Relationship of Melatonin Levels in Blood and Urine with Sleep Quality in Children Admitted to Pediatric Intensive Care Unit
Abstract
Highlights
- Children admitted to the PICU frequently fail to achieve the recommended amount of sleep for their age.
- Altered serum and urinary melatonin levels are significantly associated with poorer sleep quality.
- In the PICU, circadian rhythm is disrupted. Among modifiable factors that contribute to this, are the environmental factors such as artificial lighting, noise, and nocturnal interventions.
- Melatonin is a potential non-invasive biomarker of circadian dysfunction in the PICU setting.
Abstract
1. Introduction
2. Materials and Methods
- Patient characteristics: demographic data, location within the PICU (room type), clinical interventions received, and medications administered during the study period, including enteral abstinence medication.
- Sleep characteristics: total sleep duration during the day and night (including naps), the duration of continuous nighttime sleep episodes, and characteristics of sleep interruptions (frequency, duration, and causes). The sleep data were obtained through clinical observation and routine nursing documentation recorded in patient charts. Sleep quality was categorized as either adequate or inadequate based on whether the patient met the 2nd and 50th percentiles of expected sleep duration for their age, according to the normative values published by Iglowstein et al. [13].
- Environmental factors were assessed through objective measurements of noise and light levels:
- ○
- Noise was measured continuously using the PCE-322A® sound level meter, which operates within a range of 30 to 130 decibels (dB) and offers a measurement accuracy of ±1.4 dB.
- ○
- Light intensity was measured every two hours using the PCE-174® lux meter, with a measuring range of 400 to 4000 lux and an accuracy of ±5% for levels below 10,000 lux and ±10% for levels above 10,000 lux.
- Melatonin levels were evaluated through the collection of biological samples on the first day of observation. Specifically:
- ○
- Blood and urine samples were collected early in the morning (around 7:00 a.m. or upon the patient’s awakening).
- ○
- An additional urine sample was obtained in the late afternoon (between 7:00 and 8:00 p.m.).
- Elevated morning serum melatonin: Values > 30 pg/mL at 7:00 a.m. were considered abnormal. At this time of day, melatonin levels are physiologically low. Elevated levels suggest a disruption in the normal circadian secretion pattern of melatonin.
- Reduced urinary 6-SMEL at 7:00 a.m.: Concentrations < 58 mcg/L were considered low. Morning urine typically contains melatonin metabolites accumulated during nighttime sleep; therefore, reduced levels are indicative of decreased nocturnal melatonin secretion.
- Elevated urinary 6-SMEL at 7:00 p.m.: Concentrations > 58 mcg/L were considered elevated. Since this sample reflects melatonin metabolism during the daytime, lower levels would be expected. Elevated values may suggest abnormal daytime melatonin secretion, which could be associated with increased daytime sleepiness or circadian rhythm dysregulation.
Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Sleep Characteristics
3.3. Melatonin Levels in Blood and Urine
3.4. Relationship Between Melatonin Levels and Sleep Characteristics
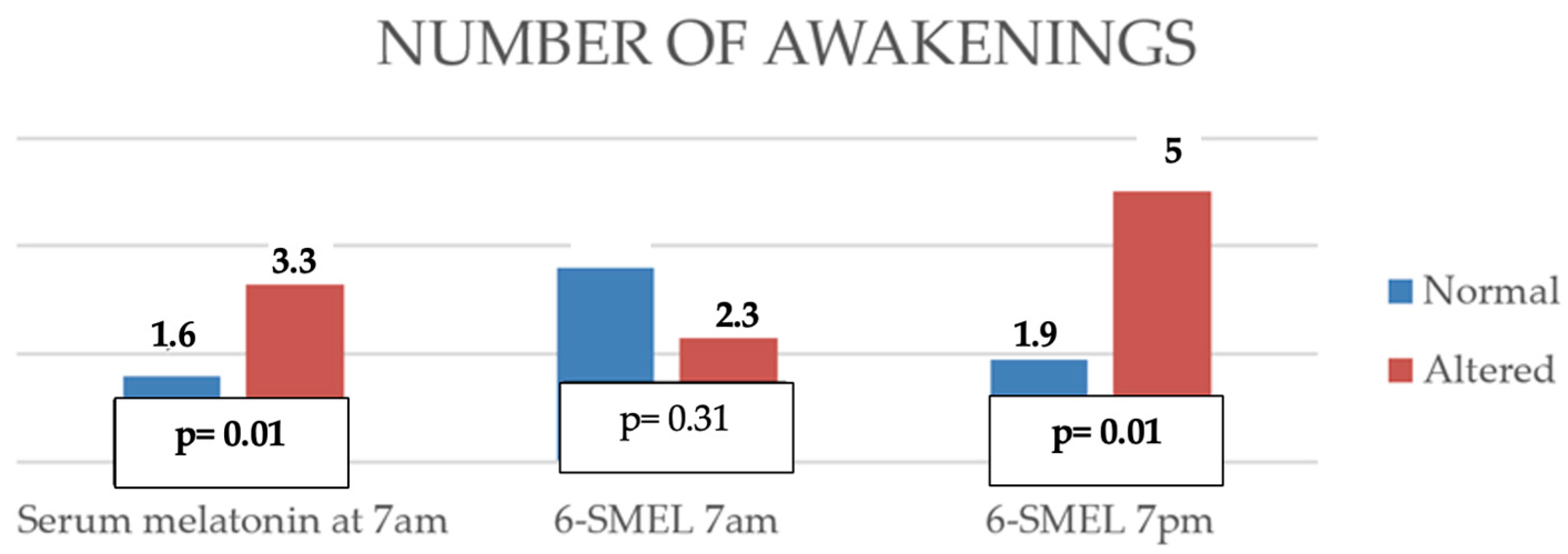
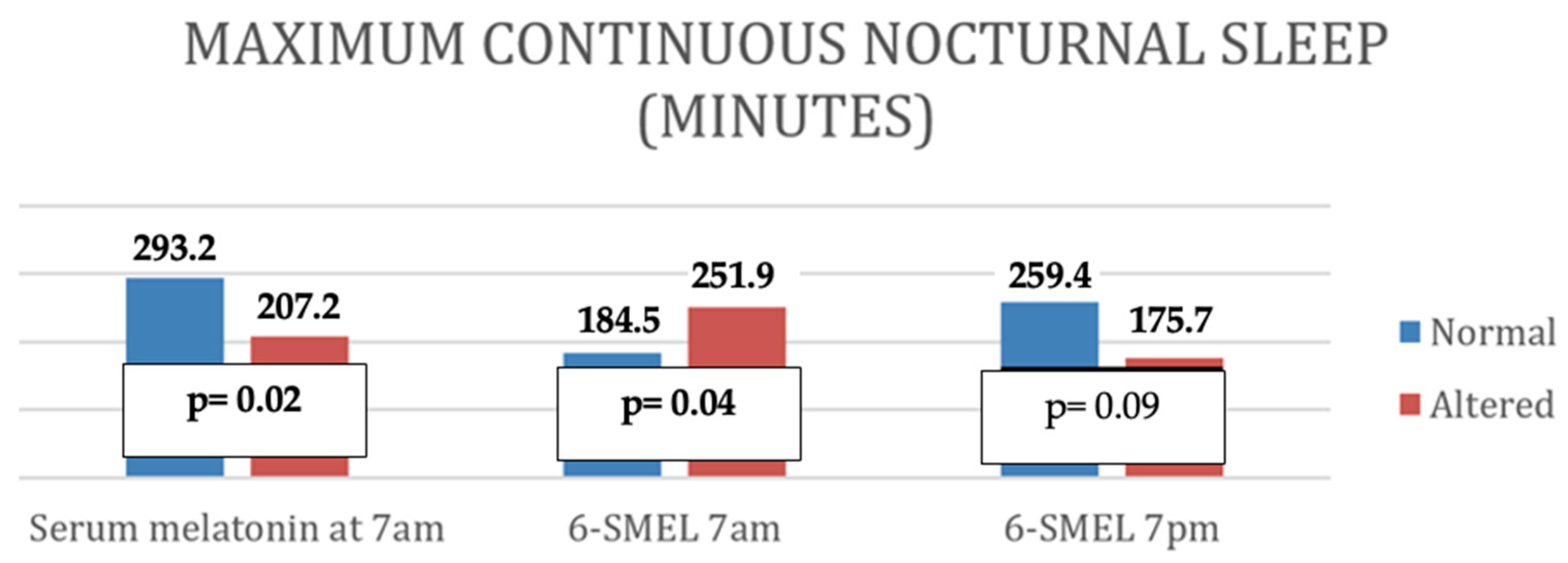
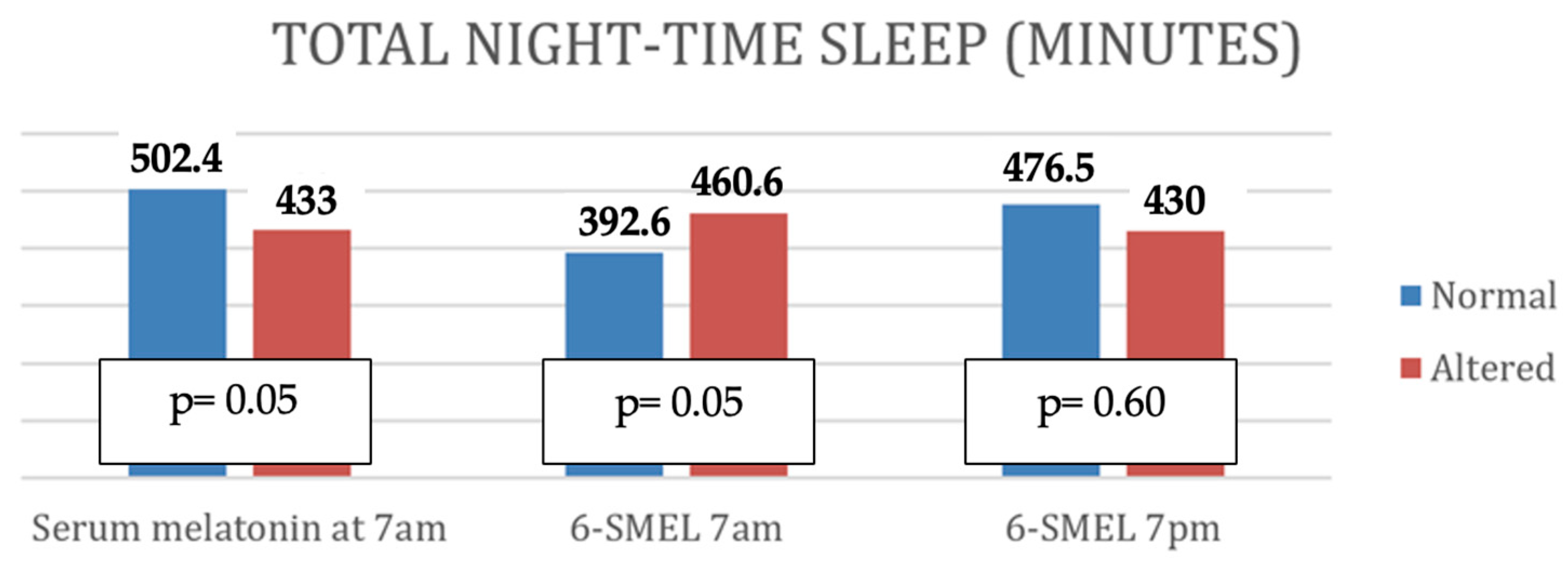
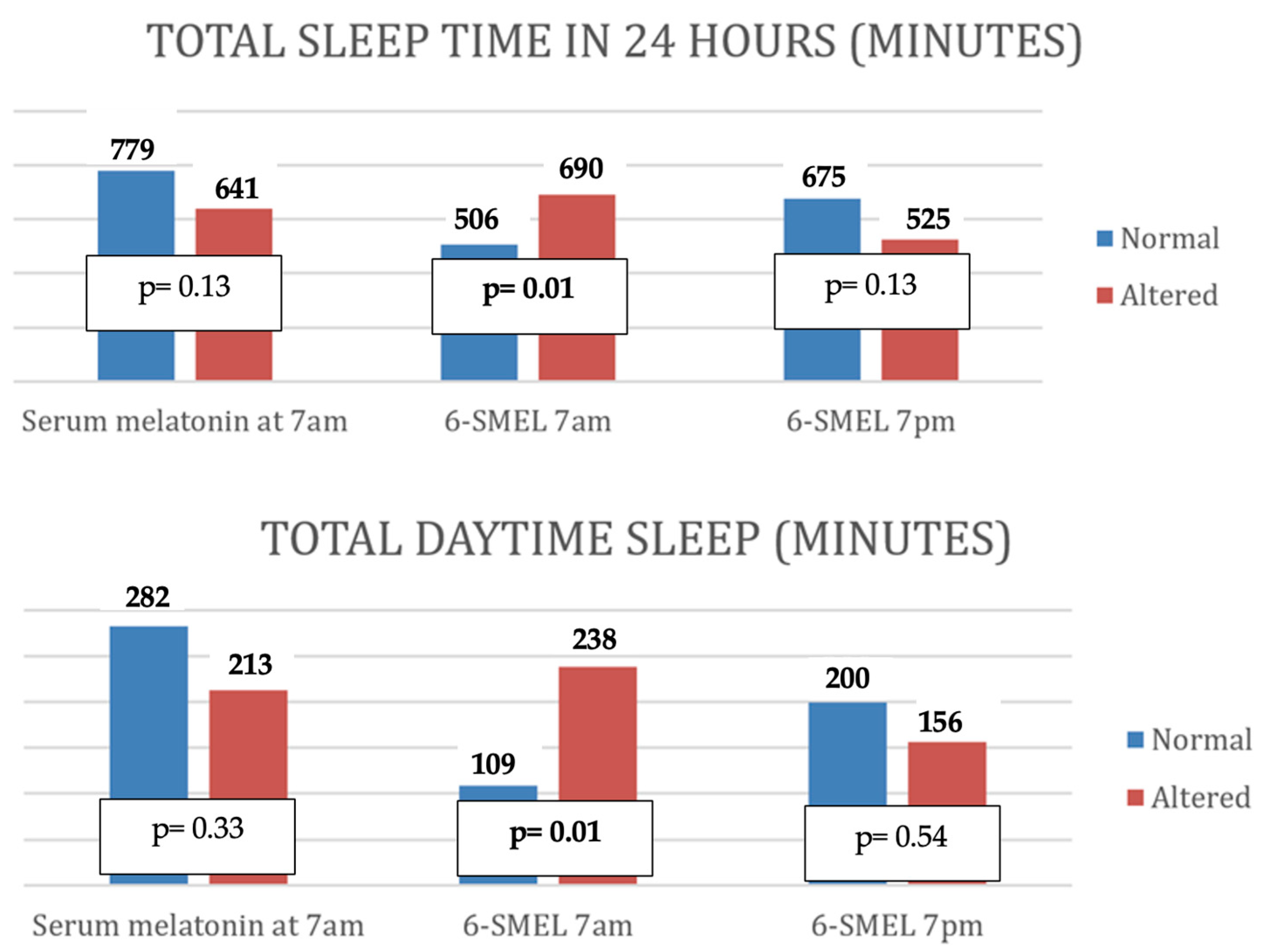
- Altered serum melatonin was associated with shorter nighttime sleep duration, more frequent awakenings, shorter total nighttime sleep, and reduced continuous sleep episodes.
- Low morning 6-SMEL levels were associated with longer daytime sleep and increased total 24 h sleep.
- High evening 6-SMEL was associated with reduced total daily sleep duration and a higher number of nighttime awakenings.
3.5. Relationship Between Melatonin Levels and Environmental Factors
4. Discussion
4.1. Melatonin Levels in Blood and Urine
4.2. Relationship Between Melatonin Levels and Clinical Patient Characteristics
4.3. Relationship Between Melatonin Levels and Sleep Characteristics
4.4. Relationship Between Melatonin Levels and Ambient Light and Noise
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PICU | Pediatric Intensive Care unit |
| 6-SMEL | 6-sulfatoxymelatonin |
| CAPD | Cornell Assessment of Pediatric Delirium |
| PRISM | Pediatric Risk of Mortality |
| IQR | Interquartile Range |
| MV | Mechanical Ventilation |
| SD | Standard Deviation |
Appendix A
| Good Sleep | ||||
|---|---|---|---|---|
| Nighttime p2 | Nighttime p50 | 24 h p2 | 24 h p50 | |
| Enteral sedoanalgesic medication | p = 0.90 | p = 0.16 | p = 0.52 | p = 0.93 |
| Drug combination | p=0.48 | p = 0.22 | p = 0.08 | p = 0.49 |
| Serum Melatonin 7 am | 6-SMEL 1 7 am | 6-SMEL 1 7 pm | ||
|---|---|---|---|---|
| Number of awakenings | p | 0.01 | 0.10 | 0.01 |
| r | 0.35 | 0.46 | ||
| Total time of nighttime sleep (minutes) | p | 0.08 | 0.07 | 0.08 |
| r | ||||
| Maximum time of continuous sleep (minutes) | p | 0.03 | 0.10 | 0.03 |
| r | −0.29 | −0.37 | ||
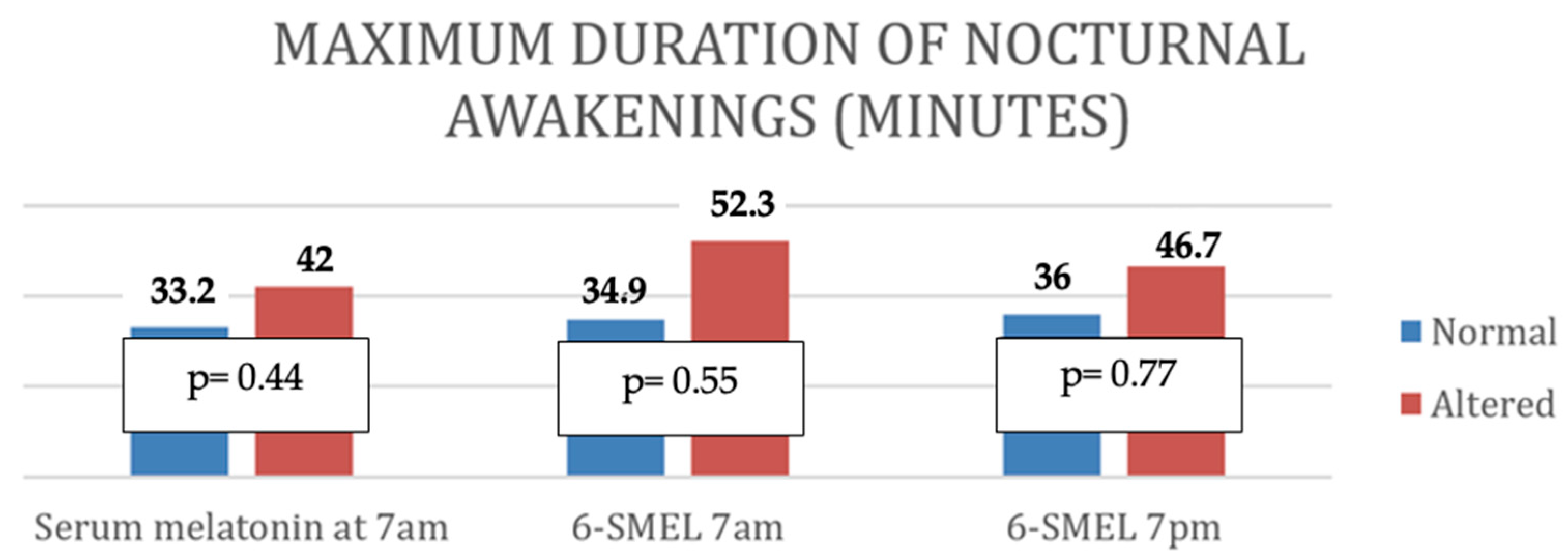
| Maximum duration of awakenings (minutes) | p | 0.38 | 0.73 | 0.97 |
| r | ||||
| Sleep in 24 h (minutes) | p | 0.14 | 0.01 | 0.04 |
| r | −0.43 | −0.38 | ||
| Daytime sleep (minutes) | p | 0.37 | 0.03 | 0.37 |
| r | −0.34 | |||
| Sleep Quality | Altered Melatonin | ||||||
|---|---|---|---|---|---|---|---|
| Serum 7 a.m. | p | Urine 7 a.m. | p | Urine 7 p.m. | p | ||
| Nighttime p2 | B | 20 | 0.09 | 12 | 0.08 | 3 | 1 |
| G | 16 | 14 | 3 | ||||
| Nighttime p50 | B | 35 | 0.01 | 21 | 0.06 | 5 | 1 |
| G | 1 | 5 | 1 | ||||
| Daytime p2 | B * | - | - | - | |||
| G | 34 | 26 | 8 | ||||
| Daytime p50 | B | 2 | 0.57 | 2 | 0.52 | 1 | 0.46 |
| G | 32 | 24 | 7 | ||||
| 24 h p2 | B | 8 | 1 | 5 | 0.08 | 4 | 0.02 |
| G | 23 | 18 | 2 | ||||
| 24 h p50 | B | 21 | 0.06 | 11 | 0.02 | 4 | 1 |
| G | 10 | 12 | 2 | ||||
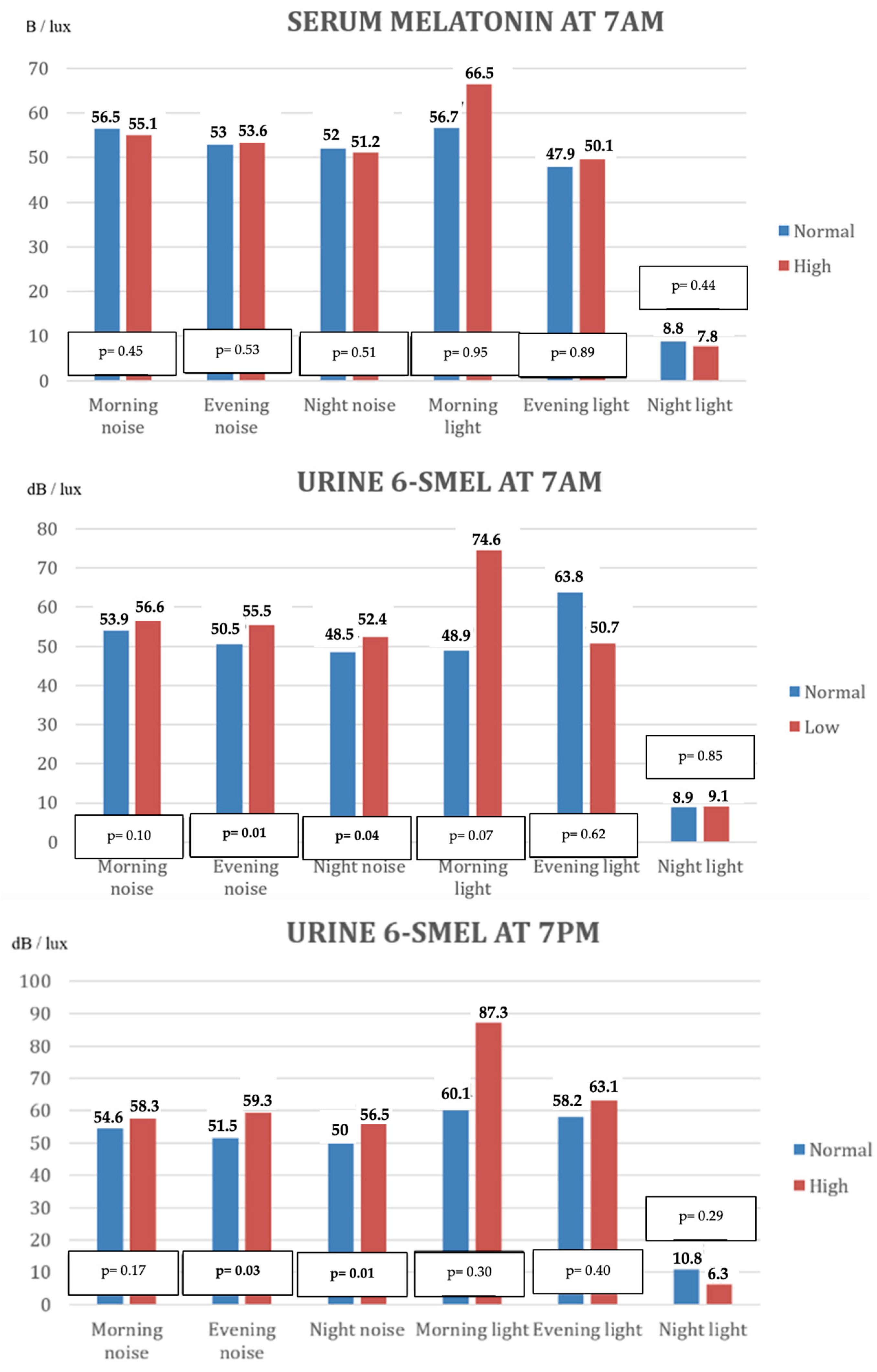
| Serum Melatonin at 7 AM | ||||||
|---|---|---|---|---|---|---|
| High | Normal | |||||
| Morning | Evening | Night | Morning | Evening | Night | |
| Noise dB (mean values ± SD) | 55.1 ± 6.1 | 53.6 ± 7.2 | 51.2 ± 6.1 | 56.5 ± 4.3 | 53 ± 4.6 | 52 ± 5.4 |
| Light lux (mean values ± SD) | 66.5 ± 49.1 | 50.1 ± 28.1 | 7.8 ± 6.9 | 56.7 ± 13.4 | 47.9 ± 28.9 | 8.8 ± 7.7 |
| Urine 6-SMEL AT 7 AM | ||||||
| Low | Normal | |||||
| Morning | Evening | Night | Morning | Evening | Night | |
| Noise dB (mean values ± SD) | 56.6 ± 6.6 | 55.5 ± 4.1 | 52.4 ± 4.6 | 53.9 ± 4.7 | 50.5 ± 8.1 | 48.9 ± 6.6 |
| Light lux (mean values ± SD) | 74.6 ± 49.7 | 50.7 ± 30.2 | 9.1 ± 7.8 | 48.9 ± 28.2 | 63.8 ± 28.6 | 8.9 ± 6.8 |
| Urine 6-SMEL at 7 PM | ||||||
| High | Normal | |||||
| Morning | Evening | Night | Morning | Evening | Night | |
| Noise dB (mean values ± SD) | 58.3 ± 4.5 | 59.3 ± 1.4 | 56.5 ± 3.1 | 54.6 ± 6.7 | 51.5 ± 6.3 | 50.0 ± 5.7 |
| Light lux (mean values ± SD) | 87.3 ± 83.8 | 63.1 ± 40.1 | 6.3 ± 3.0 | 60.1 ± 31.9 | 58.2 ± 29.1 | 10.8 ± 8.6 |
References
- Bathory, E.; Tomopoulos, S. Sleep Regulation, Physiology and Development, Sleep Duration and Patterns, and Sleep Hygiene in Infants, Toddlers, and Preschool-Age Children. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 29–42. [Google Scholar] [CrossRef]
- Mínguez, R.; Hidalgo, M.I.; Grupo de Sueño de la SEPEAP. Pediatría Integral: Los trastornos del sueño en la infancia: Importancia de su diagnóstico y tratamiento en atención primaria. Pediatr. Integr. 2018, 22, 358–371. [Google Scholar]
- Kudchadkar, S.R.; Aljohani, O.A.; Punjabi, N.M. Sleep of critically ill children in the pediatric intensive care unit: A systematic review. Sleep Med. Rev. 2014, 18, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Kotagal, S. Sleep in the pediatric intensive care unit. Sleep Med. Rev. 2014, 18, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.L. Implications of sleep deprivation for children in the pediatric intensive care unit. Pediatr. Crit. Care Med. 2004, 5, 291–293. [Google Scholar] [CrossRef]
- Carno, M.A.; Connolly, H.V. Sleep and sedation in the pediatric intensive care unit. Crit. Care Nurs. Clin. 2005, 17, 239–244. [Google Scholar] [CrossRef]
- Stremler, R.; Micsinszki, S.; Adams, S.; Parshuram, C.; Pullenayegum, E.; Weiss, S.K. Objective Sleep Characteristics and Factors Associated with Sleep Duration and Waking during Pediatric Hospitalization. JAMA Netw. Open. 2021, 4, e213924. [Google Scholar] [CrossRef]
- Rana, M.; Riffo-Allende, C.; Mesa-Latorre, T.; Rosso-Astorga, K.; Torres, A.R. Sueño en los ninos: Fisiología y actualizacion de los últimos conocimientos. Medicina 2019, 79, 25–28. [Google Scholar]
- Boivin, D.B.; Duffy, J.F.; Kronauer, R.E.; Czeisler, C.A. Dose-response relationships for resetting of human circadian clock by light. Nature 1996, 379, 540–542. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Escames-Rosa, G.; Acuña-Castroviejo, D. Melatonina, análogos sintéticos y el ritmo sueño/vigilia. Rev. Neurol. 2009, 48, 245–254. [Google Scholar] [CrossRef]
- Mundigler, G.; Delle-Karth, G.; Koreny, M.; Zehetgruber, M.; Steindl-Munda, P.; Marktl, W.; Ferti, L.; Siostrzonek, P. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit. Care Med. 2002, 30, 536–540. [Google Scholar] [CrossRef]
- Iglowstein, I.; Jenni, O.G.; Molinari, L.; Largo, R.H. Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics 2003, 111, 302–307. [Google Scholar] [CrossRef]
- Amaral, F.G.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef]
- Benloucif, S.; Burgess, H.J.; Klerman, E.B.; Lewy, A.J.; Middleton, B.; Murphy, P.J.; Parry, B.L.; Revell, V.L. Measuring melatonin in humans. J. Clin. Sleep Med. 2008, 4, 66–69. [Google Scholar] [CrossRef]
- De Almeida, E.A.; Di Mascio, P.; Harumi, T.; Spence, D.W.; Moscovitch, A.; Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Measurement of melatonin in body fluids: Standards, protocols and procedures. Child’s Nerv. Syst. 2011, 27, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Molina-Carballo, A.; Muñoz-Hoyos, A.; Uberos-Fernández, J.; Acuña-Castroviejo, D.; Molina-Font, J.A. Pineal functioning (melatonin levels) in healthy children of different ages. An update and the value of pineal gland study in pediatrics. An. Esp. Pediatr. 1996, 45, 33–44. [Google Scholar] [PubMed]
- Madrid-Navarro, C.J.; Sanchez-Galvez, R.; Martinez-Nicolas, A.; Marina, R.; Garcia, J.A.; Madrid, J.A.; Rol, M.A. Disruption of Circadian Rhythms and Delirium, Sleep Impairment and Sepsis in Critically ill Patients. Potential Therapeutic Implications for Increased Light-Dark Contrast and Melatonin Therapy in an ICU Environment. Curr. Pharm. Des. 2015, 21, 3453–3468. [Google Scholar] [CrossRef] [PubMed]
- Shilo, L.; Dagan, Y.; Smorjik, Y.; Weinberg, U.; Dolev, S.; Komptel, B.; Balaum, H.; Shenkman, L. Patients in the Intensive Care Unit Suffer from Severe Lack of Sleep Associated with Loss of Normal Melatonin Secretion Pattern. Am. J. Med. Sci. 1999, 317, 278–281. [Google Scholar] [CrossRef]
- Foster, J.R.; Tijssen, J.A.; Miller, M.R.; Seabrook, J.A.; Fraser, D.D. Total Daily Production and Periodicity of Melatonin Metabolite in Critically Ill Children. Pediatr. Crit. Care Med. 2020, 21, 1061–1068. [Google Scholar] [CrossRef]
- Cavallo, A. Plasma melatonin rhythm in normal puberty: Interactions of age and pubertal stages. Neuroendocrinology 1992, 55, 372–379. [Google Scholar] [CrossRef]
- Salti, R.; Galluzzi, F.; Bindi, G.; Perfetto, F.; Tarquini, R.; Halberg, F.; Cornelissen, G. Nocturnal melatonin patterns in children. J. Clin. Endocrinol. Metab. 2000, 85, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.C.; Kjær, E.K.R.; Vase, C.B.; Mathiasen, R.; Debes, N.M.; Jørgensen, N.R.; Jennum, P.J. Melatonin secretion across puberty: A systematic review and meta-analysis. Psychoneuroendocrinology 2025, 173, 107281. [Google Scholar] [CrossRef]
- Brodner, W.; Kirchlechner, V.; Waldhauser, F.; Kovács, J.; Arif, T. Measurement of Urinary Melatonin: A Useful Tool for Monitoring Serum Melatonin after Its Oral Administration. J. Clin. Endocrinol. Metab. 2014, 85, 666–670. [Google Scholar]
- Pääkkönen, T.; Mäkinen, T.M.; Leppäluoto, J.; Vakkuri, O.; Rintamäki, H.; Palinkas, L.A.; Hassi, J. Urinary melatonin: A noninvasive method to follow human pineal function as studied in three experimental conditions. J. Pineal Res. 2006, 40, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, J.G.; Rose, L.; Blackwood, B.; Akerman, E.; McGaughey, J.; Egerod, I.; Fossum, M.; Foss, H.; Georgiou, E.; Graff, H.J.; et al. Clinical practices to promote sleep in the ICU: A multinational survey. Int. J. Nurs. Stud. 2018, 81, 107–114. [Google Scholar] [CrossRef]
- Pulak, L.M.; Jensen, L. Sleep in the Intensive Care Unit: A Review. J. Intensive Care Med. 2016, 31, 14–23. [Google Scholar] [CrossRef]
- Medrzycka-Dabrowska, W.; Lewandowska, K.; Kwiecień-Jagus, K.; Czyz-Szypenbajl, K. Sleep deprivation in Intensive Care Unit-systematic review. Open Med. 2018, 13, 384–393. [Google Scholar] [CrossRef]
- Tahara, Y.; Shibata, S. Chronobiology and nutrition. Neuroscience 2013, 253, 78–88. [Google Scholar] [CrossRef]
- Pot, G.K. Sleep and dietary habits in the urban environment: The role of chrono-nutrition. Proc. Nutr. Soc. 2018, 77, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Shibata, S. Time-of-Day-Dependent Physiological Responses to Meal and Exercise. Front. Nutr. 2020, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Galindo, A.P.; Camargo-Caicedo, Y.; Vélez-Pereira, A.M. Nivel de ruido en unidades de cuidado intensivo de un hospital público universitario en Santa Marta (Colombia). Med. Intensiva 2016, 40, 403–410. [Google Scholar] [CrossRef] [PubMed]
| Number of Determinations | Melatonin Levels (Median) | Normal Reference Values | |
|---|---|---|---|
| Serum 7 am (pg/mL) | 58 | 110 (IQR 25.8–110) | <30 |
| Urine 6-SMEL 1 7 am (mcg/L) | 50 | 40.3 (IQR 16.6–125.6) | >58 |
| Urine 6-SMEL 1 7 pm (mcg/L) | 38 | 15.2 (IQR 8.2–25.7) | <58 |
| Serum 7 AM ↑ | 6-SMEL 1 7 AM ↓ | 6-SMEL 1 7 PM ↑ | |
|---|---|---|---|
| AGE (months) | p = 0.08 | p = 0.05 (ß—0.33) | p = 0.02 (ß 0.52) |
| Sex | p = 0.16 | p = 0.07 | p = 0.74 |
| Time in PICU at the beginning of study | p = 0.51 | p = 0.02 (ß 0.94) | p = 0.86 |
| Heart disease | p = 0.02 (ß—0.45) | p = 0.08 | p = 0.75 |
| Previous surgery | p = 0.04 (ß 0.37) | p = 0.15 | p = 0.10 |
| PRISM III | p = 0.82 | p = 0.86 | p = 0.18 |
| Prior MV 2 | p = 0.07 | p = 0.03 (ß 0.48) | p = 0.56 |
| Days with previous MV | p = 0.65 | p = 0.12 | p = 0.86 |
| Serum 7 AM ↑ | 6-SMEL 1 7 AM ↓ | 6-SMEL 1 7 PM ↑ | |
|---|---|---|---|
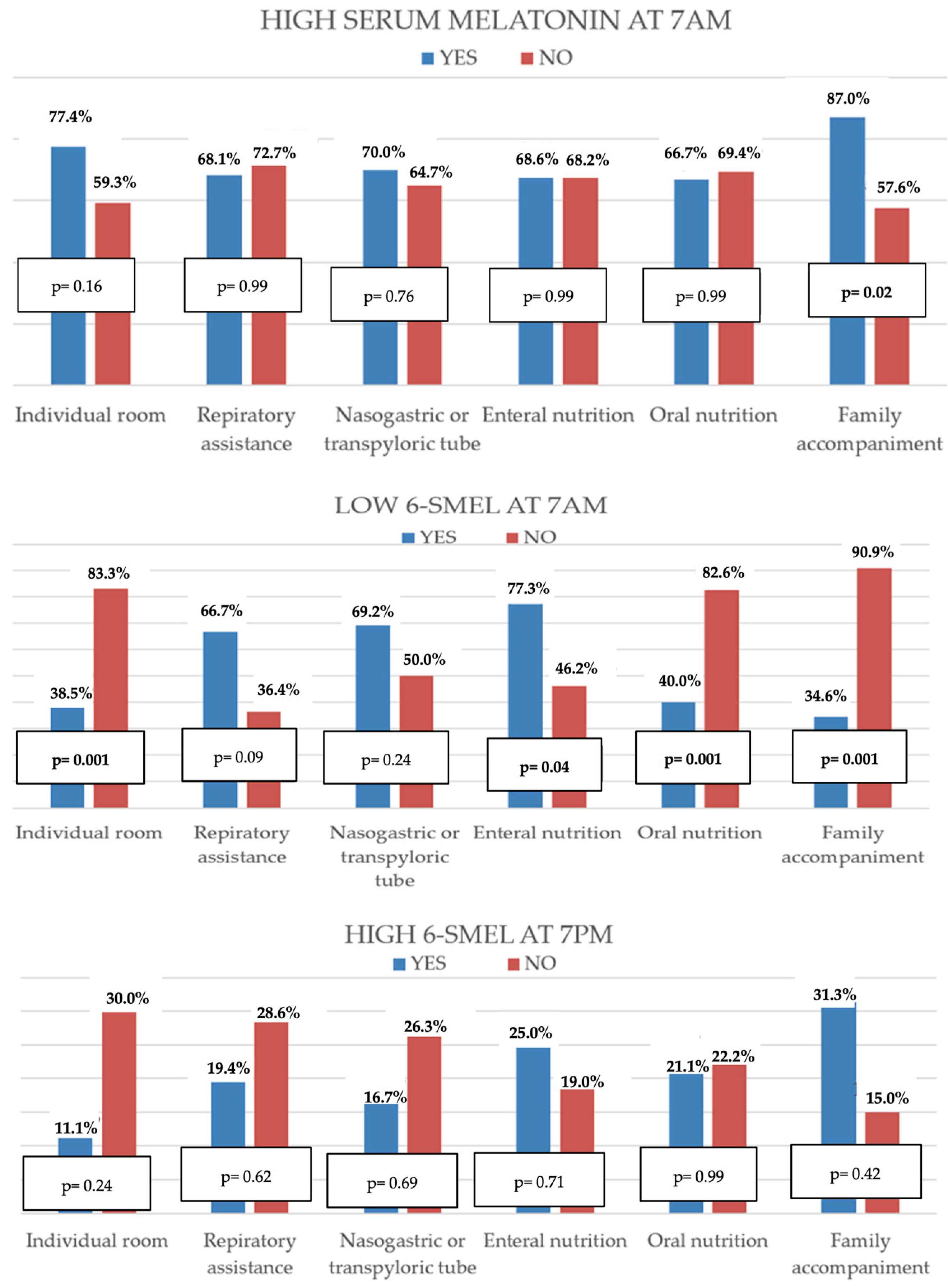
| Shared room | p = 0.11 | p = 0.001 | p = 0.20 |
| Respiratory assistance | p = 0.24 | p = 0.31 | p = 0.39 |
| Enteral nutrition | p = 0.50 | p = 0.29 | p = 0.24 |
| Family accompaniment | p = 0.03 | p = 0.001 | p = 0.40 |
| Serum 7 AM ↑ | 6-SMEL 1 7 AM ↓ | 6-SMEL 1 7 PM ↑ | |
|---|---|---|---|
| Nighttime sleep p2 | p = 0.17 | p = 0.03 | p = 0.69 |
| Nighttime sleep p50 | p = 0.99 | p = 0.99 | p = 0.99 |
| Daytime sleep p2 * | - | - | - |
| Daytime sleep p50 | p = 0.99 | p = 0.99 | p = 0.99 |
| Sleep in 24 h p2 | p = 0.99 | p = 0.99 | p = 0.99 |
| Sleep in 24 h p50 | p = 0.99 | p = 0.99 | p = 0.85 |
| High Serum Melatonin At 7 AM | Low 6-Smel 1 At 7 AM | High 6-Smel 1 At 7 PM | |
|---|---|---|---|
| Morning Noise | p = 0.53 | p = 0.89 | p = 0.54 |
| Evening Noise | p = 0.65 | p = 0.21 | p = 0.17 |
| Night Noise | p = 0.96 | p = 0.30 | p = 0.15 |
| Morning Light | p = 0.25 | p = 0.05 | p = 0.64 |
| Evening Light | p = 0.75 | p = 0.08 | p = 0.44 |
| Night Light | p = 0.76 | p = 0.42 | p = 0.12 |
| Medium Noise in 24 h | Medium Light in 24 h | |
|---|---|---|
| High Serum Melatonin at 7 AM | p = 0.04 | p = 0.50 |
| Low 6-SMEL 1 at 7 AM | p = 0.02 | p = 0.99 |
| High 6-SMEL 1 at 7 PM | p = 0.96 | p = 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-San Prudencio, M.; Manrique, G.; Cieza, R.; Corraliza, C.; Arias, P.; Medina, E.; López-Herce, J.; Mencía, S. Relationship of Melatonin Levels in Blood and Urine with Sleep Quality in Children Admitted to Pediatric Intensive Care Unit. Children 2025, 12, 1074. https://doi.org/10.3390/children12081074
García-San Prudencio M, Manrique G, Cieza R, Corraliza C, Arias P, Medina E, López-Herce J, Mencía S. Relationship of Melatonin Levels in Blood and Urine with Sleep Quality in Children Admitted to Pediatric Intensive Care Unit. Children. 2025; 12(8):1074. https://doi.org/10.3390/children12081074
Chicago/Turabian StyleGarcía-San Prudencio, Miriam, Gema Manrique, Raquel Cieza, Cristina Corraliza, Patricia Arias, Elena Medina, Jesús López-Herce, and Santiago Mencía. 2025. "Relationship of Melatonin Levels in Blood and Urine with Sleep Quality in Children Admitted to Pediatric Intensive Care Unit" Children 12, no. 8: 1074. https://doi.org/10.3390/children12081074
APA StyleGarcía-San Prudencio, M., Manrique, G., Cieza, R., Corraliza, C., Arias, P., Medina, E., López-Herce, J., & Mencía, S. (2025). Relationship of Melatonin Levels in Blood and Urine with Sleep Quality in Children Admitted to Pediatric Intensive Care Unit. Children, 12(8), 1074. https://doi.org/10.3390/children12081074





