Investigating Different Clinical Manifestations of Staphylococcus aureus Infections in Childhood—Can D-Dimer and Fibrinogen Predict Deep Tissue Invasion?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Retrospective Study
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRP | C-reactive protein |
| SAB | Staphylococcus aureus bacteremia |
| SPEs | Septic pulmonary emboli |
| CA | Community-associated |
| HA | Healthcare-associated |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| MRSA | Methicillin-resistant Staphylococcus aureus |
References
- Boyce, J.M. Community-associated methicillin-resistant Staphylococcus aureus as a cause of healthcare-associated infection. Clin. Infect. Dis. 2008, 46, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 27, 16. [Google Scholar]
- Dabaja-Younis, H.; Garra, W.; Shachor-Meyouhas, Y.; Mashiach, T.; Geffen, Y.; Kassis, I. The epidemiology of Staphylococcus aureus bacteremia in Israeli children: Community- vs hospital-associated or healthcare-related infections. Acta Paediatr. 2021, 110, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.; Ip, M.; Tang, A.; Wei, V.W.; Wong, S.Y.; Riley, S.; Read, J.M.; Kwok, K.O. Prevalence and risk factors of community-associated methicillin-resistant Staphylococcus aureus carriage in Asia-Pacific region from 2000 to 2016: A systematic review and meta-analysis. Clin. Epidemiol. 2018, 10, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Ringberg, H.; Thorén, A.; Lilja, B. Metastatic complications of Staphylococcus aureus septicemia. To seek is to find. Infection 2000, 28, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Toltzis, P.; O’Riordan, M.A.; Millstein, L.; Sands, T.; Redpath, A.; John, C. Frequency and risk factors for the deep focus of infection in children with Staphylococcus aureus bacteremia. Pediatr. Infect. Dis. J. 2008, 27, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.G. Importance of focus identification in the treatment of Staphylococcus aureus bacteremia. J. Hosp. Infect. 2002, 52, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Fredenburgh, J.C.; Eikelboom, J.W. A Test in Context: D-Dimer. J. Am. Coll. Cardiol. 2017, 70, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Suryati, B.A.; Watson, M. Staphylococcus aureus bacteremia in children: A 5-year retrospective review. J. Paediatr. Child Health 2002, 38, 290–294. [Google Scholar] [CrossRef] [PubMed]
- García de la Mària, C.; Cañas, M.A.; Fernández-Pittol, M.; Dahl, A.; García-González, J.; Hernández-Meneses, M.; Cuervo, G.; Moreno, A.; Miró, J.M.; Marco, F. Emerging issues on Staphylococcus aureus endocarditis and the role in therapy of daptomycin plus fosfomycin. Expert. Rev. Anti Infect. Ther. 2023, 52, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Mansour, C.; Veledar, E.; Khan, B.; Lerakis, S. Staphylococcus aureus bacteremia and endocarditis: The Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am. Heart J. 2004, 147, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Denniston, S.; Riordan, F.A. Staphylococcus aureus bacteremia in children and neonates: A 10-year retrospective review. J. Infect. 2006, 53, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Goswami, U.; Brenes, J.A.; Punjabi, G.V.; LeClaire, M.M.; Williams, D.N. Associations and outcomes of septic pulmonary embolism. Open Respir. Med. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Levin, H.; Lim, R.; Sangha, G. Case 1: An 11-year-old girl with bilateral hip and groin pain. Paediatr. Child Health 2015, 20, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Rassbach, C.; Ottolini, M. Streptococcal pyomyositis of the psoas: Case reports and review. Pediatr. Emerg. Care 2006, 22, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Meehan, J.; Grose, C.; Soper, R.T.; Kimura, K. Pyomyositis in an adolescent female athlete. J. Pediatr. Surg. 1995, 30, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Maravelas, R.; Melgar, T.A.; Vos, D.; Lima, N.; Sadarangani, S. Pyomyositis in the United States 2002–2014. J. Infect. 2020, 80, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, J.S.; Zauk, A.M.; Minnefor, A.B.; Boyd, L.C.; Avella, D.G.; McInerney, V.K. Pyomyositis in an HIV-positive premature infant: Case report and review of the literature. J. Pediatr. Orthop. 1990, 10, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, F.; Rodríguez, J.; Arancibia, M.E.; Walker, B.; Espinoza, A. Pyomyositis in children: Report of two cases. Rev. Chilena Infectol. 2013, 30, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Ewald, M.B. Pyomyositis: A fatal case in a healthy teenager. Pediatr. Emerg. Care 2005, 21, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Takadama, S.; Nakaminami, H.; Aoki, S.; Akashi, M.; Wajima, T.; Ikeda, M.; Mochida, A.; Shimoe, F.; Kimura, K.; Matsuzaki, Y.; et al. Prevalence of skin infections caused by Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus in Japan, particularly in Ishigaki, Okinawa. J. Infect. Chemother. 2017, 23, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Blyth, C.C.; Campbell, A.J.; Bowen, A.C. Infection characteristics and treatment of Staphylococcus aureus bacteremia at a tertiary children’s hospital. BMC Infect. Dis. 2018, 18, 387. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.F.; Dona, D.; Jacobs, M.B.; Gerber, J.S. Risk Factors for Complications in Children with Staphylococcus aureus Bacteremia. J. Pediatr. 2019, 208, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.D.; Lo, C.K.L.; Komorowski, A.S.; Suresh, M.; Guo, K.; Garg, A.; Tandon, P.; Senecal, J.; Del Corpo, O.; Stefanova, I.; et al. Staphylococcus aureus bacteremia mortality: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, H. Distinguishing Gram-positive and Gram-negative bloodstream infections through leukocytes, C-reactive protein, procalcitonin, and D-Dimer: An empirical antibiotic guidance. FEMS Microbiol. Lett. 2024, 371, fnae091. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Fu, Y.; Tang, L. Serum D-dimer as a diagnostic index of PJI and retrospective analysis of etiology in patients with PJI. Clin. Chim. Acta 2020, 506, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Zampino, R.; Iossa, D.; Ursi, M.P.; Bertolino, L.; Karruli, A.; Molaro, R.; Esposito, G.; Vitrone, M.; D’Amico, F.; Albisinni, R.; et al. Clinical Significance and Prognostic Value of Hemostasis Parameters in 337 Patients with Acute Infective Endocarditis. J. Clin. Med. 2021, 10, 5386. [Google Scholar] [CrossRef] [PubMed]
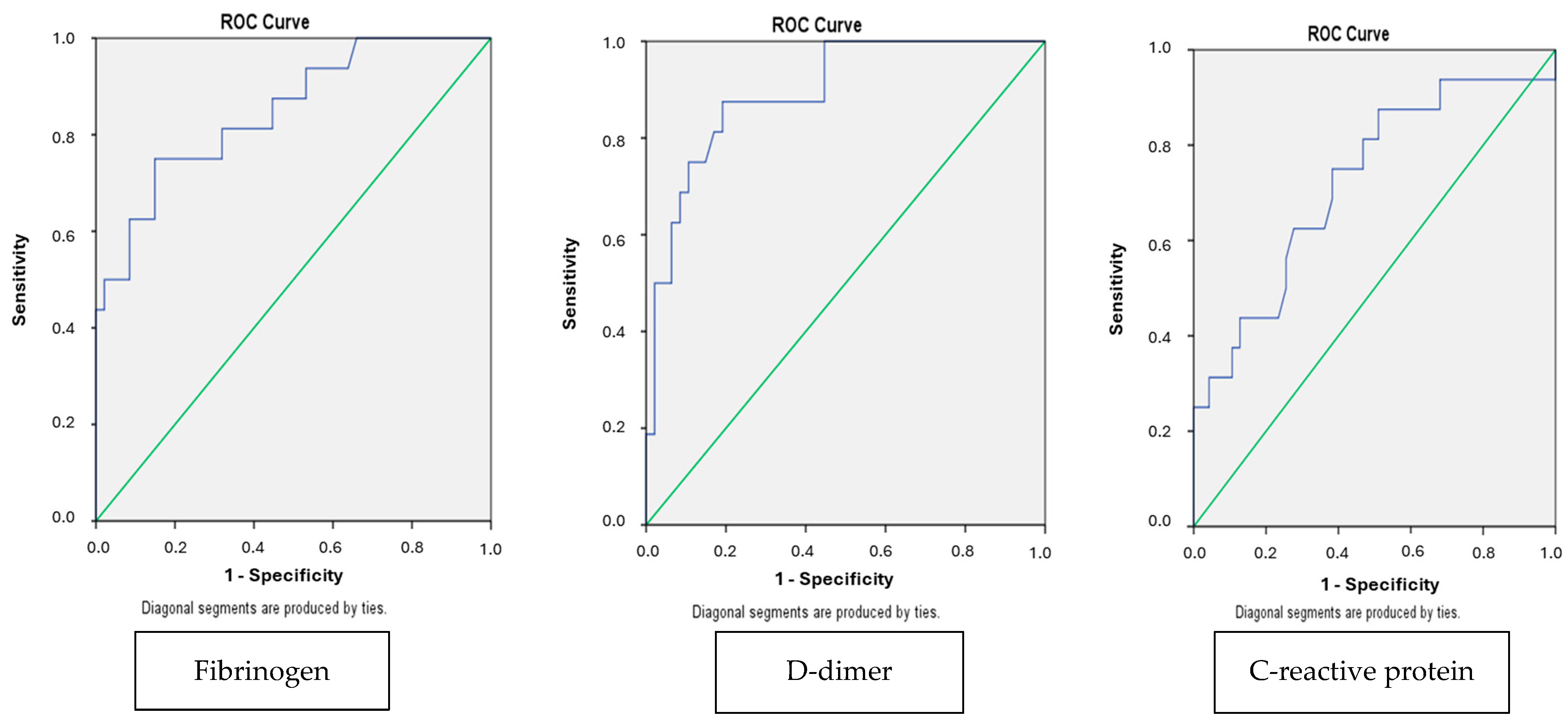

| Variables | Groups | p-Value | |||
|---|---|---|---|---|---|
| Deep-Seated Infection (n = 14) | Other (n = 47) | ||||
| N | % | N | % | ||
| Gender | 0.187 | ||||
| Male | 9 | 64.3 | 20 | 42.5 | |
| Female | 5 | 35.7 | 27 | 57.5 | |
| Community/hospital-associated infection | <0.01 | ||||
| Community | 14 | 100 | 16 | 34 | |
| Hospital | 0 | 0 | 31 | 66 | |
| Methicillin resistance | <0.01 | ||||
| Yes | 0 | 0 | 20 | 42.5 | |
| No | 14 | 100 | 27 | 57.5 | |
| Hemoculture positive >72 h | <0.01 | ||||
| Yes | 7 | 50 | 0 | 0 | |
| No | 7 | 50 | 46 | 100 | |
| Fever | 0.923 | ||||
| Yes | 9 | 64.3 | 23 | 48.9 | |
| No | 5 | 35.7 | 24 | 51.1 | |
| Intensive care unit admission | 0.957 | ||||
| Yes | 6 | 42.8 | 17 | 36.1 | |
| No | 8 | 57.2 | 30 | 63.9 | |
| Variables | Mean Values of Laboratory Values Groups | p-Value | |
|---|---|---|---|
| Deep-Seated Infection (n = 14) | Other (n = 47) | ||
| Hemoglobin (g/dL) | 11.252 ± 15.096 | 10.963 ± 17.378 | 0.562 |
| Total leucocyte count (×109/L) | 13.465 ± 7.687 | 11.470 ± 5.449 | 0.465 |
| Neutrophil count (×109/L) | 9.205 ± 6.949 | 7.199 ± 5.362 | 0.246 |
| Platelet count (×109/L) | 363.647 ± 22.420 | 343.434 ± 155.350 | 0.190 |
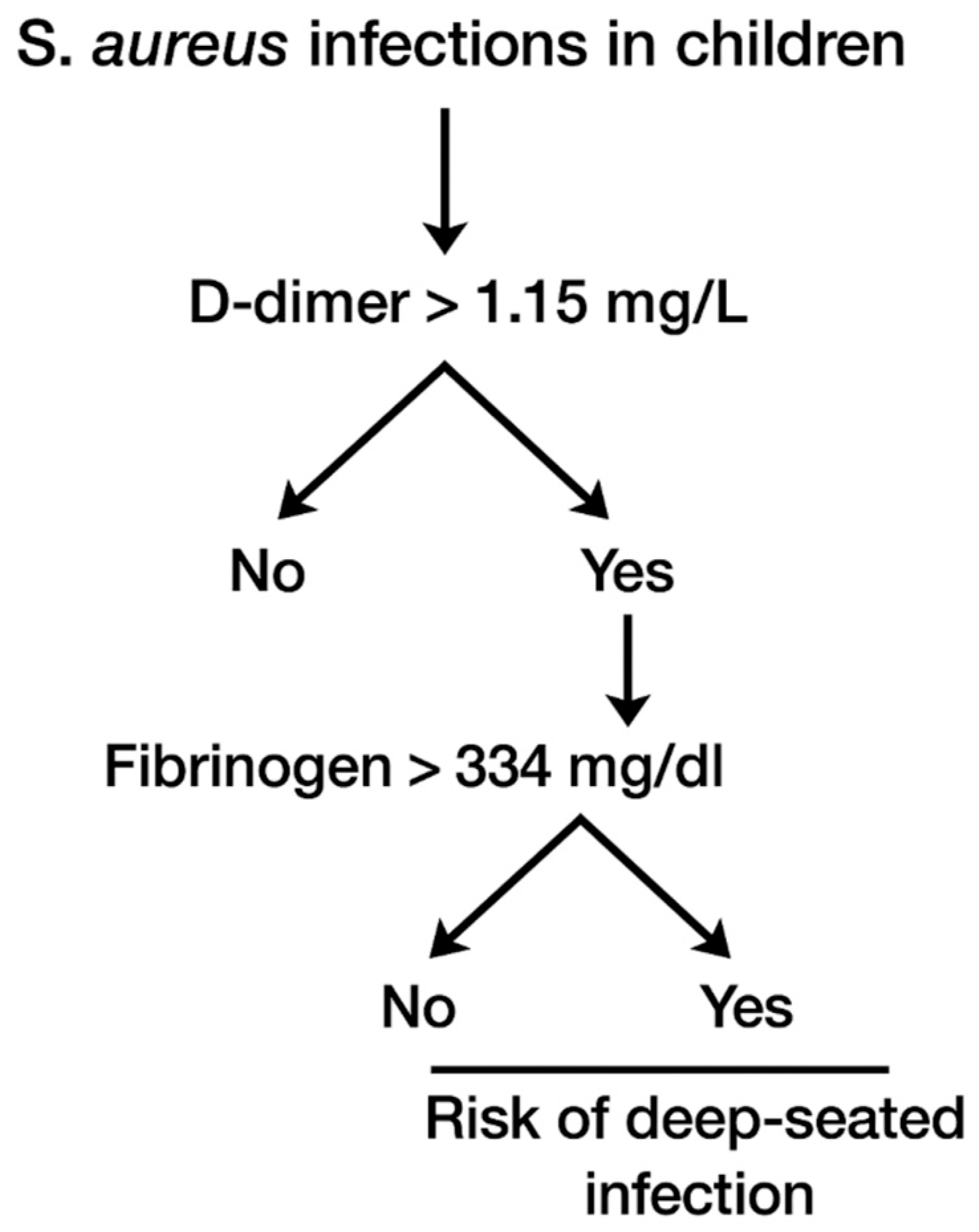
| D-dimer (mg/L) | 18.630 ± 25.653 | 2.317 ± 3.870 | 0.010 |
| Fibrinogen (mg/dL) | 492.300 ± 196.364 | 229.0 ± 118.597 | 0.015 |
| C-reactive protein (mg/L) | 134.634 ± 155.305 | 45.214 ± 64.803 | 0.020 |
| Procalcitonin (ng/dL) | 20.132 ± 38.98 | 4.949 ± 17.152 | 0.789 |
| Sedimentation rate (mm/h) | 56.633 ± 39.713 | 24 ± 22.242 | 0.018 |
| Creatinine (mg/dL) | 0.574 ± 0.651 | 3.863 ± 0.613 | 0.042 |
| Albumin (g/dL) | 3.657 ± 0.766 | 3.863 ± 0.613 | 0.315 |
| Aspartate aminotransferase (IU/L) | 42.570 ±26.997 | 53.130 ± 57.634 | 0.667 |
| Alanine aminotransferase (IU/L) | 33.052 ± 24.712 | 42.169 ± 55.612 | 0.378 |
| Variables | Groups | p-Value | |||
|---|---|---|---|---|---|
| Community-Associated (n = 29) | Healthcare-Associated (n = 32) | ||||
| N | % | N | % | ||
| Gender | 0.102 | ||||
| Male | 17 | 58.6 | 12 | 37.5 | |
| Female | 12 | 41.4 | 20 | 62.5 | |
| Deep focus involvement | <0.01 | ||||
| Yes | 14 | 48.2 | 0 | 0 | |
| No | 15 | 51.8 | 32 | 100 | |
| Methicillin resistance | 0.21 | ||||
| Yes | 5 | 17.2 | 15 | 46.9 | |
| No | 24 | 82.8 | 17 | 53.1 | |
| Hemoculture positive >72 h | 0.011 | ||||
| Yes | 7 | 24.1 | 0 | 0 | |
| No | 22 | 75.9 | 31 | 100 | |
| Fever | 0.235 | ||||
| Yes | 14 | 48.2 | 18 | 62.1 | |
| No | 15 | 51.8 | 11 | 37.9 | |
| Intensive care unit admission | 0.024 | ||||
| Yes | 7 | 24.1 | 16 | 50 | |
| No | 22 | 75.9 | 16 | 50 | |
| Variables | 1–60 (n = 31) | 61–144 (n = 16) | 145–216 (n = 14) | p-Value |
|---|---|---|---|---|
| (n,%) | (n,%) | (n,%) | ||
| Gender | 0.861 | |||
| Male | 15 (48.3%) | 7 (43.7%) | 7 (50%) | |
| Female | 16 (51.7) | 9 (56.3%) | 7 (50%) | |
| Deep focus involvement | 0.035 | |||
| Yes | 3 (9.6%) | 5 (31.2%) | 6 (42.8%) | |
| No | 28 (90.4%) | 11 (68.8%) | 8 (57.2%) | |
| Methicillin resistance | 0.368 | |||
| Yes | 12 (38.7%) | 5 (31.2%) | 2 (14.2%) | |
| No | 19 (61.3%) | 11 (68.8%) | 12 (85.8%) | |
| Hemoculture positive >72 h | 0.018 | |||
| Yes | 0 (0%) | 4 (25%) | 3 (21.4%) | |
| No | 31 (100%) | 12 (75%) | 11 (78.6%) | |
| Fever | 0.637 | |||
| Yes | 14 (45.1%) | 10 (62.5%) | 8 (57.1%) | |
| No | 17 (54.9%) | 6 (37.5%) | 6 (42.9%) | |
| Intensive care unit admission | 0.868 | |||
| Yes | 12 (38.7%) | 5 (31.2%) | 6 (42.8%) | |
| No | 19 (61.3%) | 11 (68.8%) | 8 (57.2%) |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age | 15 years | 17 years | 11 years | 15 years | 9 years |
| Gender | Female | Male | Female | Female | Male |
| Comorbidities | No | No | Atrial septal defect | No | No |
| Symptoms | Respiratory distress | Respiratory distress | Respiratory distress | Respiratory distress | Respiratory distress |
| Extrapulmonary source | Pyomyositis Arthritis | Pyomyositis Osteomyelitis Arthritis | Endocarditis | Arthritis | Arthritis |
| Thrombosis | No | Yes | No | No | Yes |
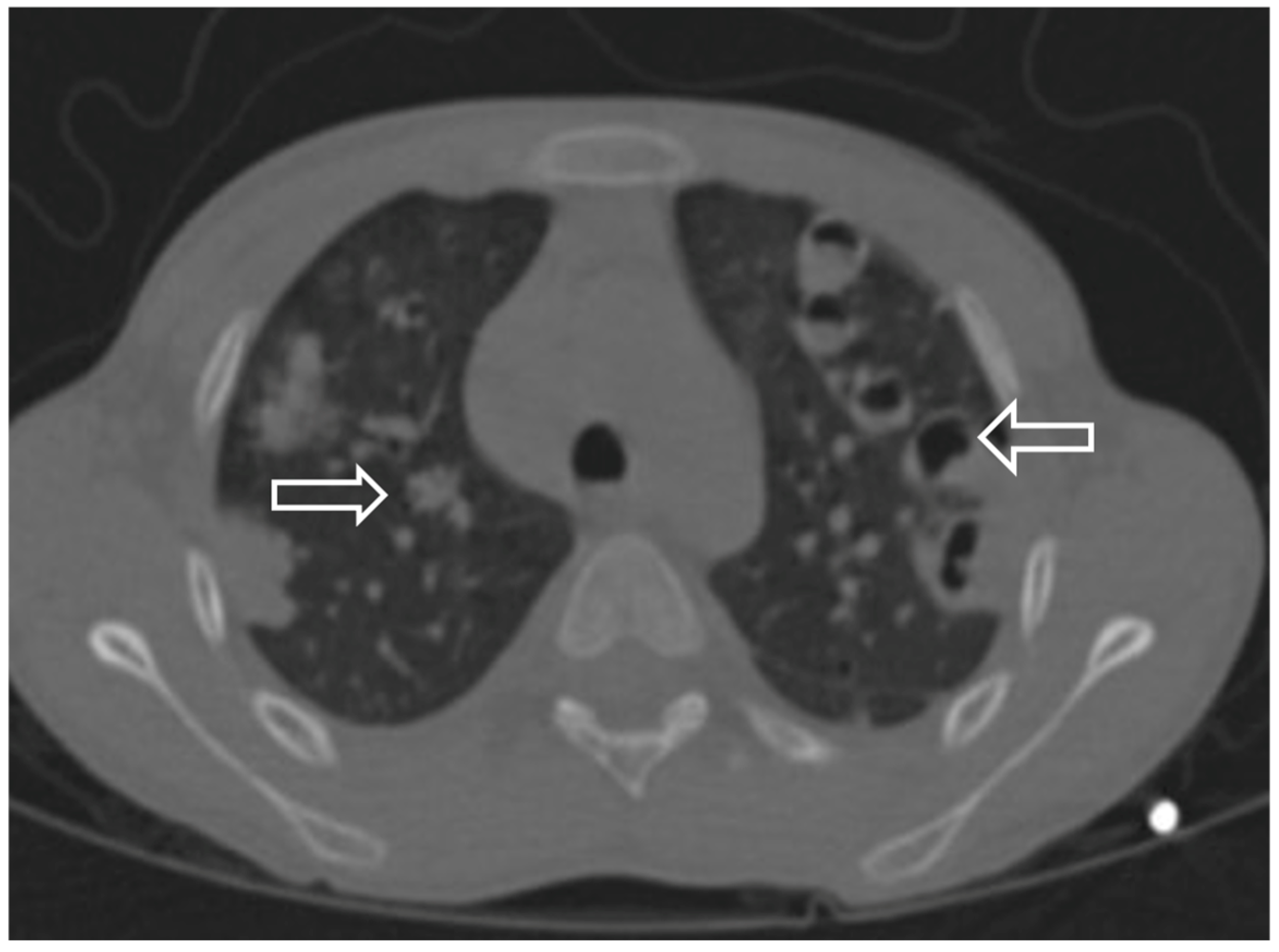
| CT | Effusion | Nodul Cavitation Reverse halo Pneumothorax | Nodul Cavitation Reverse halo | Nodul Ground glass opacity | Nodul Cavitation Reverse halo sign |
| Trauma | No | Yes | No | Yes | Yes |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age | 15 years | 13 years | 10 years | 17 years | 17 years | 15 years |
| Gender | Female | Female | Female | Male | Male | Male |
| Trauma | No | Yes | No | Yes | No | No |
| Muscles | Gluteus, Iliopsoas Pectoral, Paravertebral, Obturator, Iliac | Iliopsoas | Iliopsoas | Iliopsoas, Gluteus | Iliopsoas | Iliopsoas |
| Symptoms | Fever, respiratory distress, pain | Pain | Pain | Fever, respiratory distress, pain | Pain | Pain |
| Arthritis/OM | Yes | Yes | Yes | Yes | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Önal, P.; Sever, G.A.; Eren, B.A.; Kes, G.; Sakallı, A.A.K.; Aygün, F.; Aygün, G.; Çokuğraş, H.; Aygün, F.D. Investigating Different Clinical Manifestations of Staphylococcus aureus Infections in Childhood—Can D-Dimer and Fibrinogen Predict Deep Tissue Invasion? Children 2025, 12, 959. https://doi.org/10.3390/children12080959
Önal P, Sever GA, Eren BA, Kes G, Sakallı AAK, Aygün F, Aygün G, Çokuğraş H, Aygün FD. Investigating Different Clinical Manifestations of Staphylococcus aureus Infections in Childhood—Can D-Dimer and Fibrinogen Predict Deep Tissue Invasion? Children. 2025; 12(8):959. https://doi.org/10.3390/children12080959
Chicago/Turabian StyleÖnal, Pınar, Gözde Apaydın Sever, Beste Akdeniz Eren, Gülşen Kes, Ayşe Ayzıt Kılınç Sakallı, Fatih Aygün, Gökhan Aygün, Haluk Çokuğraş, and Fatma Deniz Aygün. 2025. "Investigating Different Clinical Manifestations of Staphylococcus aureus Infections in Childhood—Can D-Dimer and Fibrinogen Predict Deep Tissue Invasion?" Children 12, no. 8: 959. https://doi.org/10.3390/children12080959
APA StyleÖnal, P., Sever, G. A., Eren, B. A., Kes, G., Sakallı, A. A. K., Aygün, F., Aygün, G., Çokuğraş, H., & Aygün, F. D. (2025). Investigating Different Clinical Manifestations of Staphylococcus aureus Infections in Childhood—Can D-Dimer and Fibrinogen Predict Deep Tissue Invasion? Children, 12(8), 959. https://doi.org/10.3390/children12080959





