Evaluation of Serum Complement Components in Pediatric IgA Vasculitis: A Case-Control Study
Abstract
Highlights
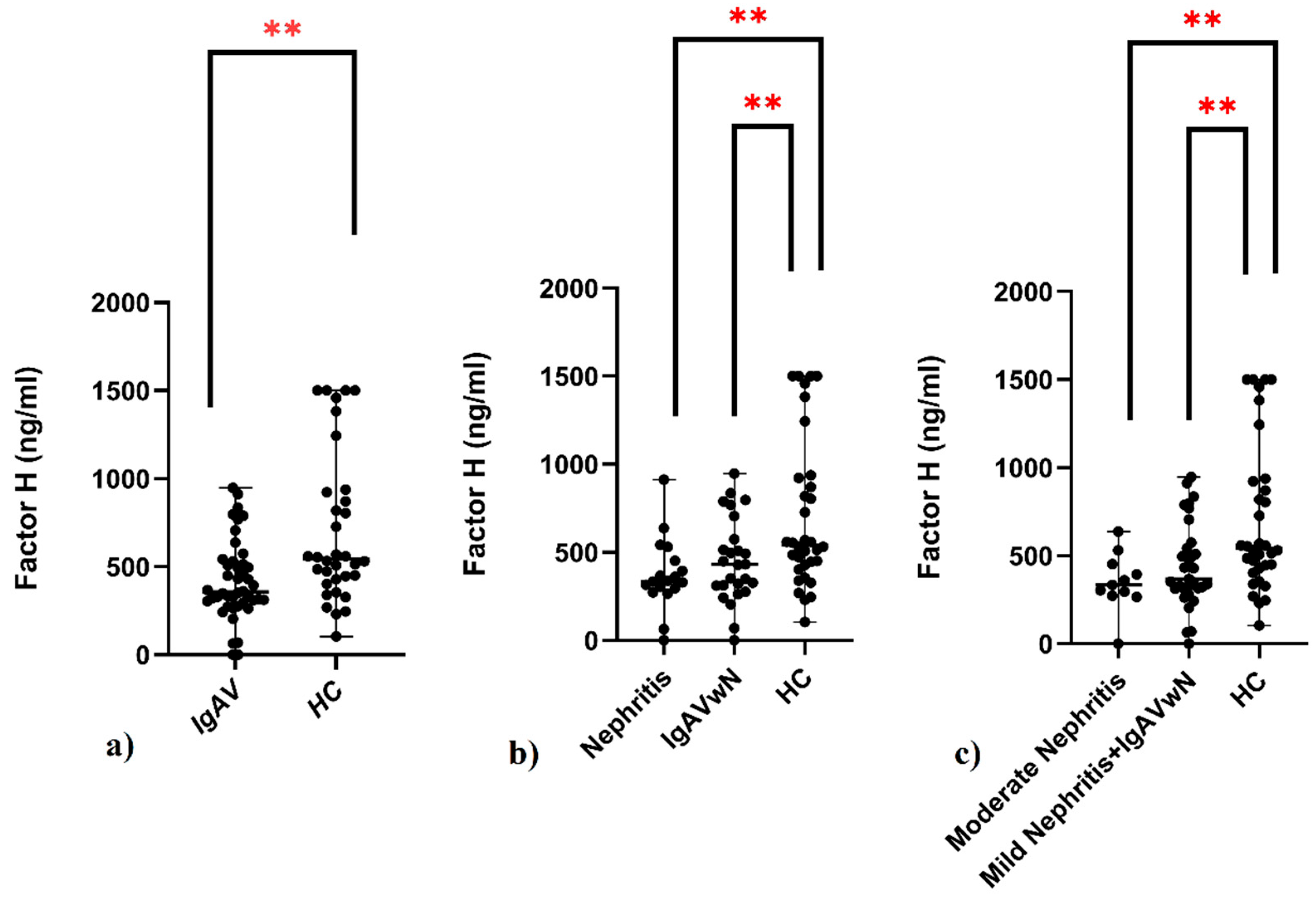
- Pediatric IgAV patients showed significantly reduced serum CFH levels compared to controls, regardless of nephritis status.
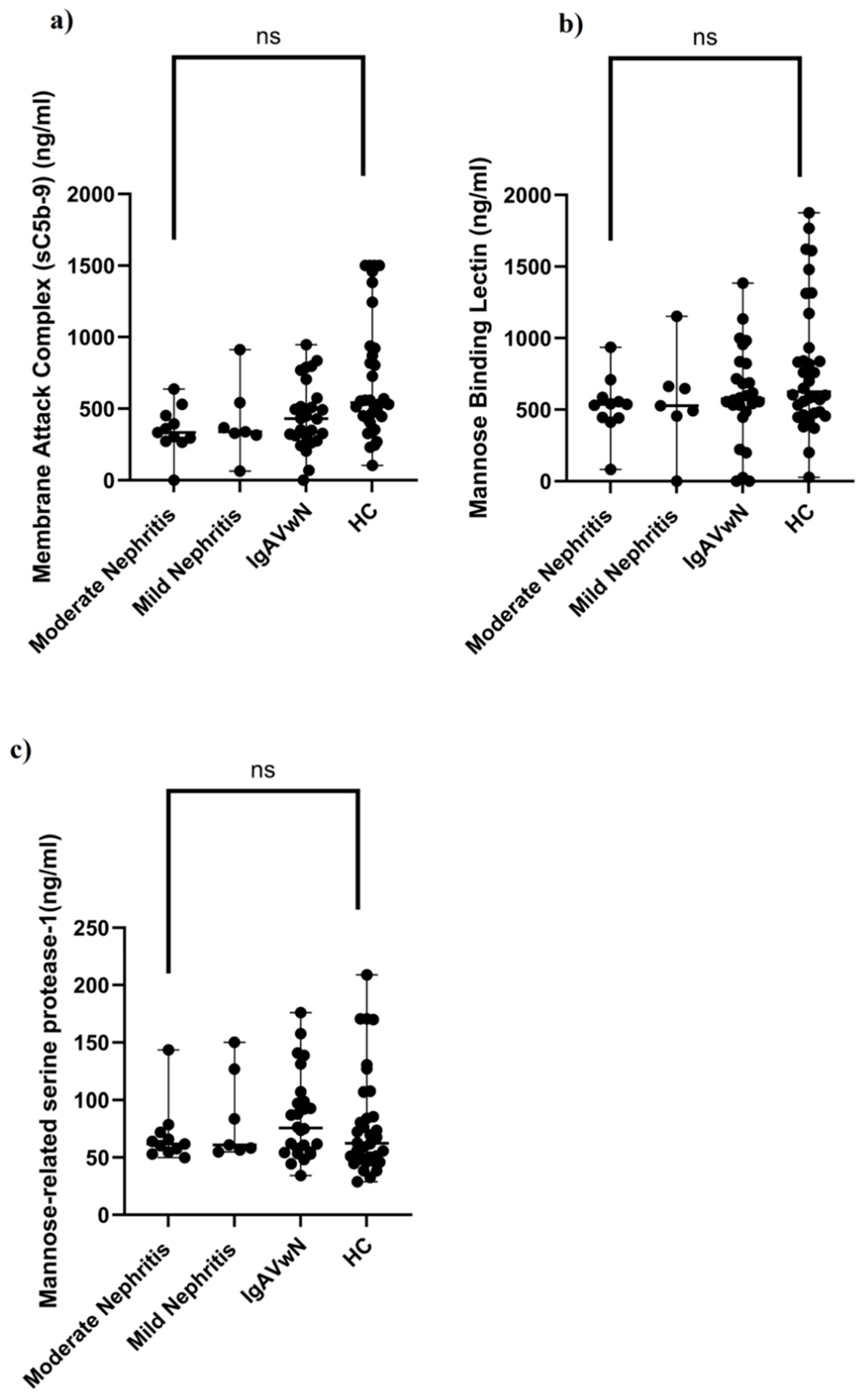
- Serum levels of sC5b-9, MBL, and MASP-1 did not differ between patients and healthy children.
- Reduced CFH levels suggest a role of alternative complement pathway dysregulation in IgAV pathogenesis.
- sC5b-9, MBL, and MASP-1 are unlikely to serve as noninvasive biomarkers for renal involvement in IgAV.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
- Mesangial hypercellularity: M0 (≤50% glomeruli), M1 (>50%);
- Endocapillary hypercellularity: E0 (absent), E1 (present);
- Segmental glomerulosclerosis: S0 (absent), S1 (present);
- Tubular atrophy/interstitial fibrosis: T0 (0–25%), T1 (26–50%), T2 (>50%);
- Crescents: C0 (none), C1 (<25% glomeruli), C2 (≥25%) [18].
2.3. Statistical Analysis
2.4. Ethical Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amatruda, M.; Carucci, N.S.; Chimenz, R.; Conti, G. Immunoglobulin A vasculitis nephritis: Current understanding of pathogenesis and treatment. World J. Nephrol. 2023, 12, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Huang, X.; Yu, G.; Qiao, J.; Cheng, J.; Wu, J.; Chen, J. Pathogenesis of IgA Vasculitis: An Up-To-Date Review. Front. Immunol. 2021, 12, 771619. [Google Scholar] [CrossRef]
- Oni, L.; Sampath, S. Childhood IgA Vasculitis (Henoch Schonlein Purpura)-Advances and Knowledge Gaps. Front. Pediatr. 2019, 7, 257. [Google Scholar] [CrossRef]
- Damman, J.; Mooyaart, A.L.; Bosch, T.P.P.V.D.; Seelen, M.A.; Doorn, M.B.V. Lectin and alternative complement pathway activation in cutaneous manifestations of IgA-vasculitis: A new target for therapy? Mol. Immunol. 2022, 143, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.J.; Williams, D.G.; Peters, D.K.; Sissons, J.G.; Boulton-Jones, J.M.; Ogg, C.S.; Cameron, J.S.; Hoffbrand, B.I. Glomerular deposition of properdin in Henoch-Schönlein syndrome and idiopathic focal nephritis. Br. Med. J. 1973, 3, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Hisano, S.; Matsushita, M.; Fujita, T.; Iwasaki, H. Activation of the lectin complement pathway in Henoch-Schönlein purpura nephritis. Am. J. Kidney Dis. 2005, 45, 295–302. [Google Scholar] [CrossRef]
- Endo, M.; Ohi, H.; Ohsawa, I.; Fujita, T.; Matsushita, M. Complement activation through the lectin pathway in patients with Henoch-Schönlein purpura nephritis. Am. J. Kidney Dis. 2000, 35, 401–407. [Google Scholar] [CrossRef]
- Roos, A.; Rastaldi, M.P.; Calvaresi, N.; Oortwijn, B.D.; Schlagwein, N.; van Gijlswijk-Janssen, D.J.; Stahl, G.L.; Matsushita, M.; Fujita, T.; van Kooten, C.; et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J. Am. Soc. Nephrol. 2006, 17, 1724–1734. [Google Scholar] [CrossRef]
- Dumont, C.; Mérouani, A.; Ducruet, T.; Benoit, G.; Clermont, M.J.; Lapeyraque, A.L.; Phan, V.; Patey, N. Clinical relevance of membrane attack complex deposition in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr. Nephrol. 2020, 35, 843–850. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Williams, C.E.C.; Toner, A.; Wright, R.D.; Oni, L. A systematic review of urine biomarkers in children with IgA vasculitis nephritis. Pediatr. Nephrol. 2021, 36, 3033–3044. [Google Scholar] [CrossRef]
- Kesarwani, V.; Bukhari, M.H.; Kahlenberg, J.M.; Wang, S. Urinary complement biomarkers in immune-mediated kidney diseases. Front. Immunol. 2024, 15, 1357869. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Martin, A.; Chantler, C.; Williams, D.G. Serum complement components in Henoch-Schönlein purpura. Arch. Dis. Child. 1978, 53, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Tsai, I.J.; Chang, C.J.; Chuang, Y.H.; Hsu, H.Y.; Chiang, B.L. The interaction between circulating complement proteins and cutaneous microvascular endothelial cells in the development of childhood Henoch-Schönlein Purpura. PLoS ONE 2015, 10, e0120411. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Barratt, J.; Beck, L.H., Jr.; Fakhouri, F.; Gale, D.P.; Goicoechea de Jorge, E.; Mosca, M.; Noris, M.; Pickering, M.C.; Susztak, K.; et al. The role of complement in kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2024, 106, 369–391. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Ruperto, N.; Dillon, M.J.; Bagga, A.; Barron, K.; Davin, J.C.; Kawasaki, T.; Lindsley, C.; Petty, R.E.; Prieur, A.M.; et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann. Rheum. Dis. 2006, 65, 936–941. [Google Scholar] [CrossRef]
- Ozen, S.; Marks, S.D.; Brogan, P.; Groot, N.; de Graeff, N.; Avcin, T.; Bader-Meunier, B.; Dolezalova, P.; Feldman, B.M.; Kone-Paut, I.; et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin A vasculitis-the SHARE initiative. Rheumatology 2019, 58, 1607–1616. [Google Scholar] [CrossRef]
- Jimenez, A.; Chen, A.; Lin, J.J.; South, A.M. Does MEST-C score predict outcomes in pediatric Henoch-Schönlein purpura nephritis? Pediatr. Nephrol. 2019, 34, 2583–2589. [Google Scholar] [CrossRef]
- Zhu, L.; Zhai, Y.L.; Wang, F.M.; Hou, P.; Lv, J.C.; Xu, D.M.; Shi, S.F.; Liu, L.J.; Yu, F.; Zhao, M.H.; et al. Variants in Complement Factor H and Complement Factor H-Related Protein Genes, CFHR3 and CFHR1, Affect Complement Activation in IgA Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 1195–1204. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, H.; Khosravi, M.; Cui, H.; Qian, X.; Kelly, J.A.; Kaufman, K.M.; Langefeld, C.D.; Williams, A.H.; Comeau, M.E.; et al. Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet. 2011, 7, e1002079. [Google Scholar] [CrossRef]
- Heurich, M.; McCluskey, G. Complement and coagulation crosstalk—Factor H in the spotlight. Immunobiology 2023, 228, 152707. [Google Scholar] [CrossRef] [PubMed]
- Józsi, M.; Tortajada, A.; Uzonyi, B.; Goicoechea de Jorge, E.; Rodríguez de Córdoba, S. Factor H-related proteins determine complement-activating surfaces. Trends Immunol. 2015, 36, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Banda, N.K.; Moreland, L.W.; Deane, K.D.; Simberg, D.; Scheinman, R.I.; Frank, R.M.; Seifert, J.A.; Accelerating Medicines Partnership (AMP) RA/SLE Network; Lau, R.; Pitzalis, C.; et al. Analysis of human factor H-related gene and protein expressed in rheumatoid arthritis synovium identifies a novel mechanism promoting dysregulated complement pathway activation. Sci. Rep. 2025, 15, 19633. [Google Scholar] [CrossRef]
- Medjeral-Thomas, N.R.; Lomax-Browne, H.J.; Beckwith, H.; Willicombe, M.; McLean, A.G.; Brookes, P.; Pusey, C.D.; Falchi, M.; Cook, H.T.; Pickering, M.C. Circulating complement factor H-related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int. 2017, 92, 942–952. [Google Scholar] [CrossRef]
- Sánchez-Corral, P.; Pouw, R.B.; López-Trascasa, M.; Józsi, M. Self-Damage Caused by Dysregulation of the Complement Alternative Pathway: Relevance of the Factor H Protein Family. Front. Immunol. 2018, 9, 1607. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhu, L.; Zhai, Y.L.; Chen, P.; Xu, B.Y.; Guo, W.Y.; Shi, S.F.; Liu, L.J.; Lv, J.C.; Zhang, H. Variation in complement factor H affects complement activation in immunoglobulin A vasculitis with nephritis. Nephrology 2020, 25, 40–47. [Google Scholar] [CrossRef]
- Marro, J.; Chetwynd, A.J.; Hawkes, J.; Northey, S.J.; Oni, L. Urinary markers of the alternative and lectin complement pathway are increased in IgA vasculitis nephritis. Clin. Kidney J. 2023, 16, 2703–2711. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhu, L.; Shi, M.; Yu, S.; Jin, Y.; Wang, Z.; Ma, J.; Yang, M.; Zhang, X.; Pan, X.; et al. A Rare Genetic Defect of MBL2 Increased the Risk for Progression of IgA Nephropathy. Front. Immunol. 2019, 10, 537. [Google Scholar] [CrossRef]
- Guo, W.Y.; Zhu, L.; Meng, S.J.; Shi, S.F.; Liu, L.J.; Lv, J.C.; Zhang, H. Mannose-Binding Lectin Levels Could Predict Prognosis in IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 3175–3181. [Google Scholar] [CrossRef]
- Wijaya, C.; Burns, C.; Hall, S.; Farmer, M.; Jones, D.; Rowlandson, M.; Choi, P.; Formby, M.; de Malmanche, T. Measurement of Complement Activation via Plasma-Soluble C5b-9 Comparison with Terminal Complement Complex Staining in a Series of Kidney Biopsies. Kidney Blood Press. Res. 2023, 48, 220–230. [Google Scholar] [CrossRef]
| Parameters | IgAV (n:44) | HC (n:34) | p-Value |
|---|---|---|---|
| Gender n (%) | |||
| Male | 20 (45.5%) | 18 (61%) | 0.14 * |
| Female | 24 (54.5%) | 16 (39%) | |
| Age (years), median (IQR) | 8 (8) | 7 (3) | 0.22 ** |
| Skin involvement, n (%) | 44 (100%) | ||
| Gastrointestinal involvement, n (%) | 18 (40.9%) | ||
| Joint involvement, n (%) | 10 (22.7%) | ||
| Kidney involvement, n (%) | 18 (40.9%) | ||
| Mild nephritis, n (%) | 7 (15.9%) | ||
| Moderate nephritis, n (%) | 11 (25%) | ||
| Kidney biopsy, n (%) | 11 (25%) | ||
| Membrane attack complex (ng/mL), median (IQR) | 0 (219.33) | 17.58 (929.97) | 0.11 + |
| Factor H (ng/mL), median (IQR) | 357.31 (228.32) | 543.08 (504.05) | <0.001 + |
| Mannose-binding lectin (ng/mL) median (IQR) | 555 (255) | 626.92 (520) | 0.07 + |
| Mannose-related serine protease-1 (ng/mL), median (IQR) | 65 (40.76) | 62.36 (42.94) | 0.24 + |
| Parameters (ng/mL) | Moderate Nefrit (n:11) | Mild Nephritis (n:7) | IgAVwN (n:26) | HC (n:34) | p-Value |
|---|---|---|---|---|---|
| Membrane attack complex, median (IQR) | 0 (0) | 0 (243.58) | 1.84 (301.52) | 17.58(929.97) | 0.08 * |
| Factor H, median (IQR) | 334.56 (178.86) | 338.49 (226.46) | 430.21 (305.61) | 543.08 (504.05) | 0.009 * |
| Mannose-binding lectin, median (IQR) | 537.31 (142) | 527.41 (206) | 574.88 (350) | 626.92 (520) | 0.21 * |
| Mannose-related serine protease-1, median (IQR) | 61.87 (17.33) | 60.90 (70.23) | 75.74 (46.19) | 62.36 (42.94) | 0.48 * |
| Pathological Features | n (%) | Membrane Attack Complex (sC5b-9) (ng/mL) (Median (IQR) |
|---|---|---|
| Mesangial hypercellularity | ||
| M0 | 7 (63.6%) | 0 (0) |
| M1 | 4 (36.4%) | 0 (311.86) |
| Endocapillary hypercellularity | ||
| E0 | 5 (45.5%) | 0 |
| E1 | 6 (54.5%) | 0 (103.95) |
| Segmental glomerulosclerosis | ||
| S0 | 10 (90.1%) | 0 (0) |
| S1 | 1 (9.1%) | - |
| Tubular atrophy/interstitial fibrosis | ||
| T0 | 10 (90.1%) | 0 (0) |
| T1&2 | 1 (9.1%) | - |
| Crescent | ||
| C0 | 5 (45.5%) | 0 (0) |
| C1&2 | 6 (54.5%) | 0 (104.12) |
| IF | ||
| IgA ++ | 4 (36.4%) | 0 (0) |
| IgA +++ | 3 (27.3%) | 0 (0) |
| IgA ++++ | 4 (36.4%) | 0 (0) |
| IF | ||
| C3 + | 7 (63.6%) | 0 (0) |
| C3 - | 4 (36.4%) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taşkın, R.B.; Aksu, G.; Şen, S.; Hakverdi, G.; Dörtkardeşler, B.E.; Conkar Tunçay, S. Evaluation of Serum Complement Components in Pediatric IgA Vasculitis: A Case-Control Study. Children 2025, 12, 1090. https://doi.org/10.3390/children12081090
Taşkın RB, Aksu G, Şen S, Hakverdi G, Dörtkardeşler BE, Conkar Tunçay S. Evaluation of Serum Complement Components in Pediatric IgA Vasculitis: A Case-Control Study. Children. 2025; 12(8):1090. https://doi.org/10.3390/children12081090
Chicago/Turabian StyleTaşkın, Raziye Burcu, Güzide Aksu, Sait Şen, Gülden Hakverdi, Burçe Emine Dörtkardeşler, and Secil Conkar Tunçay. 2025. "Evaluation of Serum Complement Components in Pediatric IgA Vasculitis: A Case-Control Study" Children 12, no. 8: 1090. https://doi.org/10.3390/children12081090
APA StyleTaşkın, R. B., Aksu, G., Şen, S., Hakverdi, G., Dörtkardeşler, B. E., & Conkar Tunçay, S. (2025). Evaluation of Serum Complement Components in Pediatric IgA Vasculitis: A Case-Control Study. Children, 12(8), 1090. https://doi.org/10.3390/children12081090





