Impact of Heart Rate Monitoring Using Dry-Electrode ECG Immediately After Birth on Time to Start Ventilation: A Randomized Trial
Abstract
Highlights
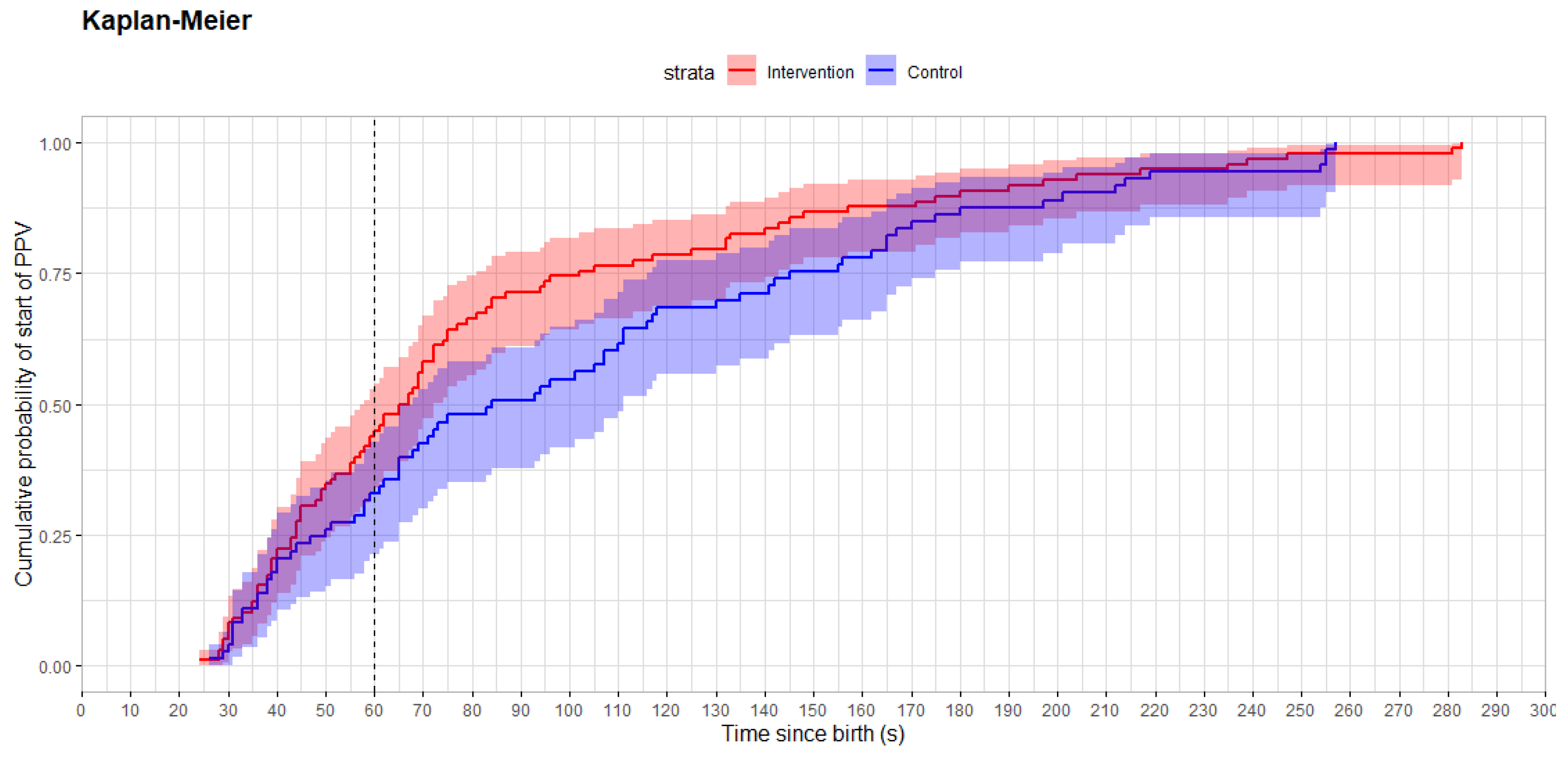
- Ventilation was provided within 60 s to 45% of newborns in the ECG heart rate-visible group versus 33% in the control group, p = 0.12. Time from birth to start of PPV was a median of 66 (44, 102) s in the intervention and 84 (49, 148) s in the control group, p = 0.058.
- Only 36% of resuscitated newborns were bradycardic with HR < 100 beats per minute at the start of PPV.
- Use of dry-electrode ECG HR monitoring did not change the proportion of newborns that received PPV within 60 s after birth, but early termination due to employee protest over video recording rendered the trial inadequately powered to detect a difference. Starting ventilation within 60 s after birth remains challenging.
- All newborns that received PPV were apneic or ineffectively breathing at the start of PPV, but only about a third were bradycardic. This reinforces current resuscitation guideline recommendations, highlighting that respiratory status is the most important indicator for starting PPV.
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Design
2.3. Study Participants
2.4. Intervention
2.5. Data Collection
2.6. Outcome Measures
2.7. Statistical Analysis and Power Calculation
2.8. Early Termination of the Trial
3. Results
3.1. Baseline Characteristics
3.2. Resuscitative Interventions and Short-Term Outcomes
3.3. Heart Rate Acquisition
3.4. Safety Outcomes
3.5. Protocol Violations
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bpm | beats per minute |
| ECG | electrocardiogram |
| HCP | healthcare provider |
| HR | heart rate |
| ILCOR | The International Liaison Committee on Resuscitation |
| PPV | positive pressure ventilation |
| RCT | randomized controlled trial |
References
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; de Almeida, M.F.; Fabres, J.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2020, 142 (Suppl. 1), S185–S221. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Greif, R.; Morley, P.T.; Ng, K.C.; Olasveengen, T.M.; Singletary, E.M.; Soar, J.; Cheng, A.; Drennan, I.R.; Liley, H.G.; et al. 2022 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Resuscitation 2022, 181, 208–288. [Google Scholar] [CrossRef]
- Berg, K.M.; Bray, J.E.; Ng, K.C.; Liley, H.G.; Greif, R.; Carlson, J.N.; Morley, P.T.; Drennan, I.R.; Smyth, M.; Scholefield, B.R.; et al. 2023 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Circulation 2023, 148, e187–e280. [Google Scholar] [CrossRef]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rudiger, M.; Skare, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Lee, H.C.; Escobedo, M.B.; Hoover, A.V.; Kamath-Rayne, B.D.; Kapadia, V.S.; Magid, D.J.; Niermeyer, S.; Schmolzer, G.M.; Szyld, E.; et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142 (Suppl. 2), S524–S550. [Google Scholar] [CrossRef] [PubMed]
- Pike, H.; Kolstad, V.; Eilevstjonn, J.; Davis, P.G.; Langli Ersdal, H.; Irene Rettedal, S. Newborn resuscitation timelines: Accurately capturing treatment in the delivery room. Resuscitation 2024, 197, 110156. [Google Scholar] [CrossRef] [PubMed]
- Bjorland, P.A.; Ersdal, H.L.; Oymar, K.; Rettedal, S.I. Compliance with Guidelines and Efficacy of Heart Rate Monitoring during Newborn Resuscitation: A Prospective Video Study. Neonatology 2020, 117, 175–181. [Google Scholar] [CrossRef]
- Niles, D.E.; Cines, C.; Insley, E.; Foglia, E.E.; Elci, O.U.; Skare, C.; Olasveengen, T.; Ades, A.; Posencheg, M.; Nadkarni, V.M.; et al. Incidence and characteristics of positive pressure ventilation delivered to newborns in a US tertiary academic hospital. Resuscitation 2017, 115, 102–109. [Google Scholar] [CrossRef]
- Rettedal, S.; Eilevstjonn, J.; Kibsgaard, A.; Kvaloy, J.T.; Ersdal, H. Comparison of Heart Rate Feedback from Dry-Electrode ECG, 3-Lead ECG, and Pulse Oximetry during Newborn Resuscitation. Children 2021, 8, 1092. [Google Scholar] [CrossRef]
- van Vonderen, J.J.; Hooper, S.B.; Kroese, J.K.; Roest, A.A.; Narayen, I.C.; van Zwet, E.W.; te Pas, A.B. Pulse oximetry measures a lower heart rate at birth compared with electrocardiography. J. Pediatr. 2015, 166, 49–53. [Google Scholar] [CrossRef]
- Johnson, P.A.; Schmolzer, G.M. Heart Rate Assessment during Neonatal Resuscitation. Healthcare 2020, 8, 43. [Google Scholar] [CrossRef]
- Bush, J.B.; Cooley, V.; Perlman, J.; Chang, C. NeoBeat offers rapid newborn heart rate assessment. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 550–552. [Google Scholar] [CrossRef]
- Bjorland, P.A.; Ersdal, H.L.; Eilevstjonn, J.; Oymar, K.; Davis, P.G.; Rettedal, S.I. Changes in heart rate from 5 s to 5 min after birth in vaginally delivered term newborns with delayed cord clamping. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 106, 311–315. [Google Scholar] [CrossRef] [PubMed]
- van Twist, E.; Salverda, H.H.; Pas, A.B.T. Comparing pulse rate measurement in newborns using conventional and dry-electrode ECG monitors. Acta Paediatr. 2022, 111, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Pike, H.; Eilevstjonn, J.; Bjorland, P.; Linde, J.; Ersdal, H.; Rettedal, S. Heart rate detection properties of dry-electrode ECG compared to conventional 3-lead gel-electrode ECG in newborns. BMC Res. Notes 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Rettedal, S.; Kibsgaard, A.; Eilevstjonn, J.; Kvaloy, J.T.; Bjorland, P.A.; Markhus Pike, H.; Haynes, J.; Tysland, T.B.; Stordal, K.; Holte, K.; et al. Impact of immediate and continuous heart rate feedback by dry electrode ECG on time to initiation of ventilation after birth: Protocol for a randomised controlled trial. BMJ Open 2022, 12, e061839. [Google Scholar] [CrossRef]
- Bjorland, P.A.; Oymar, K.; Ersdal, H.L.; Rettedal, S.I. Incidence of newborn resuscitative interventions at birth and short-term outcomes: A regional population-based study. BMJ Paediatr. Open 2019, 3, e000592. [Google Scholar] [CrossRef]
- O’Brien, P.C.; Fleming, T.R. A multiple testing procedure for clinical trials. Biometrics 1979, 35, 549–556. [Google Scholar] [CrossRef]
- Ersdal, H.L.; Mduma, E.; Svensen, E.; Perlman, J.M. Early initiation of basic resuscitation interventions including face mask ventilation may reduce birth asphyxia related mortality in low-income countries: A prospective descriptive observational study. Resuscitation 2012, 83, 869–873. [Google Scholar] [CrossRef]
- Linde, J.E.; Perlman, J.M.; Oymar, K.; Schulz, J.; Eilevstjonn, J.; Thallinger, M.; Kusulla, S.; Kidanto, H.L.; Ersdal, H.L. Predictors of 24-h outcome in newborns in need of positive pressure ventilation at birth. Resuscitation 2018, 129, 1–5. [Google Scholar] [CrossRef]
- Vadla, M.S.; Mduma, E.R.; Kvaloy, J.T.; Mdoe, P.; Hhoki, B.H.; Sarangu, S.; Michael, P.; Oftedal, B.; Ersdal, H. Increase in Newborns Ventilated Within the First Minute of Life and Reduced Mortality After Clinical Data-Guided Simulation Training. Simul. Healthc. 2023, 19, 271–280. [Google Scholar] [CrossRef]
- Patterson, J.K.; Ishoso, D.; Eilevstjonn, J.; Bauserman, M.; Haug, I.; Iyer, P.; Kamath-Rayne, B.D.; Lokangaka, A.; Lowman, C.; Mafuta, E.; et al. Delayed and Interrupted Ventilation with Excess Suctioning after Helping Babies Breathe with Congolese Birth Attendants. Children 2023, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Katheria, A.C.; Morales, A.; Shashank, S.; Rich, W.D.; Finer, N.N. A Pilot Randomized Trial of Heart Rate Monitoring Using Conventional Versus a New Electrocardiogram Algorithm during Neonatal Resuscitation at Birth. J. Pediatr. 2022, 242, 245–247.e1. [Google Scholar] [CrossRef] [PubMed]
- Kapadia VS, K.M.; Strand, M.; Gately, C.; Costa-Nobre, D.T.; Davis, P.G.; de Almeida, M.F.; El-Naggar, W.; Fabres, J.G.; Fawke, J.; Finan, E.; et al. Methods of Heart Rate Monitoring in the Delivery Room and Neonatal Outcomes (NLS #5201). International Liaison Committee on Resuscitation (ILCOR) Neonatal Life Support Task Force 2022. Available online: http://ilcor.org (accessed on 13 August 2025).
- Patterson, J.K.; Ishoso, D.; Lokangaka, A.; Iyer, P.; Lowman, C.; Eilevstjonn, J.; Haug, I.; Kamath-Rayne, B.D.; Mafuta, E.; Myklebust, H.; et al. Neonatal outcomes and resuscitation practices following the addition of heart rate-guidance to basic resuscitation. PLoS ONE 2025, 20, e0317199. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Berkelhamer, S.; Ishoso, D.; Iyer, P.; Lowman, C.; Bauserman, M.; Eilevstjonn, J.; Haug, I.; Lokangaka, A.; Kamath-Rayne, B.; et al. Effect of resuscitation training and implementation of continuous electronic heart rate monitoring on identification of stillbirth. Resuscitation 2022, 171, 57–63. [Google Scholar] [CrossRef]
- Ersdal, H.L.; Eilevstjonn, J.; Linde, J.E.; Yeconia, A.; Mduma, E.R.; Kidanto, H.; Perlman, J. Fresh stillborn and severely asphyxiated neonates share a common hypoxic-ischemic pathway. Int. J. Gynaecol. Obstet. 2018, 141, 171–180. [Google Scholar] [CrossRef]
- Shah, B.A.; Wlodaver, A.G.; Escobedo, M.B.; Ahmed, S.T.; Blunt, M.H.; Anderson, M.P.; Szyld, E.G. Impact of electronic cardiac (ECG) monitoring on delivery room resuscitation and neonatal outcomes. Resuscitation 2019, 143, 10–16. [Google Scholar] [CrossRef]
- Katheria, A.; Arnell, K.; Brown, M.; Hassen, K.; Maldonado, M.; Rich, W.; Finer, N. A pilot randomized controlled trial of EKG for neonatal resuscitation. PLoS ONE 2017, 12, e0187730. [Google Scholar] [CrossRef]
- Abbey, N.V.; Mashruwala, V.; Weydig, H.M.; Steven Brown, L.; Ramon, E.L.; Ibrahim, J.; Mir, I.N.; Wyckoff, M.H.; Kapadia, V. Electrocardiogram for heart rate evaluation during preterm resuscitation at birth: A randomized trial. Pediatr. Res. 2022, 91, 1445–1451. [Google Scholar] [CrossRef]
- Linde, J.E.; Schulz, J.; Perlman, J.M.; Oymar, K.; Francis, F.; Eilevstjonn, J.; Ersdal, H.L. Normal Newborn Heart Rate in the First Five Minutes of Life Assessed by Dry-Electrode Electrocardiography. Neonatology 2016, 110, 231–237. [Google Scholar] [CrossRef]
- Kibsgaard, A.; Ersdal, H.; Kvaloy, J.T.; Eilevstjonn, J.; Rettedal, S. Newborns requiring resuscitation: Two thirds have heart rate >/=100 beats/minute in the first minute after birth. Acta Paediatr. 2023, 112, 697–705. [Google Scholar] [CrossRef]
- Rettedal, S.; Kibsgaard, A.; Kvaloy, J.T.; Eilevstjonn, J.; Ersdal, H.L. Prevalence of bradycardia in 4876 newborns in the first minute after birth and association with positive pressure ventilation: A population-based cross-sectional study. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 109, 371–377. [Google Scholar] [CrossRef]
- Patterson, J.; Niermeyer, S. Delayed cord clamping and the response to bradycardia immediately after birth. Arch. Dis. Child. Fetal Neonatal Ed. 2024, 109, 346–347. [Google Scholar] [CrossRef]
- Skare, C.; Kramer-Johansen, J.; Steen, T.; Odegaard, S.; Niles, D.E.; Nakstad, B.; Solevag, A.L.; Nadkarni, V.M.; Olasveengen, T.M. Incidence of Newborn Stabilization and Resuscitation Measures and Guideline Compliance during the First Minutes of Life in Norway. Neonatology 2015, 108, 100–107. [Google Scholar] [CrossRef]
- Kolstad, V.; Garcia-Torres, J.; Brunner, S.; Johannessen, A.; Foglia, E.; Ersdal, H.; Meinich-Bache, O.; Rettedal, S. Detection of time of birth and cord clamping using thermal video in the delivery room. Front. Pediatr. 2024, 12, 1342415. [Google Scholar] [CrossRef]

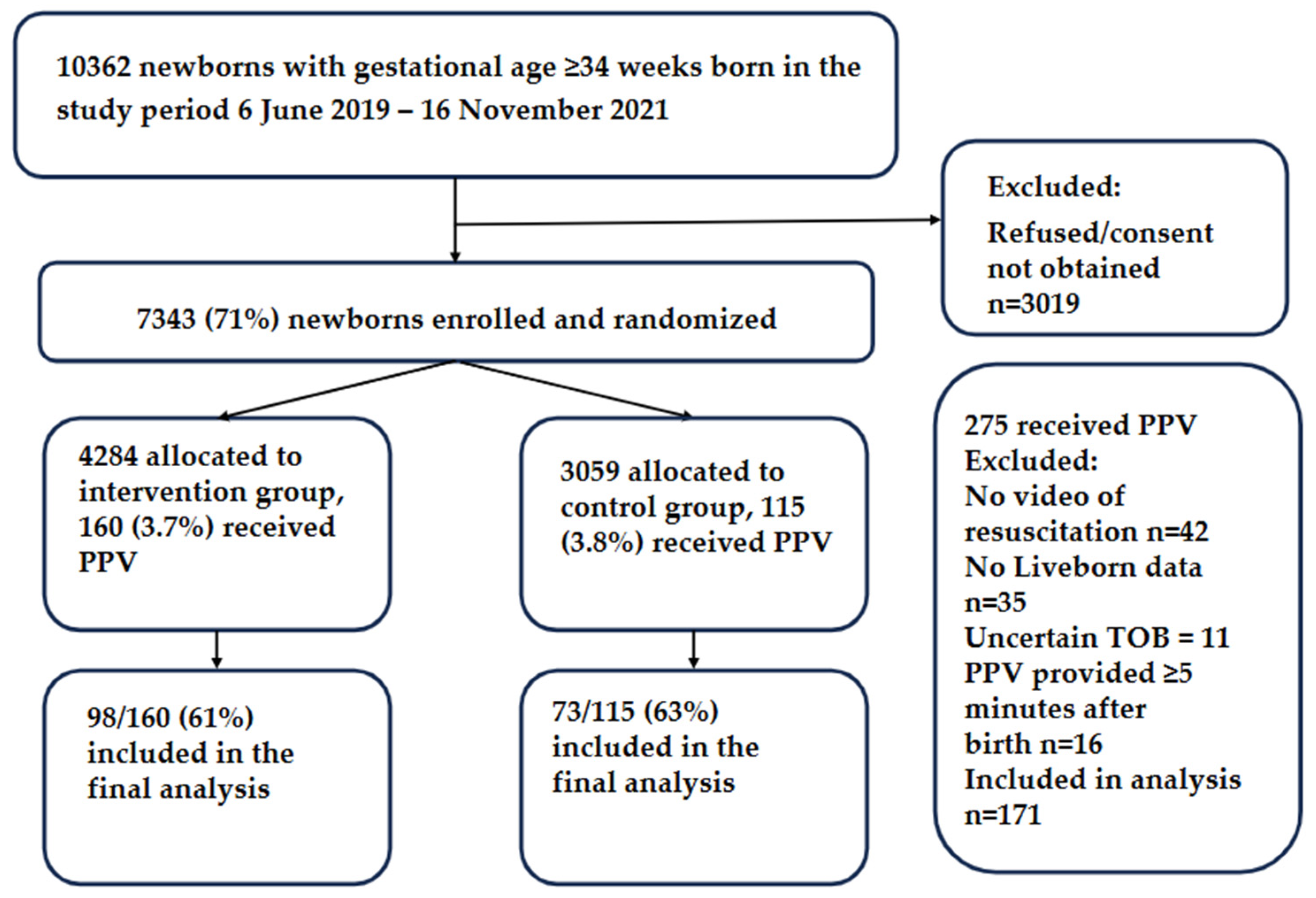
| Participant Characteristics | Intervention, n = 98 | Control, n = 73 | p-Value |
|---|---|---|---|
| Maternal age | 31.0 (4.3) | 31.8 (4.9) | 0.2 |
| Pre-eclampsia | 3 (3.1%) | 3 (4.1%) | 0.7 |
| Gestational diabetes | 7 (7.1%) | 4 (5.5%) | 0.8 |
| Parity | 0.5 | ||
| Nulliparous | 56 (57%) | 37 (51%) | |
| Primiparous | 33 (34%) | 26 (36%) | |
| Multiparous | 9 (9.2%) | 10 (14%) | |
| Mode of delivery | 0.2 | ||
| Vaginal | 13 (13%) | 18 (25%) | |
| Vacuum or forceps | 32 (33%) | 19 (26%) | |
| Elective caesarean section | 2 (2.0%) | 4 (5.5%) | |
| Emergency caesarean section | 43 (44%) | 28 (38%) | |
| Breech | 8 (8.2%) | 4 (5.5%) | |
| Gestational age (weeks) | 39.2 (1.8) | 39.3 (1.8) | 0.8 |
| Birth weight (grams) | 3416 (736) | 3540 (660) | 0.4 |
| Female | 46 (47%) | 32 (44%) | 0.8 |
| Twins | 7 (7.1%) | 4 (5.5%) | 0.8 |
| Umbilical arterial pH | 7.16 (0.12) | 7.19 (0.09) | 0.3 |
| Missing | 18 | 14 | |
| Apgar 1 min | 5 (3, 6) | 5 (4, 6) | >0.9 |
| Apgar 5 min | 8 (6, 9) | 8 (7, 9) | 0.4 |
| Apgar 10 min | 9 (8, 10) | 9 (8,10) | 0.6 |
| Intervention, n = 98 | Control, n = 73 | p-Value | |
|---|---|---|---|
| Resuscitation outcomes | |||
| PPV provided within 60 s after birth | 44 (45%) | 24 (33%) | 0.12 |
| Time from birth to start of PPV (s) | 66 (44, 101) | 84 (50, 145) | 0.058 |
| Duration of PPV (s) | 141 (66, 223) | 122 (67, 231) | 0.7 |
| Missing | 12 | 4 | |
| HR data | |||
| Heart rate at 60 s (bpm) | 147 (88, 167) | 139 (102, 168) | 0.8 |
| Missing | 42 | 29 | |
| Heart rate at start of PPV | 146 (86, 177) | 106 (75, 157) | 0.065 |
| Missing | 49 | 29 | |
| ≥10 s of HR < 100 bpm in first 5 min after birth | 37 (43%) | 35 (60%) | 0.06 |
| Missing | 12 | 15 | |
| Tidal volumes | |||
| Mean tidal volume first 30 s (mL/kg) | 4.6 (3.1, 6.1) | 4.8 (2.9, 7.9) | 0.6 |
| Mean tidal volume first 60 s (mL/kg) | 4.7 (3.2, 6.4) | 4.9 (2.8, 7.3) | 0.6 |
| Proportion of inflations with tidal volume ≥ 4 mL/kg first 30 s | 0.6 (0.4, 0.8) | 0.5 (0.2, 0.7) | 0.12 |
| Proportion of inflations with tidal volume ≥ 4 mL/kg first 60 s | 0.6 (0.4, 0.8) | 0.6 (0.3, 0.8) | 0.7 |
| Proportion of inflations with tidal volume ≥ 6 mL/kg first 30 s | 0.3 (0.1, 0.5) | 0.3 (0.0, 0.6) | 0.3 |
| Proportion of inflations with tidal volume ≥ 6 mL/kg first 60 s | 0.3 (0.1, 0.5) | 0.4 (0.1, 0.6) | 0.8 |
| Proportion of inflations with tidal volume ≥ 8 mL/kg first 30 s | 0.1 (0.0, 0.3) | 0.1 (0.0, 0.6) | 0.6 |
| Proportion of inflations with tidal volume ≥ 8 mL/kg first 60 s | 0.2 (0.0, 0.3) | 0.2 (0.0, 0.6) | 0.5 |
| Missing for all tidal volume data | 46 | 35 | |
| Short term outcomes | |||
| Apgar at 5 min | 0.4 | ||
| Apgar ≥ 7 | 49 (50%) | 41 (56%) | |
| Apgar < 7 | 49 (50%) | 32 (44%) | |
| Apgar at 10 min | 0.8 | ||
| Apgar ≥ 7 | 80 (82%) | 58 (79%) | |
| Apgar < 7 | 18 (18%) | 15 (21%) | |
| Intubation | 5 (5.1%) | 1 (1.4%) | 0.2 |
| Chest compressions | 4 (4.1%) | 2 (2.7%) | >0.9 |
| Death before discharge | 1 (1.0%) | 0 (0%) | >0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rettedal, S.; Kibsgaard, A.; Buskov, F.; Eilevstjønn, J.; Kolstad, V.; Kvaløy, J.T.; Bjorland, P.A.; Pike, H.; Haynes, J.; Tysland, T.B.; et al. Impact of Heart Rate Monitoring Using Dry-Electrode ECG Immediately After Birth on Time to Start Ventilation: A Randomized Trial. Children 2025, 12, 1082. https://doi.org/10.3390/children12081082
Rettedal S, Kibsgaard A, Buskov F, Eilevstjønn J, Kolstad V, Kvaløy JT, Bjorland PA, Pike H, Haynes J, Tysland TB, et al. Impact of Heart Rate Monitoring Using Dry-Electrode ECG Immediately After Birth on Time to Start Ventilation: A Randomized Trial. Children. 2025; 12(8):1082. https://doi.org/10.3390/children12081082
Chicago/Turabian StyleRettedal, Siren, Amalie Kibsgaard, Frederikke Buskov, Joar Eilevstjønn, Vilde Kolstad, Jan Terje Kvaløy, Peder Aleksander Bjorland, Hanne Pike, Joanna Haynes, Thomas Bailey Tysland, and et al. 2025. "Impact of Heart Rate Monitoring Using Dry-Electrode ECG Immediately After Birth on Time to Start Ventilation: A Randomized Trial" Children 12, no. 8: 1082. https://doi.org/10.3390/children12081082
APA StyleRettedal, S., Kibsgaard, A., Buskov, F., Eilevstjønn, J., Kolstad, V., Kvaløy, J. T., Bjorland, P. A., Pike, H., Haynes, J., Tysland, T. B., Davis, P. G., & Ersdal, H. (2025). Impact of Heart Rate Monitoring Using Dry-Electrode ECG Immediately After Birth on Time to Start Ventilation: A Randomized Trial. Children, 12(8), 1082. https://doi.org/10.3390/children12081082









