Impact of COVID-19 Lockdown on the Incidence of Common Pregnancy Complications—Is the Diagnosis of FGR Made Too Generously?
Abstract
1. Introduction
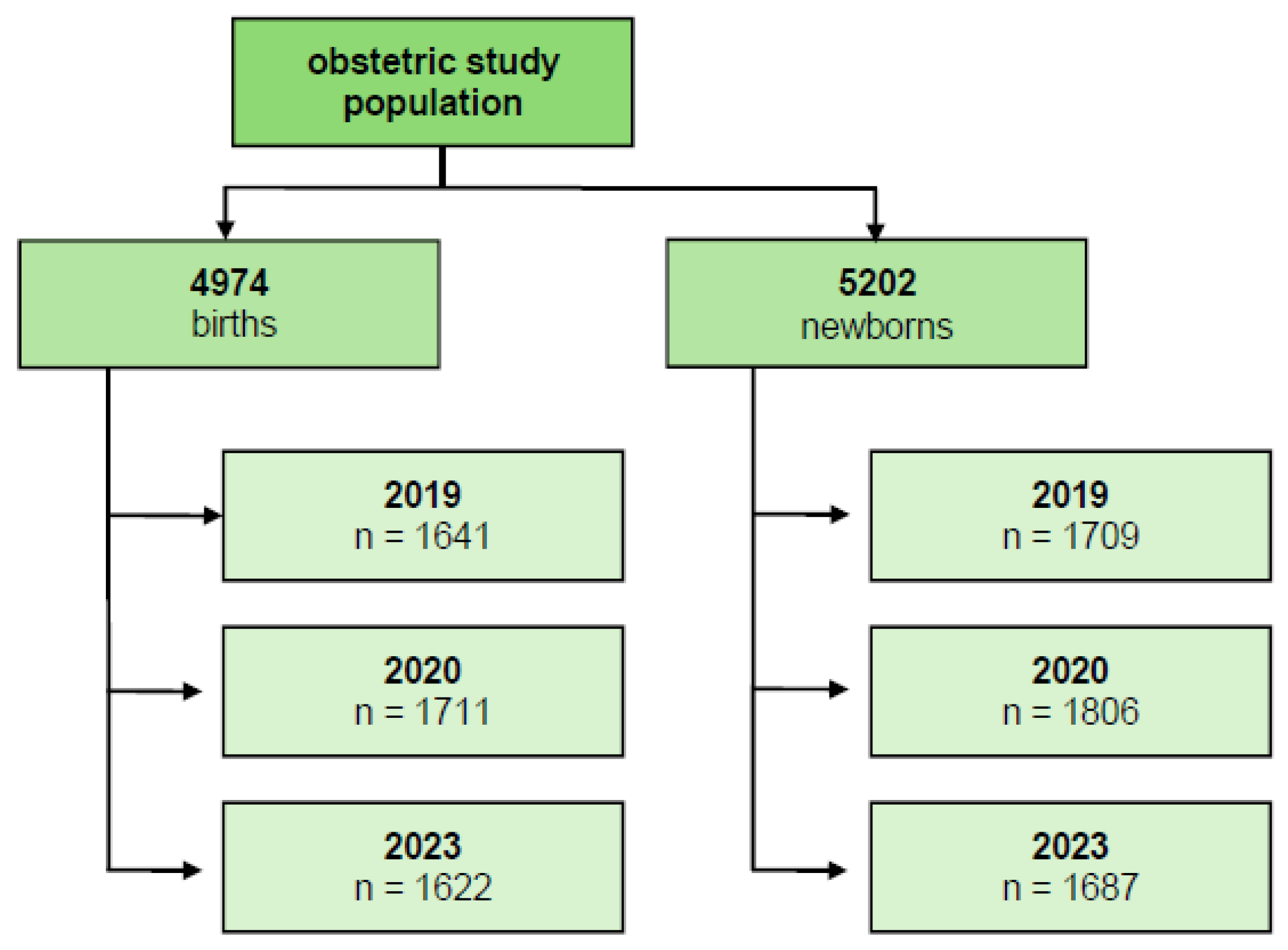
2. Material and Methods
3. Results
4. Discussion
5. Strength and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease 2019 |
| IQR | interquartile range |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SD | standard deviation |
| WG | weeks of gestation |
References
- Available online: https://www.tagesschau.de/inland/coronavirus-deutschland-erster-fall-101.html (accessed on 15 January 2024).
- Kabesch, M.; Roth, S.; Brandstetter, S.; Häusler, S.; Juraschko, E.; Weigl, M.; Wellmann, S.; Lang, T.; Schmidt, B.; Salzberger, B.; et al. Successful containment of COVID-19 outbreak in a large maternity and perinatal center while continuing clinical service. Pediatr. Allergy Immunol. 2020, 31, 560–564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Czuba, B.; Mlodawski, J.; Kajdy, A.; Sys, D.; Cnota, W.; Mlodawska, M.; Kwiatkowski, S.; Guzik, P.; Wielgos, M.; Rybak-Krzyszkowska, M.; et al. Implementation of the Publicly Funded Prenatal Screening Programme in Poland during the COVID-19 Pandemic: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 1317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mor, M.; Kugler, N.; Jauniaux, E.; Betser, M.; Wiener, Y.; Cuckle, H.; Maymon, R. Impact of the COVID-19 Pandemic on Excess Perinatal Mortality and Morbidity in Israel. Am. J. Perinatol. 2021, 38, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Dell’uTri, C.; Manzoni, E.; Cipriani, S.; Spizzico, C.; Dell’Acqua, A.; Barbara, G.; Parazzini, F.; Kustermann, A. Effects of SARS-CoV-2 epidemic on the obstetrical and gynecological emergency service accesses. What happened and what shall we expect now? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 64–68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khalil, A.; Kalafat, E.; Benlioglu, C.; O’BRien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Heath, P.; Ladhani, S.; et al. SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis of clinical features and pregnancy outcomes. eClinicalMedicine 2020, 25, 100446. [Google Scholar] [CrossRef] [PubMed]
- Roberton, T.; Carter, E.D.; Chou, V.B.; Stegmuller, A.R.; Jackson, B.D.; Tam, Y.; Sawadogo-Lewis, T.; Walker, N. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: A modelling study. Lancet Glob. Health 2020, 8, e901–e908. [Google Scholar] [CrossRef] [PubMed Central]
- Been, J.V.; Ochoa, L.B.; Bertens, L.C.M.; Schoenmakers, S.; Steegers, E.A.P.; Reiss, I.K.M. Impact of COVID-19 mitigation measures on the incidence of preterm birth: A national quasi-experimental study. Lancet Public Health 2020, 5, e604–e611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caniglia, E.C.; Magosi, L.E.; Zash, R.; Diseko, M.; Mayondi, G.; Mabuta, J.; Powis, K.; Dryden-Peterson, S.; Mosepele, M.; Luckett, R.; et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am. J. Obstet. Gynecol. 2021, 224, 615.e1–615.e12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Curtis, M.; Villani, L.; Polo, A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hedermann, G.; Hedley, P.L.; Bækvad-Hansen, M.; Hjalgrim, H.; Rostgaard, K.; Poorisrisak, P.; Breindahl, M.; Melbye, M.; Hougaard, D.M.; Christiansen, M.; et al. Danish premature birth rates during the COVID-19 lockdown. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 93–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kc, A.; Gurung, R.; Kinney, M.V.; Sunny, A.K.; Moinuddin, M.; Basnet, O.; Paudel, P.; Bhattarai, P.; Subedi, K.; Shrestha, M.P.; et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: A prospective observational study. Lancet Glob. Health 2020, 8, e1273–e1281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khalil, A.; von Dadelszen, P.; Draycott, T.; Ugwumadu, A.; O’Brien, P.; Magee, L. Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic. JAMA 2020, 324, 705–706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Handley, S.C.; Mullin, A.M.; Elovitz, M.A.; Gerson, K.D.; Montoya-Williams, D.; Lorch, S.A.; Burris, H.H. Changes in Preterm Birth Phenotypes and Stillbirth at 2 Philadelphia Hospitals During the SARS-CoV-2 Pandemic, March–June 2020. JAMA 2021, 325, 87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cosma, S.; Carosso, A.R.; Cusato, J.; Borella, F.; Carosso, M.; Bovetti, M.; Filippini, C.; D’Avolio, A.; Ghisetti, V.; Di Perri, G.; et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: A case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2021, 224, 391.e1–391.e7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, M.; Puri, M.; Yadav, R.; Biswas, R.; Singh, M.; Chaudhary, V.; Jaiswal, N.; Meena, D. Stillbirths and the COVID-19 pandemic: Looking beyond SARS-CoV-2 infection. Int. J. Gynecol. Obstet. 2021, 153, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Mehta, K.; Choudhary, R. COVID-19 outbreak and decreased hospitalisation of pregnant women in labour. Lancet Glob. Health 2020, 8, e1116–e1117. [Google Scholar] [CrossRef] [PubMed Central]
- Main, E.K.; Chang, S.-C.; Carpenter, A.M.; Wise, P.H.; Stevenson, D.K.; Shaw, G.M.; Gould, J.B. Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am. J. Obstet. Gynecol. 2021, 224, 239–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer, R.; Bart, Y.; Tsur, A.; Yinon, Y.; Friedrich, L.; Maixner, N.; Levin, G. A marked decrease in preterm deliveries during the coronavirus disease 2019 pandemic. Am. J. Obstet. Gynecol. 2021, 224, 234–237. [Google Scholar] [CrossRef] [PubMed Central]
- Philip, R.K.; Purtill, H.; Reidy, E.; Daly, M.; Imcha, M.; McGrath, D.; O’Connell, N.H.; Dunne, C.P. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: A ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob. Health 2020, 5, e003075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koren, G.; Boskovic, R.; Hard, M.; Maltepe, C.; Navioz, Y.; Einarson, A. Motherisk—PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. Am. J. Obstet. Gynecol. 2002, 186 (Suppl. S5), S228–S231. [Google Scholar] [CrossRef] [PubMed]
- Bührer, C.; Felderhoff-Müser, U.; Gembruch, U.; Hecher, K.; Kainer, F.; Kehl, S.; Kidszun, A.; Kribs, A.; Krones, T.; Lipp, V.; et al. Frühgeborene an der Grenze der Lebensfähigkeit (Entwicklungsstufe S2k, AWMF-Leitlinien-Register Nr. 024/019, Juni 2020). Z. Geburtshilfe Neonatol. 2020, 224, 244–254. (In German) [Google Scholar] [CrossRef] [PubMed]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet. Gynecol. 2020, 135, 1492–1495. [CrossRef] [PubMed]
- Pecks, U.; Baumann, M.; Binder, J.; Contini, C.; Dathan-Stumpf, A.; Dechend, R.; Enna-Kirchmair, B.; Fischer, T.; Girard, T.; Greve, S.; et al. Hypertensive Disorders in Pregnancy (HDP): Diagnostics and Therapy. Guideline of the DGGG, OEGGG and SGGG (S2k-Level, AWMF Registry No. 015/018, June 2024). Geburtshilfe Frauenheilkd. 2025, 85, 810–850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kehl, S.; Dötsch, J.; Hecher, K.; Schlembach, D.; Schmitz, D.; Stepan, H.; Gembruch, U. Intrauterine Growth Restriction. Guideline of the German Society of Gynecology and Obstetrics (S2k-Level, AWMF Registry No. 015/080, October 2016). Geburtshilfe Frauenheilkd. 2017, 77, 1157–1173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Sacinti, K.G.; Kalafat, E.; Sukur, Y.E.; Koc, A. Increased incidence of first-trimester miscarriage during the COVID-19 pandemic. Ultrasound Obstet. Gynecol. 2021, 57, 1013–1014. [Google Scholar] [CrossRef] [PubMed Central]
- Justman, N.; Shahak, G.; Gutzeit, O.; Ben Zvi, D.; Ginsberg, Y.; Solt, I.; Vitner, D.; Beloosesky, R.; Weiner, Z.; Zipori, Y. Lockdown with a Price: The impact of the COVID-19 Pandemic on Prenatal Care and Perinatal Outcomes in a Tertiary Care Center. Isr. Med. Assoc. J. 2020, 22, 533–537. [Google Scholar] [PubMed]
- Stumpfe, F.M.; Schneider, M.O.; Hein, A.; Faschingbauer, F.; Kehl, S.; Hermanek, P.; Böhm, J.; Scharl, A.; Beckmann, M.W.; Staerk, C.; et al. Limited Effects of SARS-CoV-2 Pandemic-related Lockdowns and Reduced Population Mobility on Preterm Birth Rates: A Secondary Analysis of Bavarian Obstetric Quality Parameters from 2010 to 2020. Geburtshilfe Und Frauenheilkd. 2022, 82, 842–851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papageorghiou, A.T.; Deruelle, P.; Gunier, R.B.; Rauch, S.; García-May, P.K.; Mhatre, M.; Usman, M.A.; Abd-Elsalam, S.; Etuk, S.; Simmons, L.E.; et al. Preeclampsia and COVID-19: Results from the INTERCOVID prospective longitudinal study. Am. J. Obstet. Gynecol. 2021, 225, 289.e1–289.e17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radan, A.-P.; Baud, D.; Favre, G.; Papadia, A.; Surbek, D.; Baumann, M.; Raio, L. Low placental weight and altered metabolic scaling after severe acute respiratory syndrome coronavirus type 2 infection during pregnancy: A prospective multicentric study. Clin. Microbiol. Infect. 2022, 28, 718–722. [Google Scholar] [CrossRef]
- Świercz, G.; Zmelonek-Znamirowska, A.; Armańska, J.; Stanisławska, M.; Mlodawska, M.; Młodawski, J. Prevalence of pregnancy complications in the Świętokrzyskie province: A follow-up of a cohort of patients participating in first-trimester prenatal screening tests. Med. Stud. 2024, 40, 341–346. [Google Scholar] [CrossRef]
- Stumpfe, F.M.; Schneider, M.O.; Antoniadis, S.; Mayr, A.; Fleckenstein, T.; Staerk, C.; Kehl, S.; Hermanek, P.; Böhm, J.; Scharl, A.; et al. Lack of evidence for effects of lockdowns on stillbirth rates during the SARS-CoV-2 pandemic in Bavaria: Analysis of the Bavarian perinatal survey from 2010 to 2020. Arch. Gynecol. Obstet. 2022, 308, 1457–1462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen Tran, T.N.; Nguyen, H.T.; Cao, N.T.; Nguyen, P.N.; Truong Thi, L.G.; Le, M.T.; Nguyen Vu, Q.H. Umbilical cord coiling index in predicting neonatal outcomes: A single-center cross-sectional study from Vietnam. J. Matern. Fetal Neonatal Med. 2025, 38, 2517763. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.zeit.de/gesellschaft/zeitgeschehen/2020-12/coronavirus-erste-impfungen-impfstoff-halberstadt (accessed on 5 December 2024).

| Pre-Pandemic Period Maternal Age (Years) Mean (SD) Median (IQR) n = Total Number | Pandemic Period Maternal Age (Years) Mean (SD) Median (IQR) n = Total Number | p-Value | |
|---|---|---|---|
| Whole cohort of patients with pathology | 32.0 (5.3) 32.0 (28.0–36.0) n = 282 | 31.8 (5.7) 31.0 (28.0–35.5) n = 257 | 0.67 |
| Complication/Outcome: | |||
| Hyperemesis | 29.9 (5.6) 31.0 (25.0–33.5) n = 29 | 28.7 (5.8) 27.5 (24.5–34.5) n = 16 | 0.50 |
| Miscarriage < 14 + 0 WG | 34.1 (5.7) 33.5 (30.3–38.0) n = 52 | 33.1 (5.3) 34.0 (29.0–37.0) n = 43 | 0.38 |
| Miscarriage ≥ 14 + 0 WG | 33.1 (5.1) 32.0 (29.0–37.0) n = 11 | 28.9 (3.2) 30.0 (26.0–32.0) n = 10 | 0.04 * |
| preterm birth (<37 + 0 WG) | 31.7 (5.0) 31.0 (28.0–35.0) n = 190 | 31.9 (5.8) 31.0 (28.5–35.0) n = 188 | 0.72 |
| Preterm birth subgroups: | |||
| Preterm with suspected FGR | 31.0 (5.8) 30.5 (27.5–36.0) n = 23 | 32.9 (4.7) 31.0 (29.5–36.0) n = 9 | 0.39 |
| Preterm with preeclampsia | 30.5 (4.3) 29.0 (27.5–33.5) n = 24 | 33.4 (5.9) 34.0 (28.0–36.3) n = 27 | 0.053 |
| Complications/Outcome | Pre-Pandemic Period n (%) | Pandemic Period n (%) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| Hyperemesis (pre-pandemic: births = 1641 pandemic: births = 1711) | 29 (1.8%) | 16 (0.9%) | 0.04 * | 1.90 (1.03–3.52) |
| Miscarriage < 14 + 0 WG (pre-pandemic: births = 1641 pandemic: births = 1711) | 52 (3.2%) | 43 (2.6%) | 0.25 | 1.27 (0.84–1.91) |
| Miscarriage ≥ 14 + 0 WG (pre-pandemic: births = 1641 pandemic: births = 1711) | 11 (0.7%) | 10 (0.5%) | 0.75 | 1.14 (0.49–2.71) |
| Preterm birth (<37 + 0 WG) (pre-pandemic: newborns = 1709 pandemic: newborns = 1806) | 224 (13.1%) | 217 (12.0%) | 0.33 | 1.10 (0.90–1.34) |
| Preterm birth subgroups: | ||||
| Preterm with suspected FGR (pre-pandemic: newborns = 1709 pandemic: newborns = 1806) | 23 (1.5%) | 9 (0.5%) | 0.01 * | 2.72 (1.26–5.90) |
| Preterm with preeclampsia (pre-pandemic: newborns = 1709 pandemic: newborns = 1806) | 24 (1.3%) | 27 (1.5%) | 0.82 | 0.93 (0.53–1.63) |
| Birth Weight (g) | Pre-Pandemic Period n (%) (Total Number of Preterms 224) | Pandemic Period n (%) (Total Number of Preterms 217) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| <1000 | 14 (6.3%) | 15 (6.9%) | 0.93 | 0.90 (0.42–1.90) |
| 1000–<1500 | 22 (9.8%) | 20 (9.2%) | 0.95 | 1.07 (0.57–2.02) |
| 1500–<2000 | 42 (18.7%) | 29 (13.4%) | 0.16 | 1.50 (0.89–2.50) |
| 2000–2500 | 68 (30.4%) | 67 (30.9%) | 0.99 | 0.97 (0.65–1.46) |
| >2500 | 78 (34.8%) | 86 (39.6%) | 0.34 | 0.81 (0.55–1.20) |
| Pre-Pandemic Period (Total Number of Newborns = 1709) n (%) | Pandemic Period (Total Number of Newborns = 1806) n (%) | Post-Pandemic Period (Total Number of Newborns = 1687) n (%) | p-Value | |
|---|---|---|---|---|
| Preterm born babies with FGR | 23 (1.3%) | 9 (0.5%) | 26 (1.5%) | 0.005 * |
| Preterm born babies with preeclampsia | 24 (1.4%) | 27 (1.5%) | 13 (0.8%) | 0.145 |
| Pre-Pandemic Period (n = 23) n (%) | Pandemic Period (n = 9) n (%) | Post-Pandemic Period (n = 26) n (%) | p-Value | |
|---|---|---|---|---|
| Early-onset | 11 (48%) | 3 (33.3%) | 7 (27.9%) | 0.31 |
| Late-onset | 12 (52%) | 6 (66.7%) | 19 (72.1%) |
| Pre-Pandemic Period n (%) | Pandemic Period n (%) | Post-Pandemic Period n (%) | p-Value | |
|---|---|---|---|---|
| Stillbirths (pre-pandemic newborns = 1709 pandemic newborns = 1806 post-pandemic newborns = 1687) | 8 (0.47%) | 9 (0.50%) | 8 (0.47%) | 0.86 |
| Pre-Pandemic Period (n = 23) | Pandemic Period (n = 9) | Post-Pandemic Period (n = 26) | p-Value | |
|---|---|---|---|---|
| Mode of delivery | ||||
| Cesarean section | 20 (87%) | 7 (77.8%) | 20 (76.9%) | 0.66 |
| Vaginal birth | 3 (13%) | 2 (22.2%) | 6 (23.1%) | |
| Birth weight | ||||
| <10th percentile | 18 (78.2%) | 9 (100%) | 14 (53.8%) | 0.019 * |
| ≥10th percentile | 5 (21.7%) | 0 (0%) | 12 (46.2%) | |
| Indication for delivery | ||||
| FGR | 14 (60.8%) | 6 (66.7%) | 11 (42.3%) | 0.045 * |
| Preeclampsia | 5 (21.7%) | 2 (22.2%) | 4 (15.4%) | |
| PPROM/contractions | 4 (17.5%) | 1 (11.1%) | 10 (38.5%) | |
| Others | 0 (0%) | 0 (0%) | 1 (3.8%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauh, M.; Suttner, S.; Bartl, C.; Weigl, M.; Wellmann, S.; Kappelmeyer, M.; Schmidt, B.; Solano, M.E.; Köninger, A. Impact of COVID-19 Lockdown on the Incidence of Common Pregnancy Complications—Is the Diagnosis of FGR Made Too Generously? Children 2025, 12, 1085. https://doi.org/10.3390/children12081085
Rauh M, Suttner S, Bartl C, Weigl M, Wellmann S, Kappelmeyer M, Schmidt B, Solano ME, Köninger A. Impact of COVID-19 Lockdown on the Incidence of Common Pregnancy Complications—Is the Diagnosis of FGR Made Too Generously? Children. 2025; 12(8):1085. https://doi.org/10.3390/children12081085
Chicago/Turabian StyleRauh, Maximilian, Silvia Suttner, Claudia Bartl, Marco Weigl, Sven Wellmann, Maurice Kappelmeyer, Börge Schmidt, Maria Emilia Solano, and Angela Köninger. 2025. "Impact of COVID-19 Lockdown on the Incidence of Common Pregnancy Complications—Is the Diagnosis of FGR Made Too Generously?" Children 12, no. 8: 1085. https://doi.org/10.3390/children12081085
APA StyleRauh, M., Suttner, S., Bartl, C., Weigl, M., Wellmann, S., Kappelmeyer, M., Schmidt, B., Solano, M. E., & Köninger, A. (2025). Impact of COVID-19 Lockdown on the Incidence of Common Pregnancy Complications—Is the Diagnosis of FGR Made Too Generously? Children, 12(8), 1085. https://doi.org/10.3390/children12081085





