglu, R.; Greco, M.; Ariceta, G.; Bettini, C.; Bockenhauer, D.; Veys, K.; Pape, L.; et al. An international cohort study spanning five decades assessed outcomes of nephropathic cystinosis. Kidney Int. 2021, 100, 1112–1123. [Google Scholar] [CrossRef]
- Ariceta, G.; Camacho, J.A.; Fernández-Obispo, M.; Fernández-Polo, A.; Gamez, J.; García-Villoria, J.; Monteczuma, E.L.; Leyes, P.; Martín-Begué, N.; Oppenheimer, F.; et al. Cystinosis in adult and adolescent patients: Recommendations for the comprehensive care of cystinosis. Nefrologia 2015, 35, 304–321. [Google Scholar] [CrossRef][Green Version]
- Levtchenko, E.; Servais, A.; Hulton, S.A.; Ariceta, G.; Emma, F.; Game, D.S.; Lange, K.; Lapatto, R.; Liang, H.; Sberro-Soussan, R.; et al. Expert guidance on the multidisciplinary management of cystinosis in adolescent and adult patients. Clin. Kidney J. 2022, 15, 1675–1684. [Google Scholar] [CrossRef]
- Jamalpoor, A.; Othman, A.; Levtchenko, E.N.; Masereeuw, R.; Janssen, M.J. Molecular Mechanisms and Treatment Options of Nephropathic Cystinosis. Trends Mol. Med. 2021, 27, 673–686. [Google Scholar] [CrossRef]
- Gahl, W.A.; Balog, J.Z.; Kleta, R. Nephropathic cystinosis in adults: Natural history and effects of oral cysteamine therapy. Ann. Intern. Med. 2007, 147, 242–250. [Google Scholar] [CrossRef]
- Brodin-Sartorius, A.; Tête, M.-J.; Niaudet, P.; Antignac, C.; Guest, G.; Ottolenghi, C.; Charbit, M.; Moyse, D.; Legendre, C.; Lesavre, P.; et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012, 81, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Levtchenko, E.N.; van Dael, C.M.; de Graaf-Hess, A.C.; Wilmer, M.J.G.; Heuvel, L.P.v.D.; Monnens, L.A.; Blom, H.J. Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr. Nephrol. 2006, 21, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Agency, E.M. Cystadrops. 2024. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cystadrops (accessed on 22 February 2017).
- Martín-Sabroso, C.; Alonso-González, M.; Fernández-Carballido, A.; Aparicio-Blanco, J.; Córdoba-Díaz, D.; Navarro-García, F.; Córdoba-Díaz, M.; Torres-Suárez, A.I. Limitations and Challenges in the Stability of Cysteamine Eye Drop Compounded Formulations. Pharmaceuticals 2021, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Langman, C.B.; Greenbaum, L.A.; Sarwal, M.; Grimm, P.; Niaudet, P.; Deschênes, G.; Cornelissen, E.; Morin, D.; Cochat, P.; Matossian, D.; et al. A randomized controlled crossover trial with delayed-release cysteamine bitartrate in nephropathic cystinosis: Effectiveness on white blood cell cystine levels and comparison of safety. Clin. J. Am. Soc. Nephrol. 2012, 7, 1112–1120. [Google Scholar] [CrossRef]
- van Stein, C.; Klank, S.; Grüneberg, M.; Ottolenghi, C.; Grebe, J.; Reunert, J.; Harms, E.; Marquardt, T. A comparison of immediate release and delayed release cysteamine in 17 patients with nephropathic cystinosis. Orphanet J. Rare Dis. 2021, 16, 387. [Google Scholar] [CrossRef]
- Langman, C.B.; Greenbaum, L.A.; Grimm, P.; Sarwal, M.; Niaudet, P.; Deschenes, G.; Cornelissen, E.A.; Morin, D.; Cochat, P.; Elenberg, E.; et al. Quality of life is improved and kidney function preserved in patients with nephropathic cystinosis treated for 2 years with delayed-release cysteamine bitartrate. J. Pediatr. 2014, 165, 528–533.e1. [Google Scholar] [CrossRef][Green Version]
- Ahlenstiel-Grunow, T.; Kanzelmeyer, N.K.; Froede, K.; Kreuzer, M.; Drube, J.; Lerch, C.; Pape, L. Switching from immediate- to extended-release cysteamine in nephropathic cystinosis patients: A retrospective real-life single-center study. Pediatr. Nephrol. 2017, 32, 91–97. [Google Scholar] [CrossRef]
- Vaisbich, M.H.; Ferreira, J.C.; Price, H.; Young, K.D.; Sile, S.; Checani, G.; Langman, C.B. Cysteamine bitartrate delayed-release capsules control leukocyte cystine levels and promote statural growth and kidney health in an open-label study of treatment-naïve patients <6 years of age with nephropathic cystinosis. JIMD Rep. 2022, 63, 66–79. [Google Scholar]
- Bjerre, A.; Aase, S.A.; Radtke, M.; Siva, C.; Gudmundsdottir, H.; Forsberg, B.; Woldseth, B.; Brackman, D. The effects of transitioning from immediate release to extended release cysteamine therapy in Norwegian patients with nephropathic cystinosis: A retrospective study. Pediatr. Nephrol. 2023, 38, 3671–3679. [Google Scholar] [CrossRef]
- Cystagon EU Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/cystagon-epar-product-information_en.pdf (accessed on 16 October 2007).
- Taking Procysbi Designed to Deliver Continuous Control. Available online: https://www.procysbi.com/taking-procysbi (accessed on 1 January 2024).
- Klank, S.; van Stein, C.; Grüneberg, M.; Ottolenghi, C.; Rauwolf, K.K.; Grebe, J.; Reunert, J.; Harms, E.; Marquardt, T. Enteric-Coated Cysteamine Bitartrate in Cystinosis Patients. Pharmaceutics 2023, 15, 1851. [Google Scholar] [CrossRef]
- PROCYSBI Gastro-Resistant Hard Capsules—EU Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/procysbi-epar-product-information_en.pdf (accessed on 1 January 2024).
- Ariceta, G.; Santos, F.; Muñiz, A.L.; Hermida, A.; Matoses, M.L.; Ventura, A.; Martin-Moreno, P.L.; González, E.; Acuña, L.; Giner, E.; et al. Switching from immediate- to extended-release cysteamine in patients with nephropathic cystinosis: From clinical trials to clinical practice. Clin. Kidney J. 2024, 17, sfae049. [Google Scholar] [CrossRef]
- Raina, R.; Wang, J.; Krishnappa, V. Structured Transition Protocol for Children with Cystinosis. Front. Pediatr. 2017, 5, 191. [Google Scholar] [CrossRef]
- Dabirzadeh, A.; Dahhou, M.; Zhang, X.; Sapir-Pichhadze, R.; Cardinal, H.; White, M.; Johnston, O.; Blydt-Hansen, T.D.; Tibbles, L.A.; Hamiwka, L.; et al. Care processes and structures associated with higher medication adherence in adolescent and young adult transplant recipients. Pediatr. Transplant. 2021, 25, e14106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleid, H.; AlShareef, T.; Kaddourah, A.; Zeinelabdin, M.; Alkadi, M.M.; Al Ghaithi, B.; Abdelkawy, Y.; Al Khasawneh, E.; Levtchenko, E.; Alhasan, K. Local Guidance on the Management of Nephropathic Cystinosis in the Gulf Cooperation Council (GCC) Region. Children 2025, 12, 992. https://doi.org/10.3390/children12080992
Aleid H, AlShareef T, Kaddourah A, Zeinelabdin M, Alkadi MM, Al Ghaithi B, Abdelkawy Y, Al Khasawneh E, Levtchenko E, Alhasan K. Local Guidance on the Management of Nephropathic Cystinosis in the Gulf Cooperation Council (GCC) Region. Children. 2025; 12(8):992. https://doi.org/10.3390/children12080992
Chicago/Turabian StyleAleid, Hassan, Turki AlShareef, Ahmad Kaddourah, Maryam Zeinelabdin, Mohamad M. Alkadi, Badria Al Ghaithi, Yasser Abdelkawy, Eihab Al Khasawneh, Elena Levtchenko, and Khalid Alhasan. 2025. "Local Guidance on the Management of Nephropathic Cystinosis in the Gulf Cooperation Council (GCC) Region" Children 12, no. 8: 992. https://doi.org/10.3390/children12080992
APA StyleAleid, H., AlShareef, T., Kaddourah, A., Zeinelabdin, M., Alkadi, M. M., Al Ghaithi, B., Abdelkawy, Y., Al Khasawneh, E., Levtchenko, E., & Alhasan, K. (2025). Local Guidance on the Management of Nephropathic Cystinosis in the Gulf Cooperation Council (GCC) Region. Children, 12(8), 992. https://doi.org/10.3390/children12080992






