Health Technology Assessment of Continuous Glucose Monitoring Systems for Paediatric Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Inclusion Criteria
2.2. Health Technology Assessment Process
2.2.1. Definition of the Problem and Identification of Technologies
2.2.2. Evidence Gathering
2.2.3. Hierarchy Construction
2.2.4. Alternatives Performances Evaluation
2.2.5. Weighting of Criteria
2.2.6. Integration of Results
2.2.7. Sensitivity Analysis
3. Results
3.1. Definition of the Problem and Identification of Technologies
3.2. Evidence Gathering and Hierarchy Construction
3.3. Alternatives Performances Evaluation
3.4. Weighting of Criteria
3.5. Integration of Results
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CGM | Continuous Glucose Monitoring |
| SMBG | Self-monitoring of blood glucose. |
| HTA | Health Technology Assessment |
| QALYs | Quality-Adjusted Life Years |
| KPIs | Key Performance Indicators |
| MCDA | Multicriteria Decision Analysis |
| AHP | Analytic Hierarchy Process |
| EHR | Electronic Health Records |
| DKA | Diabetic Ketoacidosis |
References
- Patterson, C.C.; Harjutsalo, V.; Rosenbauer, J.; Neu, A.; Cinek, O.; Skrivarhaug, T.; Rami-Merhar, B.; Soltesz, G.; Svensson, J.; Parslow, R.C. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: A multicentre prospective registration study. Diabetologia 2019, 62, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Wagenknecht, L.E.; Lawrence, J.M.; Isom, S.; Jensen, E.T.; Dabelea, D.; Liese, A.D.; Dolan, L.M.; Shah, A.S.; Bellatorre, A.; Sauder, K. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002–2018: Results from the population-based SEARCH for Diabetes in Youth study. Lancet Diabetes Endocrinol. 2023, 11, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.W.; Plotnick, L. Type 1 diabetes mellitus in pediatrics. Pediatr. Rev. 2008, 29, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Sochett, E.; Daneman, D. Early diabetes-related complications in children and adolescents with type 1 diabetes: Implications for screening and intervention. Endocrinol. Metab. Clin. North. Am. 1999, 28, 865–882. [Google Scholar] [CrossRef]
- White, N.H. Long-term outcomes in youth with diabetes mellitus. Pediatr. Clin. N. Am. 2015, 62, 889. [Google Scholar] [CrossRef]
- Nathan, D.M.; Group, D.E.R. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef]
- Wagner, J.; Malchoff, C.; Abbott, G. Invasiveness as a barrier to self-monitoring of blood glucose in diabetes. Diabetes Technol. Ther. 2005, 7, 612–619. [Google Scholar] [CrossRef]
- Al Hayek, A.A.; Robert, A.A.; Al Dawish, M.A. Evaluation of FreeStyle Libre flash glucose monitoring system on glycemic control, health-related quality of life, and fear of hypoglycemia in patients with type 1 diabetes. Clin. Med. Insights Endocrinol. Diabetes 2017, 10, 1179551417746957. [Google Scholar] [CrossRef]
- Bailey, T.; Bode, B.W.; Christiansen, M.P.; Klaff, L.J.; Alva, S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol. Ther. 2015, 17, 787–794. [Google Scholar] [CrossRef]
- Dovc, K.; Lanzinger, S.; Cardona-Hernandez, R.; Tauschmann, M.; Marigliano, M.; Cherubini, V.; Preikša, R.; Schierloh, U.; Clapin, H.; AlJaser, F. Association of achieving time in range clinical targets with treatment modality among youths with type 1 diabetes. JAMA Netw. Open 2023, 6, e230077. [Google Scholar] [CrossRef]
- Mauras, N.; Fox, L.; Englert, K.; Beck, R.W. Continuous glucose monitoring in type 1 diabetes. Endocrine 2013, 43, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rachmiel, M.; Landau, Z.; Boaz, M.; Mazor Aronovitch, K.; Loewenthal, N.; Ben-Ami, M.; Levy-Shraga, Y.; Modan-Moses, D.; Haim, A.; Abiri, S. The use of continuous glucose monitoring systems in a pediatric population with type 1 diabetes mellitus in real-life settings: The AWeSoMe Study Group experience. Acta Diabetol. 2015, 52, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Hommel, E.; Olsen, B.; Battelino, T.; Conget, I.; Schütz-Fuhrmann, I.; Hoogma, R.; Schierloh, U.; Sulli, N.; Gough, H.; Castañeda, J. Impact of continuous glucose monitoring on quality of life, treatment satisfaction, and use of medical care resources: Analyses from the SWITCH study. Acta Diabetol. 2014, 51, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Biester, T.; Berget, C.; Boughton, C.; Cudizio, L.; Ekhlaspour, L.; Hilliard, M.E.; Reddy, L.; Sap Ngo Um, S.; Schoelwer, M.; Sherr, J.L. ISPAD clinical practice consensus guidelines 2024: Diabetes technologies: Insulin delivery. Horm. Res. Paediatr. 2024, 97, 636–662. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L. 7. Diabetes technology: Standards of care in diabetes—2023. Diabetes Care 2023, 46, S111–S127. [Google Scholar] [CrossRef]
- Holt, R.I.; DeVries, J.H.; Hess-Fischl, A.; Hirsch, I.B.; Kirkman, M.S.; Klupa, T.; Ludwig, B.; Nørgaard, K.; Pettus, J.; Renard, E. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2021, 44, 2589–2625. [Google Scholar] [CrossRef]
- Chobot, A.; Piona, C.; Bombaci, B.; Kamińska-Jackowiak, O.; Mancioppi, V.; Passanisi, S. Exploring the continuous glucose monitoring in pediatric diabetes: Current practices, innovative metrics, and future implications. Children 2024, 11, 907. [Google Scholar] [CrossRef]
- Aljuhani, R.; Adas, M.; Alnaami, R.; Alshehri, R.; Alqarni, R.; NoorSaeed, S.; Al-Agha, A.; Aljuhani, R.; Alqarni, R.M.; NoorSaed, S. Comparing real-time continuous glucose monitoring to self-monitoring of blood glucose: Advantages and limitations for children and adolescents with type 1 diabetes. Cureus 2024, 16, e51496. [Google Scholar] [CrossRef]
- Fanzola, V.; Riboni, S.; Cannalire, G.; Metti, M.; Bensi, G.; Granata, C.; Biasucci, G. The impact of new continuous glucose monitoring (CGM) devices versus self-management of blood glucose (SMBG) on the daily life of parents and children affected by type 1 diabetes mellitus. J. Pediatr. Neonatal Individ. Med. 2022, 11, e110111. [Google Scholar]
- Tauschmann, M.; Cardona-Hernandez, R.; DeSalvo, D.J.; Hood, K.; Laptev, D.N.; Lindholm Olinder, A.; Wheeler, B.J.; Smart, C.E. International society for pediatric and adolescent diabetes clinical practice consensus guidelines 2024 diabetes technologies: Glucose monitoring. Horm. Res. Paediatr. 2025, 97, 615–635. [Google Scholar] [CrossRef]
- Zhou, Y.; Sardana, D.; Kuroko, S.; Haszard, J.J.; de Block, M.I.; Weng, J.; Jefferies, C.; Wheeler, B.J. Comparing the glycaemic outcomes between real-time continuous glucose monitoring (rt-CGM) and intermittently scanned continuous glucose monitoring (isCGM) among adults and children with type 1 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabet. Med. 2024, 41, e15280. [Google Scholar] [PubMed]
- Chase, H.P.; Beck, R.W.; Xing, D.; Tamborlane, W.V.; Coffey, J.; Fox, L.A.; Ives, B.; Keady, J.; Kollman, C.; Laffel, L. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol. Ther. 2010, 12, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: Evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care 2010, 33, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Tamborlane, W.; Beck, R.; Bode, B.; Buckingham, B.; Chase, H.; Clemons, R.; Fiallo-Scharer, R.; Fox, L.; Gilliam, L.; Hirsch, I. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N. Eng. J. Med. 2008, 359, 1464–1476. [Google Scholar]
- Laffel, L.M.; Kanapka, L.G.; Beck, R.W.; Bergamo, K.; Clements, M.A.; Criego, A.; DeSalvo, D.J.; Goland, R.; Hood, K.; Liljenquist, D. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: A randomized clinical trial. JAMA 2020, 323, 2388–2396. [Google Scholar] [CrossRef]
- Morales-Dopico, L.; MacLeish, S.A. Expanding the horizon of continuous glucose monitoring into the future of pediatric medicine. Pediatr. Res. 2024, 96, 1464–1474. [Google Scholar] [CrossRef]
- Battelino, T.; Liabat, S.; Veeze, H.; Castaneda, J.; Arrieta, A.; Cohen, O. Routine use of continuous glucose monitoring in 10 501 people with diabetes mellitus. Diabet. Med. 2015, 32, 1568–1574. [Google Scholar] [CrossRef]
- Bergenstal, R.M.; Tamborlane, W.V.; Ahmann, A.; Buse, J.B.; Dailey, G.; Davis, S.N.; Joyce, C.; Perkins, B.A.; Welsh, J.B.; Willi, S.M. Sensor-augmented pump therapy for A1C reduction (STAR 3) study: Results from the 6-month continuation phase. Diabetes Care 2011, 34, 2403–2405. [Google Scholar] [CrossRef]
- Foster, N.C.; Miller, K.M.; Tamborlane, W.V.; Bergenstal, R.M.; Beck, R.W. Continuous glucose monitoring in patients with type 1 diabetes using insulin injections. Diabetes Care 2016, 39, e81. [Google Scholar] [CrossRef]
- Głowińska-Olszewska, B.; Tobiaszewska, M.; Łuczyński, W.; Bossowski, A. Monthly use of a real-time continuous glucose monitoring system as an educational and motivational tool for poorly controlled type 1 diabetes adolescents. Adv. Med. Sci. 2013, 58, 344–352. [Google Scholar] [CrossRef]
- Tsalikian, E.; Fox, L.; Weinzimer, S.; Buckingham, B.; White, N.H.; Beck, R.; Kollman, C.; Xing, D.; Ruedy, K.; Group, D.R.i.C.N.S. Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr. Diabetes 2012, 13, 301–307. [Google Scholar] [CrossRef]
- Wong, J.C.; Foster, N.C.; Maahs, D.M.; Raghinaru, D.; Bergenstal, R.M.; Ahmann, A.J.; Peters, A.L.; Bode, B.W.; Aleppo, G.; Hirsch, I.B. Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care 2014, 37, 2702–2709. [Google Scholar] [CrossRef]
- Miller, K.M.; Beck, R.W.; Bergenstal, R.M.; Goland, R.S.; Haller, M.J.; McGill, J.B.; Rodriguez, H.; Simmons, J.H.; Hirsch, I.B.; T1D Exchange Clinic Network. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care 2013, 36, 2009–2014. [Google Scholar] [CrossRef]
- Floyd, B.; Chandra, P.; Hall, S.; Phillips, C.; Alema-Mensah, E.; Strayhorn, G.; Ofili, E.O.; Umpierrez, G.E. Comparative analysis of the efficacy of continuous glucose monitoring and self-monitoring of blood glucose in type 1 diabetes mellitus. J. Diabetes Sci. Technol. 2012, 6, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, C.; Na, H.; Zeng, W.; Li, X. Effect of different glucose monitoring Methods on bold glucose control: A systematic review and meta-analysis. Comput. Math. Methods Med. 2022, 2022, 2851572. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Norman, G.; Sonabend, R.; Chao, L.; Kamboj, M.; Golden, L.; Bekx, M.T.; Hseih, S.; Levy, C.; Sanchez, J. An observational crossover study of people using real-time continuous glucose monitors versus self-monitoring of blood glucose: Real-world evidence using electronic medical record data from more than 12,000 people with type 1 diabetes. J. Diabetes Sci. Technol. 2025, 19, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Seibold, C.U.; Holder, M.; Rami, B.; Raile, K.; Heidtmann, B.; Holl, R.W.; DPV Science Initiative, the German Working Group for insulin pump treatment in pediatric patients and the German BMBF Competence Network Diabetes. Continuous glucose monitoring in children, adolescents, and adults with type 1 diabetes mellitus: Analysis from the prospective DPV diabetes documentation and quality management system from Germany and Austria. Pediatr. Diabetes 2012, 13, 12–14. [Google Scholar] [CrossRef]
- Azari, S.; Salehpour, S. A Comparative Study of Two Glycemic Control Methods (SMBG vs. CGM) in Children and Adolescents Aged from 4 to 18 Years with Type 1 Diabetes. J. Pediatr. Perspect. 2022, 10, 15331–15339. [Google Scholar]
- Lewis, K.R.; McCrone, S.; Deiriggi, P.; Bendre, S. Effectiveness of continuous glucose monitoring in children, adolescents, and young adults with poorly controlled type 1 diabetes. J. Spec. Pediatr. Nurs. 2017, 22, e12162. [Google Scholar] [CrossRef]
- Ritrovato, M.; Faggiano, F.C.; Tedesco, G.; Derrico, P. Decision-Oriented Health Technology Assessment: One Step Forward in Supporting the Decision-Making Process in Hospitals. Value Health 2015, 18, 505–511. [Google Scholar] [CrossRef]
- Kristensen, F.B.; Lampe, K.; Wild, C.; Cerbo, M.; Goettsch, W.; Becla, L. The HTA Core Model®—10 years of developing an international framework to share multidimensional value assessment. Value Health 2017, 20, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Lampe, K.; Mäkelä, M.; Garrido, M.V.; Anttila, H.; Autti-Rämö, I.; Hicks, N.J.; Hofmann, B.; Koivisto, J.; Kunz, R.; Kärki, P. The HTA core model: A novel method for producing and reporting health technology assessments. Int. J. Technol. Assess. Health Care 2009, 25, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Saaty, T.L. The Analytic Hierarchy Process: Planning, Priority Setting, Resource Allocation; McGraw-Hill: New York, NY, USA; London, UK, 1980. [Google Scholar]
- Saaty, T.L. Decision making with the analytic hierarchy process. Int. J. Serv. Sci. 2008, 1, 83–98. [Google Scholar] [CrossRef]
- Triantaphyllou, E.; Sánchez, A. A sensitivity analysis approach for some deterministic multi-criteria decision-making methods. Decis. Sci. 1997, 28, 151–194. [Google Scholar] [CrossRef]
- Saaty, T.L. The Modern Science of Multicriteria Decision Making and Its Practical Applications: The AHP/ANP Approach. Oper. Res. 2013, 61, 1101–1118. [Google Scholar] [CrossRef]
- Champakanath, A.; Akturk, H.K.; Alonso, G.T.; Snell-Bergeon, J.K.; Shah, V.N. Continuous glucose monitoring initiation within first year of type 1 diabetes diagnosis is associated with improved glycemic outcomes: 7-year follow-up study. Diabetes Care 2022, 45, 750–753. [Google Scholar] [CrossRef]
- Haslund-Thomsen, H.; Hasselbalch, L.A.; Laugesen, B. Parental experiences of continuous glucose monitoring in Danish children with type 1 diabetes mellitus. J. Pediatr. Nurs. 2020, 53, e149–e155. [Google Scholar] [CrossRef]
- Commissariat, P.V.; DiMeglio, L.A.; Kanapka, L.G.; Laffel, L.M.; Miller, K.M.; Anderson, B.J.; Hilliard, M.E.; the Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group; Woerner, S. Twelve-month psychosocial outcomes of continuous glucose monitoring with behavioural support in parents of young children with type 1 diabetes. Diabet. Med. 2023, 40, e15120. [Google Scholar] [CrossRef]
- Burckhardt, M.-A.; Roberts, A.; Smith, G.J.; Abraham, M.B.; Davis, E.A.; Jones, T.W. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: A randomized crossover trial. Diabetes Care 2018, 41, 2641–2643. [Google Scholar] [CrossRef]

| Clinical Effectiveness | CGM (Mean ± Sd) | SMBG (Mean ± Sd) | p Value |
|---|---|---|---|
| Glycaemic variability | 56.75 (±10.1) | 79.3 (±21.1) | 0.003 * |
| HbA1c (mmol/mol) | 53.5 (±12.2) | 70.2 (±14.2) | 0.004 * |
| Glycaemic average | 153.4 (±17.1) | 186.4 (±43.6) | 0.02 * |
| Severe hypoglycaemic events rate | 0.22% (±0.3%) | 0.3% (±0.5%) | 0.687 |
| Severe hyperglycaemic events rates | 13% (±12%) | 18% (±15%) | 0.408 |
| Health-related quality of life | 91.4 (±4.4) | 76.3 (±4.9) | 0.09 * |
| Weight System | Performance CGM | Performance SBGM | ||
|---|---|---|---|---|
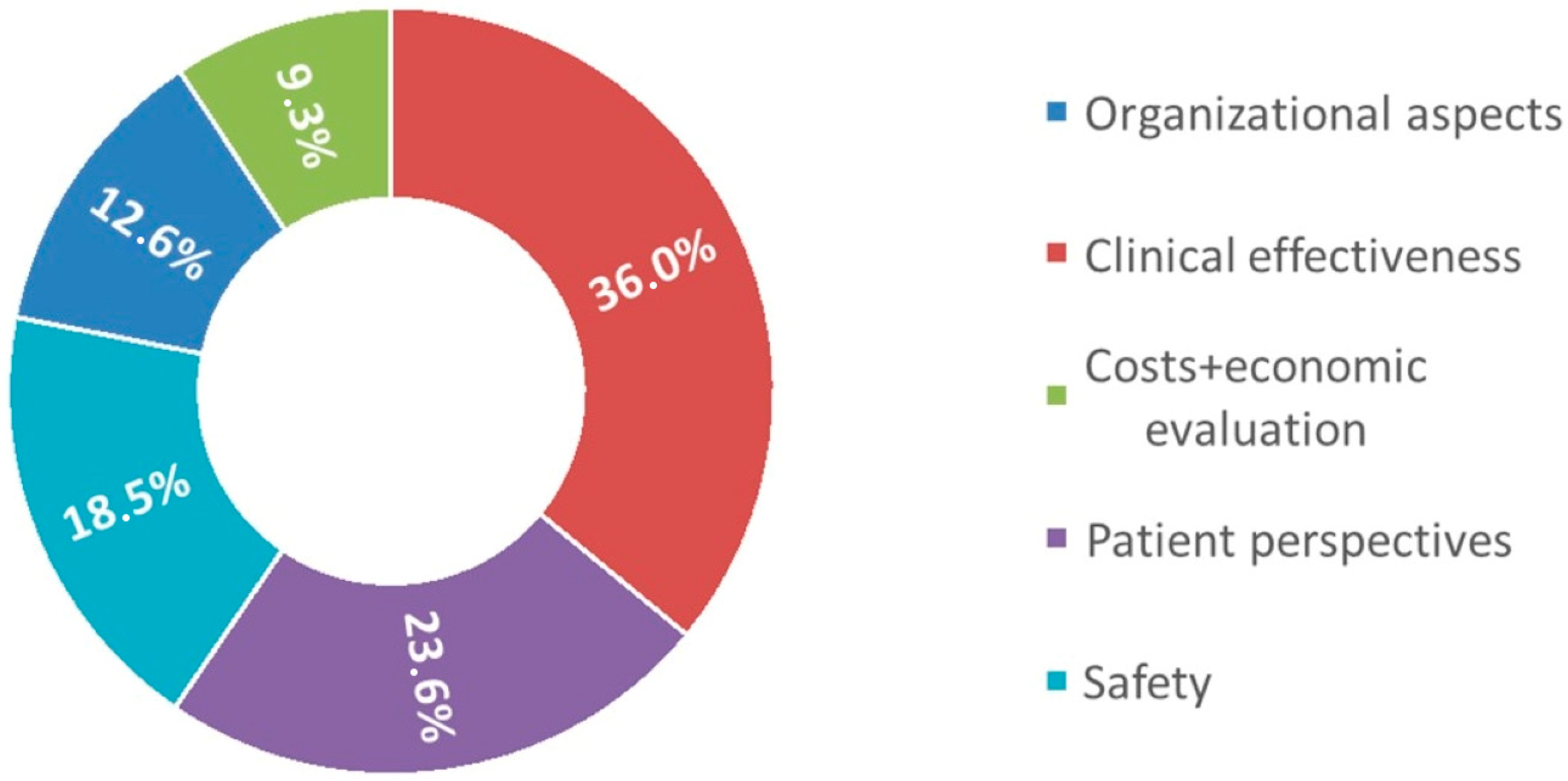
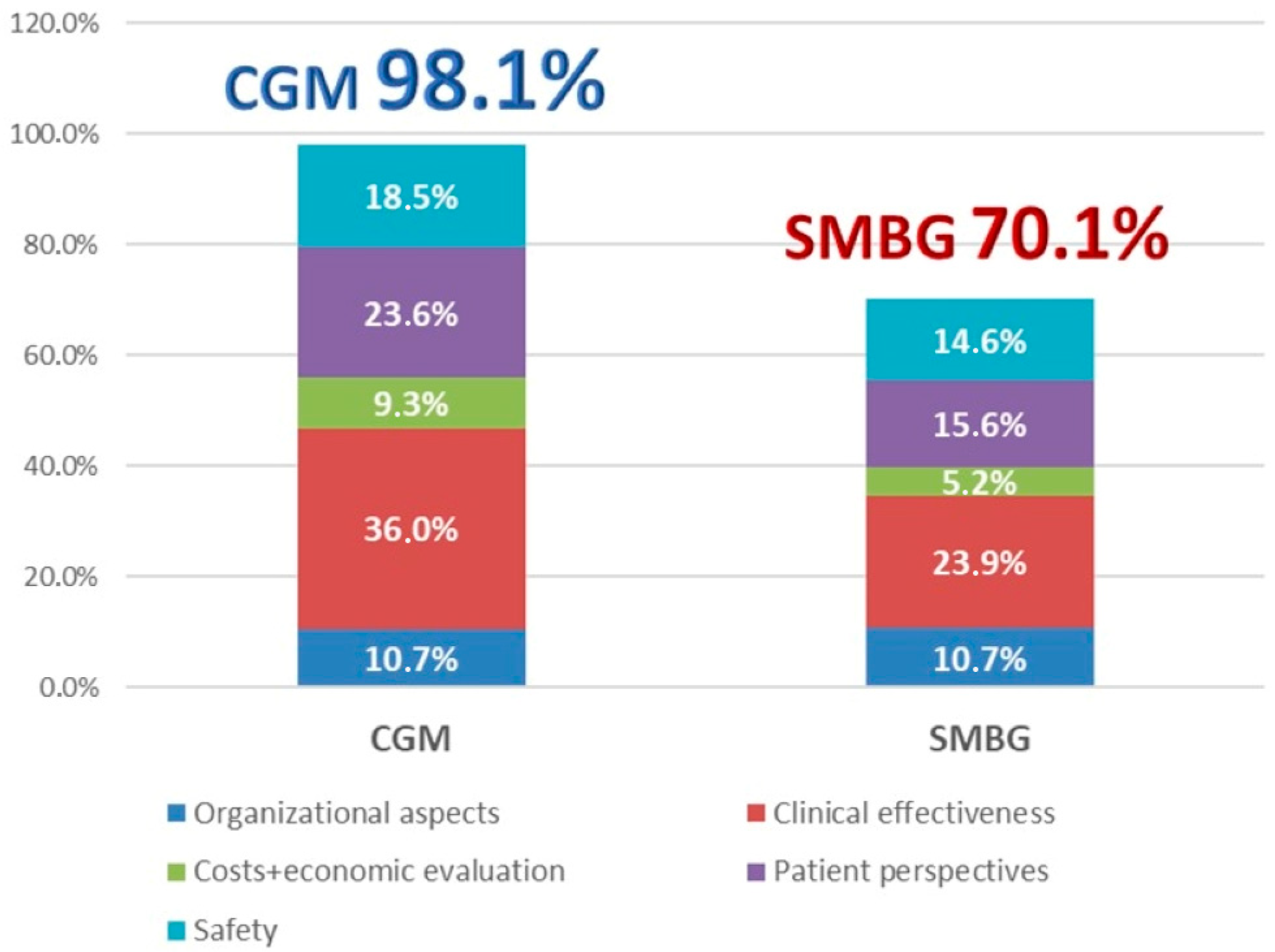
| ORGANIZATIONAL ASPECTS | 12.6% | 10.7% | 10.7% | |
| Workflow | 6.6% | 6.4% | 5.2% | |
| Hospitalization rate | 2.2% | 2.2% | 1.2% | |
| Length of stay | 0.8% | 0.8% | 0.6% | |
| Time per consultation | 1.0% | 0.9% | 1.0% | |
| Number of telephone consultation | 1.3% | 1.2% | 1.3% | |
| Number of extra visits | 1.3% | 1.3% | 1.0% | |
| Training | 6.0% | 4.3% | 5.5% | |
| Time to download | 1.1% | 1.1% | 0.6% | |
| Time for data evaluation | 3.6% | 2.4% | 3.6% | |
| Staff on training | 1.3% | 0.9% | 1.3% | |
| CLINICAL EFFECTIVENESS | 36.0% | 36.0% | 23.9% | |
| Behavioural outcomes | 12.9% | 12.9% | 10.1% | |
| Adherence to exercise | 3.8% | 3.8% | 2.9% | |
| Adherence to glucose monitoring. | 9.1% | 9.1% | 7.2% | |
| Clinical outcomes | 23.1% | 23.1% | 13.8% | |
| Glycated hemoglobin levels | 2.1% | 2.1% | 0.7% | |
| Number of severe hyperglycaemias | 2.2% | 2.2% | 1.5% | |
| Number of visits at emergency department | 3.1% | 3.1% | 1.0% | |
| Number of hypoglycaemic events | 5.4% | 5.4% | 4.8% | |
| Number of DKA | 3.6% | 3.6% | 2.0% | |
| Glycaemic variability | 3.7% | 3.7% | 2.1% | |
| Glycaemic average | 3.0% | 3.0% | 1.7% | |
| COSTS AND ECONOMIC EVALUATION | 9.3% | 9.3% | 5.2% | |
| CEE | 9.3% | 9.3% | 5.2% | |
| Cost-effectiveness | 9.3% | 9.3% | 5.2% | |
| PATIENT PERSPECTIVES | 23.6% | 23.6% | 15.6% | |
| Patients | 23.6% | 23.6% | 15.6% | |
| Adherence to exercise | 4.0% | 4.0% | 3.1% | |
| Adherence to glucose monitoring. | 6.6% | 6.6% | 5.2% | |
| Health related quality of life | 13.0% | 13.0% | 7.3% | |
| SAFETY | 18.5% | 18.5% | 14.6% | |
| Technology related risks | 18.5% | 18.5% | 14.6% | |
| Adverse events | 18.5% | 18.5% | 14.6% | |
| TOTAL | 100.0% | 98.1% | 70.1% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andellini, M.; Schiaffini, R.; Angelini, M.; Pecchia, L.; Ritrovato, M. Health Technology Assessment of Continuous Glucose Monitoring Systems for Paediatric Patients. Children 2025, 12, 1088. https://doi.org/10.3390/children12081088
Andellini M, Schiaffini R, Angelini M, Pecchia L, Ritrovato M. Health Technology Assessment of Continuous Glucose Monitoring Systems for Paediatric Patients. Children. 2025; 12(8):1088. https://doi.org/10.3390/children12081088
Chicago/Turabian StyleAndellini, Martina, Riccardo Schiaffini, Massimiliano Angelini, Leandro Pecchia, and Matteo Ritrovato. 2025. "Health Technology Assessment of Continuous Glucose Monitoring Systems for Paediatric Patients" Children 12, no. 8: 1088. https://doi.org/10.3390/children12081088
APA StyleAndellini, M., Schiaffini, R., Angelini, M., Pecchia, L., & Ritrovato, M. (2025). Health Technology Assessment of Continuous Glucose Monitoring Systems for Paediatric Patients. Children, 12(8), 1088. https://doi.org/10.3390/children12081088






