Assessment of Azathioprine-Associated Lymphopenia Incidence Rates in Polish Children with Inflammatory Bowel Disease and Autoimmune Hepatitis: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. The Prevalence Rate of Lymphopenia in the Study Group
3.2. Differences Between IBD (CU, CD) and AIH Patients with Lymphopenia and the No-Lymphopenia Subjects
3.3. The Prevalence of Lymphopenia in Relation to Disease Activity
3.4. Associations Between Lymphopenia, AZA Treatment, Disease Duration, AZA Onset Time Point, AZA Dose, Blood Cell 6-TGN Concentration, Additional Treatment, and the Patient’s Gender
3.5. Comparison of the Patients with and Without Lymphopenia in Terms of Infection Occurrence
3.6. Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vögelin, M.; Biedermann, L.; Frei, P.; Vavricka, S.R.; Scharl, S.; Zeitz, J.; Sulz, M.C.; Fried, M.; Rogler, G.; Scharl, M.; et al. The Impact of Azathioprine-Associated Lymphopenia on the Onset of Opportunistic Infections in Patients with Inflammatory Bowel Disease. PLoS ONE 2016, 11, e0155218. [Google Scholar] [CrossRef]
- González-Lama, Y.; Gisbert, J.P. Monitoring thiopurine metabolites in inflammatory bowel disease. Frontline Gastroenterol. 2016, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Chevaux, J.B.; Peyrin-Biroulet, L.; Sparrow, M.P. Optimizing thiopurine therapy in inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Stocco, G.; Londero, M.; Campanozzi, A.; Martelossi, S.; Marino, S.; Malusa, N.; Bartoli, F.; Decorti, G.; Ventura, A. Usefulness of the measurement of azathioprine metabolites in the assessment of non-adherence. J. Crohns Colitis 2010, 4, 599–602. [Google Scholar] [CrossRef]
- Walker, R.; Kammermeier, J.; Vora, R.; Mutalib, M. Azathioprine dosing and metabolite measurement in pediatric inflammatory bowel disease: Does one size fit all? Ann Gastroenterol. 2019, 32, 387–391. [Google Scholar] [CrossRef]
- Al Rifai, A.; Prasad, N.; Shuttleworth, E.; McBurney, H.; Pushpakom, S.; Robinson, A.; Newman, W.; Campbell, S. Natural history of azathioprine-associated lymphopenia in inflammatory bowel disease patients: A prospective observational study. Eur. J. Gastroenterol. Hepatol. 2011, 23, 153–158. [Google Scholar] [CrossRef]
- Stocco, G.; Martelossi, S.; Barabino, A.; Decorti, G.; Bartoli, F.; Montico, M.; Gotti, A.; Ventura, A. Glutathione-S-transferase genotypes and the adverse effects of azathioprine in young patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 57–64. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Rutgeerts, P.; Sandborn, W.J.; Sands, B.E.; Diamond, R.H.; Blank, M.; Ba, J.M.; Tang, L.; Cornillie, F.; Colombel, J.-F. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am. J. Gastroenterol. 2012, 107, 1051–1063. [Google Scholar] [CrossRef]
- Mieli-Vergani, G.; Vergani, D.; Baumann, U.; Czubkowski, P.; Debray, D.; Dezsofi, A.; Fischler, B.; Gupte, G.; Hierro, L.; Indolfi, G.; et al. Diagnosis and Management of Pediatric Autoimmune Liver Disease: ESPGHAN Hepatology Committee Position Statement. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 345–360. [Google Scholar] [CrossRef]
- Maltzman, J.; Koretzky, G. Azathioprine: Old drug, new actions. J. Clin. Investig. 2003, 111, 1122–1124. [Google Scholar] [CrossRef]
- Neurath, M. Thiopurines in IBD: What Is Their Mechanism of Action? Gastroenterol. Hepatol. 2010, 6, 435–436. [Google Scholar]
- Nguyen, T.; Gall, C.; Lachaux, A.; Boulieu, R. High thiopurine metabolite concentrations associated with lymphopenia in inflammatory bowel disease (IBD) pediatric patients receiving aminosalicylates combined with azathioprine. Int. J. Clin. Pharmacol. Ther. 2010, 48, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mahajan, R.; Kedia, S.; Dutta, A.K.; Anand, A.; Bernstein, C.N.; Desai, D.; Pai, C.G.; Makharia, G.; Tevethia, H.V.; et al. Use of thiopurines in inflammatory bowel disease: An update. Intest. Res. 2022, 20, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Zakerska-Banaszak, O.; Łykowska-Szuber, L.; Walczak, M.; Żuraszek, J.; Zielińska, A.; Skrzypczak-Zielińska, M. Cytotoxicity of thiopurine drugs in patients with inflammatory bowel disease. Toxics 2022, 10, 151. [Google Scholar] [CrossRef]
- Sousa, P.; Estevinho, M.M.; Dias, C.C.; Ministro, P.; Kopylov, U.; Danese, S.; Peyrin-Biroulet, L.; Magro, F. Thiopurines’ Metabolites and Drug Toxicity: A Meta-Analysis. J. Clin. Med. 2020, 9, 2216. [Google Scholar] [CrossRef]
- Lee, M.N.; Kang, B.; Choi, S.Y.; Kim, M.J.; Woo, S.Y.; Kim, J.-W.; Choe, Y.H.; Lee, S.-Y. Relationship between azathioprine dosage, 6-thioguanine nucleotide levels, and therapeutic response in pediatric patients with IBD treated with azathioprine. Inflamm. Bowel Dis. 2015, 21, 1054–1062. [Google Scholar] [CrossRef]
- Lee, M.N.; Kang, B.; Choi, S.Y.; Kim, M.J.; Woo, S.Y.; Kim, J.-W.; Choe, Y.H.; Lee, S.-Y. Impact of Genetic Polymorphisms on 6-Thioguanine Nucleotide Levels and Toxicity in Pediatric Patients with IBD Treated with Azathioprine. Inflamm. Bowel Dis. 2015, 21, 2897–2908. [Google Scholar] [CrossRef]
- Monasterio, C.; Kreisel, W.; Hasselblatt, P. Schwere Lymphozytopenie bei Patientin mit Morbus Crohn [Severe lymphopenia in a patient with Crohn’s disease]. Internist 2018, 59, 857–860. [Google Scholar] [CrossRef]
- Santarelli, M.D.; Davis, K.A.; Stark, R.J. Persistent Inflammation, Immunosuppression, and Catabolism Syndrome in Pediatric Populations: A Brief Perspective. Curr. Pediatr. Rev. 2024, 14. [Google Scholar] [CrossRef]
- Miele, E.; Benninga, M.A.; Broekaert, I.; Dolinsek, J.; Mas, E.; Orel, R.; Pienar, C.; Ribes-Koninckx, C.; Thomassen, R.A.; Thomson, M.; et al. Safety of Thiopurine Use in Paediatric Gastrointestinal Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 156–162. [Google Scholar] [CrossRef]
- Kirschner, B.S. Safety of azathioprine and 6-mercaptopurine in pediatric patients with inflammatory bowel disease. Gastroenterology 1998, 115, 813–821. [Google Scholar] [CrossRef]
- Freitas, C.; Castro, C.; Alba, D.; Pais, I.P.; Espinheira, M.D.C.; Trindade, E. Frequency and type of drug-related adverse effects in Pediatric Inflammatory Bowel Disease. Glob. J. Pediatr. Neonatal Care 2024, 4, 1–5. [Google Scholar] [CrossRef]
- Ingelfinger, F.; Sparano, C.; Bamert, D.; Reyes-Leiva, D.; Sethi, A.; Rindlisbacher, L.; Zwicky, P.; Kreutmair, S.; Widmer, C.C.; Mundt, S.; et al. Azathioprine therapy induces selective NK cell depletion and IFN-γ deficiency predisposing to herpesvirus reactivation. J. Allergy Clin. Immunol. 2023, 151, 280–286. [Google Scholar] [CrossRef]
- Wisniewski, A.; Kirchgesner, J.; Seksik, P.; Landman, C.; Bourrier, A.; Nion-Larmurier, I.; Marteau, P.; Cosnes, J.; Sokol, H.; Beaugerie, L.; et al. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United Eur. Gastroenterol. J. 2019, 8, 303–313. [Google Scholar] [CrossRef]
- Lukose, L.; Shantaram, P.M.; Raj, A.; Nair, G.; Shaju, A.M.; Subeesh, V.K. Purine antimetabolites associated Pneumocystis jirovecii pneumonia. Pharmacoepidemiol. Drug Saf. 2023, 32, 1244–1251. [Google Scholar] [CrossRef] [PubMed]


| Characteristic Feature | All Values | Lymphopenia | No Lymphopenia | p-Value |
|---|---|---|---|---|
| Total number of patients | 98 | 22 (22.4%) | 76 (77.5%) | |
| Females (percent) | 47 (48%) | 8 (36.4%) | 39 (51.3%) | 0.21 |
| Males (percent) | 51 | 14 (63.6%) | 37 (48.7%) | |
| The age at diagnosis (years ± SD) | 12.6 ± 3.2 | 13.3 ± 2.2 | 12.5 ± 3.4 | 0.31 |
| The age at azathioprine therapy onset | 13.6 ± 2.4 | 13.7 ± 2.2 | 13.6 ± 2.5 | 0.95 |
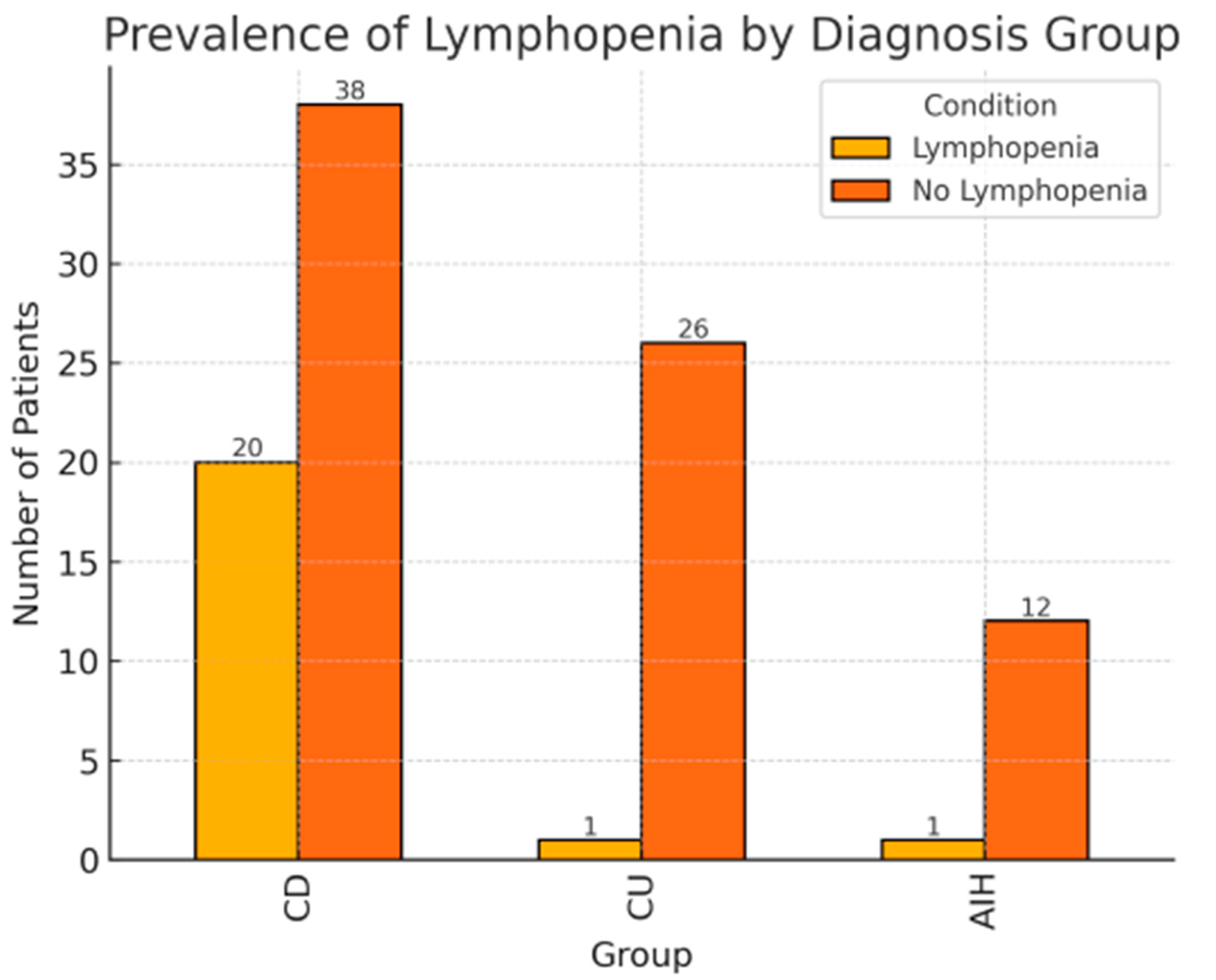
| Crohn’s disease (CD) | 58 (59%) | 20 (90%) | 38 (50%) | <0.01 |
| Ulcerative colitis (UC) | 27 (27%) | 1 (4.5%) | 26 (34%) | |
| Autoimmune hepatitis (AIH) | 13 (13.3%) | 1 (4.5%) | 12 (15.8%) | |
| Infections | 7 (7.1%) | 7 (31.8%) | 0 | <0.001 |
| Body weight SDS (mean ± SD) | 0.27 ± 1.32 | −0.33 ± 0.72 | 0.44 ± 1.41 | <0.05 |
| Body height SDS (mean ± SD) | 0.22 ± 1.52 | −0.20 ± 0.87 | 0.34 ± 1.65 | 0.14 |
| BMI SDS (mean ± SD) | 0.19 ± 1.05 | −0.29 ± 0.75 | 0.33 ± 1.09 | <0.05 |
| 6-TGN (median; interquartile range) | 417.2; 218.0–562.6 | 312.8; 235.3–504.5 | 425.7; 214.5–573.6 | 0.29 |
| Disease activity (the number of patients) | ||||
| Remission/mild | 72 | 10 | 62 | <0.001 |
| moderate/severe | 26 | 12 | 14 | |
| Treatment type: | ||||
| Azathioprine | 6 | 0 | 6 | |
| Azathioprine + Aminosalicylates | 40 | 6 | 34 | |
| Azathioprine + Aminasalicylates + Steroids | 32 | 6 | 26 | |
| Azathioprine + Aminasalicylates + Biological therapy | 20 | 10 | 10 | |
| Daily dose of azathioprine | 56.6 ± 20.9 | 53.9 ± 21.5 | 57.4 ± 20.7 | 0.77 |
| Variable | OR (95% CI) | SE | p-Value |
|---|---|---|---|
| Male sex | 1.89 (0.57–6.26) | 1.16 | 0.298 |
| Age at diagnosis | 1.01 (0.83–1.23) | 0.10 | 0.904 |
| Diagnosis of Crohn’s disease | 10.61 (2.00–56.82) | 9.08 | 0.006 |
| 6-TG concentration | 1.00 (1.00–1.00) | 0.00 | 0.456 |
| High disease activity | 6.21 (1.80–21.80) | 3.98 | 0.004 |
| BMI-SDS | 0.70 (0.36–1.36) | 0.24 | 0.294 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąk-Drabik, K.; Kaput, A.; Jarzumbek, A.; Górowska-Kowolik, K.; Szymlak, A.; Krzywicka, A.; Adamczyk, P.; Kwiecień, J. Assessment of Azathioprine-Associated Lymphopenia Incidence Rates in Polish Children with Inflammatory Bowel Disease and Autoimmune Hepatitis: A Retrospective Study. Children 2025, 12, 1093. https://doi.org/10.3390/children12081093
Bąk-Drabik K, Kaput A, Jarzumbek A, Górowska-Kowolik K, Szymlak A, Krzywicka A, Adamczyk P, Kwiecień J. Assessment of Azathioprine-Associated Lymphopenia Incidence Rates in Polish Children with Inflammatory Bowel Disease and Autoimmune Hepatitis: A Retrospective Study. Children. 2025; 12(8):1093. https://doi.org/10.3390/children12081093
Chicago/Turabian StyleBąk-Drabik, Katarzyna, Anna Kaput, Anna Jarzumbek, Katarzyna Górowska-Kowolik, Agnieszka Szymlak, Agnieszka Krzywicka, Piotr Adamczyk, and Jarosław Kwiecień. 2025. "Assessment of Azathioprine-Associated Lymphopenia Incidence Rates in Polish Children with Inflammatory Bowel Disease and Autoimmune Hepatitis: A Retrospective Study" Children 12, no. 8: 1093. https://doi.org/10.3390/children12081093
APA StyleBąk-Drabik, K., Kaput, A., Jarzumbek, A., Górowska-Kowolik, K., Szymlak, A., Krzywicka, A., Adamczyk, P., & Kwiecień, J. (2025). Assessment of Azathioprine-Associated Lymphopenia Incidence Rates in Polish Children with Inflammatory Bowel Disease and Autoimmune Hepatitis: A Retrospective Study. Children, 12(8), 1093. https://doi.org/10.3390/children12081093







