Exploring the Association Between Glucose-6-Phosphate Dehydrogenase Deficiency and Autism Spectrum Disorder: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective
2.2. Inclusion Criteria
2.2.1. Study Type
2.2.2. Population
2.2.3. Geographic Scope
2.2.4. Language
2.2.5. Time Frame
2.3. Exclusion Criteria
2.3.1. Non-Peer-Reviewed Sources
2.3.2. Duplicate Studies
2.3.3. Animal or In Vitro Studies
2.4. Literature Search Strategy
2.5. Data Extraction and Analysis
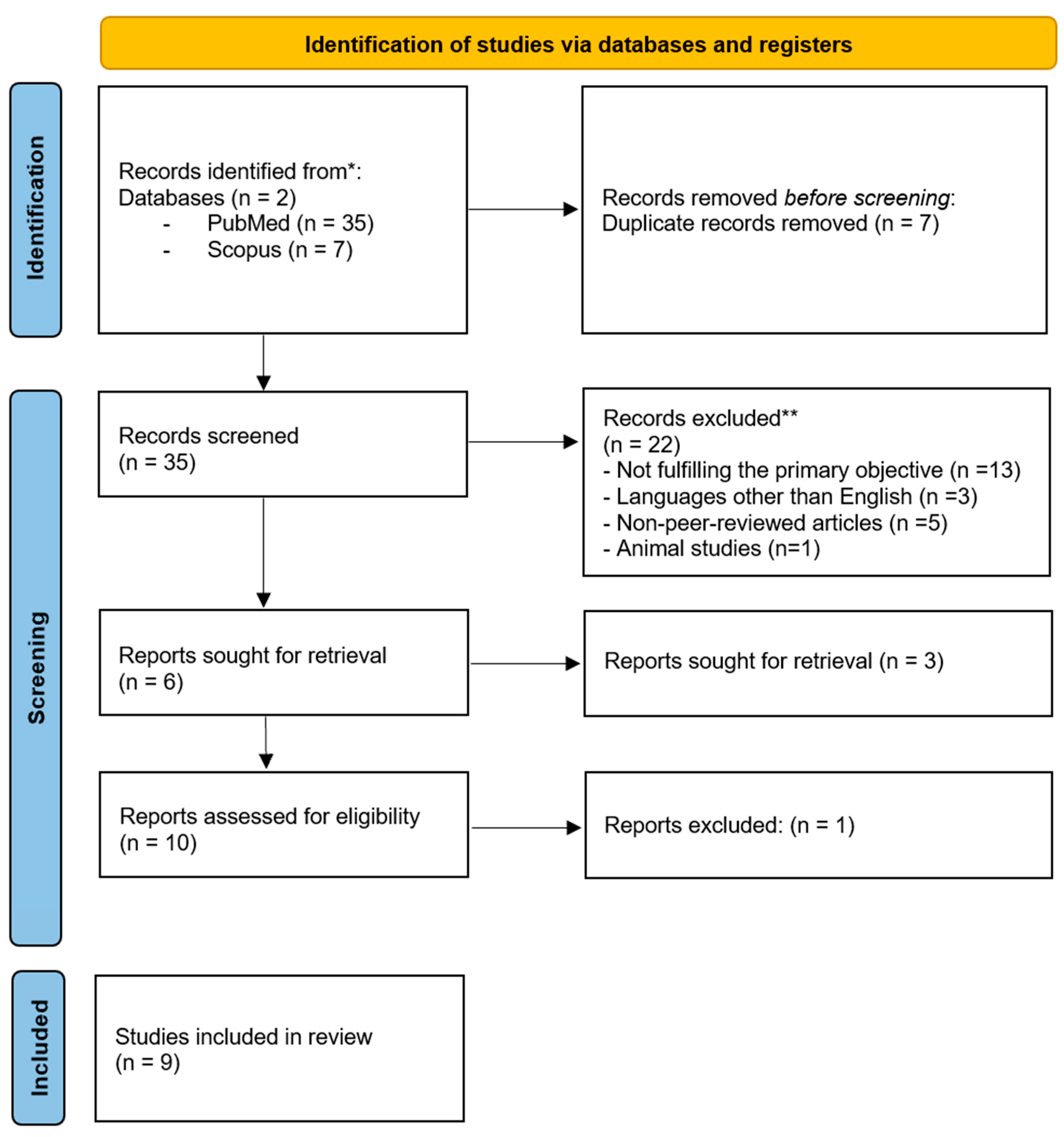
3. Results
4. Discussion
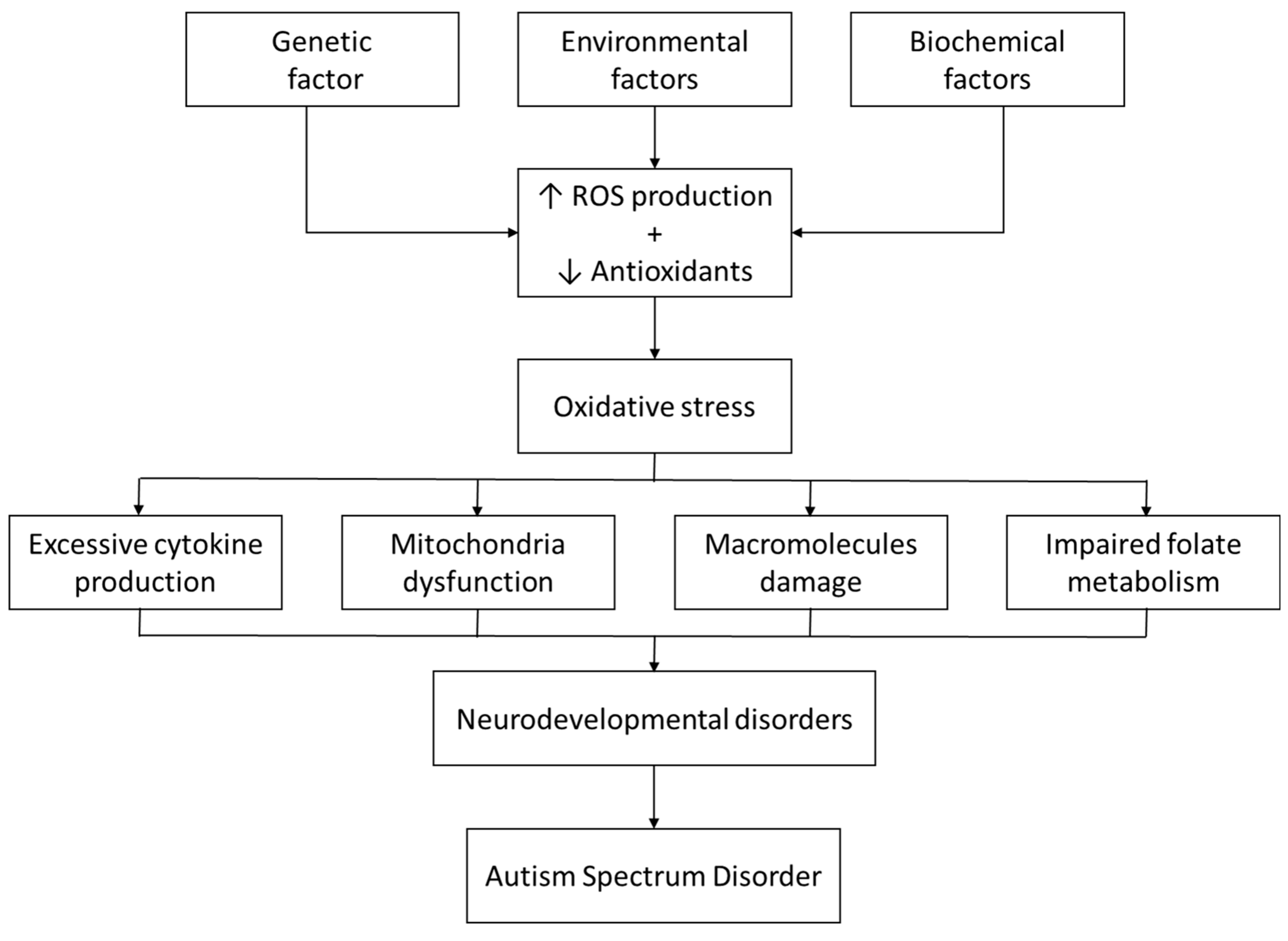
4.1. Oxidative Disequilibrium
4.2. Impaired Folate Metabolism
4.3. Neuroinflammation
5. Conclusions
Funding
Conflicts of Interest
References
- Leung, A.K.C.; Lemay, J.F. First Aid for the Psychiatry Clerkship, 3rd ed.; McGraw-Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Semple, D.; Smyth, R.; Burns, J.; Darjee, R.; McIntosh, A. Oxford Handbook of Psychiatry, 3rd ed.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Joon, P.; Kumar, A.; Parle, M. What is autism? Pharmacol. Rep. 2021, 73, 1255–1264. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Translational Pediatrics 2020, 9, S55–S65. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Stanton, J.E.; Hans, S.; Grabrucker, A.M. Autism Spectrum Disorders: Etiology and Pathology. In Exon Publications eBooks; Exon: Brisbane, Australia, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Johnson, C.P.; Myers, S.M. Identification and evaluation of children with autism spectrum disorders. Pediatrics 2007, 120, 1183–1215. [Google Scholar] [CrossRef]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar] [CrossRef]
- Lyall, K.; Croen, L.; Daniels, J.; Fallin, M.D.; Ladd-Acosta, C.; Lee, B.K.; Park, B.Y.; Snyder, N.W.; Schendel, D.; Volk, H.; et al. The changing epidemiology of autism spectrum disorders. Annu. Rev. Public Health 2017, 38, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, D.H.; State, M.W. Gene hunting in autism spectrum disorder: On the path to precision medicine. Lancet Neurol. 2015, 14, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Ecker, C.; Bookheimer, S.Y.; Murphy, D.G.M. Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. Lancet Neurol. 2015, 14, 1121–1134. [Google Scholar] [CrossRef]
- Voineagu, I.; Wang, X.; Johnston, P.G.; Lowe, J.K.; Tian, Y.; Horvath, S.; Mill, J.; Cantor, R.M.; Blencowe, B.J.; Geschwind, D.H. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011, 474, 380–384. [Google Scholar] [CrossRef]
- Roy, M.; Strate, P. Autism spectrum disorders in adulthood. Dtsch. Ärzteblatt Int. 2023, 120, 87–93. [Google Scholar] [CrossRef]
- The Royal Australian College of general Practitioners. (n.d.). Management of Mental Ill Health in People with Autism Spectrum Disorder. Australian Family Physician. Available online: https://www.racgp.org.au/afp/2015/november/management-of-mental-ill-health-in-people-with-autism-spectrum-disorder/ (accessed on 18 June 2025).
- Myers, S.M.; Johnson, C.P. Management of children with autism spectrum disorders. Pediatrics 2007, 120, 1162–1182. [Google Scholar] [CrossRef]
- Singhi, P.; Smith-Hicks, C. Early Diagnosis and Management of Autism Spectrum Disorder (ASD) in Low-Resource Countries—Challenges and Strategies. Indian J. Pediatr. 2023, 90, 362–363. [Google Scholar] [CrossRef]
- Ostrowski, J.; Religioni, U.; Gellert, B.; Sytnik-Czetwertyński, J.; Pinkas, J. Autism Spectrum Disorders: Etiology, Epidemiology, and Challenges for Public Health. Med. Sci. Monit. 2024, 30, e944161. [Google Scholar] [CrossRef]
- Nkhoma, E.T.; Poole, C.; Vannappagari, V.; Hall, S.A.; Beutler, E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. Blood Cells Mol. Dis. 2009, 42, 267–278. [Google Scholar] [CrossRef]
- Domingo, G.J.; Advani, N.; Satyagraha, A.W.; Sibley, C.H.; Rowley, E.; Kalnoky, M.; Cohen, J.; Parker, M.; Kelley, M. Addressing the gender-knowledge gap in glucose-6-phosphate dehydrogenase deficiency: Challenges and opportunities. Int. Health 2018, 11, 7–14. [Google Scholar] [CrossRef]
- Pfeffer, D.A.; Satyagraha, A.W.; Sadhewa, A.; Alam, M.S.; Bancone, G.; Boum, Y.; Brito, M.; Cui, L.; Deng, Z.; Domingo, G.J.; et al. Genetic Variants of Glucose-6-Phosphate Dehydrogenase and their associated Enzyme Activity: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, E.; Carretero, A.; Viña-Almunia, A.; Domenech-Fernandez, J.; Olaso-Gonzalez, G.; Viña, J.; Gomez-Cabrera, M.C. Glucose 6-P Dehydrogenase—An Antioxidant Enzyme with Regulatory Functions in Skeletal Muscle during Exercise. Cells 2022, 11, 3041. [Google Scholar] [CrossRef] [PubMed]
- Shash, H.; Alomari, M.; Alsaif, A.; Abualrahi, A.; AlQassab, M.; Alfaraj, A.; Alkhadhabah, A.; Alhajji, A. Parents’ Awareness and Knowledge of G6PD deficiency in the Eastern Province Saudi Arabia: A Cross-Sectional Study. Cureus 2023, 15, e50845. [Google Scholar] [CrossRef]
- Arese, P.; Gallo, V.; Pantaleo, A.; Turrini, F. Life and death of Glucose-6-Phosphate dehydrogenase (G6PD) deficient erythrocytes—Role of redox stress and Band 3 modifications. Transfus. Med. Hemotherapy 2012, 39, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Kassahun, W.; Tunta, A.; Abera, A.; Shiferaw, M. Glucose-6-phosphate dehydrogenase deficiency among neonates with jaundice in Africa; systematic review and meta-analysis. Heliyon 2023, 9, e18437. [Google Scholar] [CrossRef]
- Lee, H.Y.; Ithnin, A.; Azma, R.Z.; Othman, A.; Salvador, A.; Cheah, F.C. Glucose-6-Phosphate dehydrogenase deficiency and Neonatal hyperbilirubinemia: Insights on pathophysiology, diagnosis, and Gene variants in Disease Heterogeneity. Front. Pediatr. 2022, 10, 875877. [Google Scholar] [CrossRef]
- Orrico, F.; Laurance, S.; Lopez, A.C.; Lefevre, S.D.; Thomson, L.; Möller, M.N.; Ostuni, M.A. Oxidative stress in healthy and pathological red blood cells. Biomolecules 2023, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Bubp, J.; Jen, M.; Matuszewski, K. Caring for Glucose-6-Phosphate dehydrogenase (G6PD)—Deficient patients: Implications for pharmacy. P T 2015, 40, 572–574. [Google Scholar]
- Vives-Corrons, J.L.; Pujades, M.A.; Petit, J.; Colomer, D.; Corbella, M.; Aguilar i Bascompte, J.L.; Merino, A. Chronic nonspherocytic hemolytic anemia (CNSHA) and glucose 6 phosphate dehydrogenase (G6PD) deficiency in a patient with familial amyloidotic polyneuropathy (FAP). Hum. Genet. 1989, 81, 161–164. [Google Scholar] [CrossRef]
- Mondal, A.; Mukherjee, S.; Dar, W.; Singh, S.; Pati, S. Role of glucose 6-phosphate dehydrogenase (G6PD) deficiency and its association to Autism Spectrum Disorders. Biochim. Biophys. Acta. Mol. Basis Dis. 2021, 1867, 166185. [Google Scholar] [CrossRef]
- Al-Salehi, S.M.; Ghaziuddin, M. G6PD deficiency in autism. Eur. Child Adolesc. Psychiatry 2008, 18, 227–230. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Emokpae, A.A.; Zamora, T.G.; Slusher, T.M. Addressing the burden of neonatal hyperbilirubinaemia in countries with significant glucose-6-phosphate dehydrogenase deficiency. Acta Paediatr. 2014, 103, 1102–1109. [Google Scholar] [CrossRef]
- Lin, Q.; Zhu, D.; Chen, C.; Feng, Y.; Shen, F.; Wu, Z. Risk factors for neonatal hyperbilirubinemia: A systematic review and meta-analysis. Transl. Pediatr. 2022, 11, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, B.O.; Osibanjo, F.B.; Slusher, T.M. Risk Factors for Severe Neonatal Hyperbilirubinemia in Low and Middle-Income Countries: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117229. [Google Scholar] [CrossRef]
- Alagoz, M.; Kherad, N.; Gunger, E.; Kaymaz, S.; Yuksel, A. The New CIC Mutation Associates with Mental Retardation and Severity of Seizure in Turkish Child with a Rare Class I Glucose-6-Phosphate Dehydrogenase Deficiency. J. Mol. Neurosci. 2020, 70, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Dowd, B. Glucose-6-phosphate dehydrogenase and its relationship to mental retardation. Med. Hypotheses 1980, 6, 7–11. [Google Scholar] [CrossRef]
- Toren, A.; Brok-Simoni, F.; Ben-Bassat, I.; Holtzman, F.; Mandel, M.; Neumann, Y.; Ramot, B.; Rechavi, G.; Kende, G. Congenital haemolytic anaemia associated with adenylate kinase deficiency. Br. J. Haematol. 1994, 87, 376–380. [Google Scholar] [CrossRef]
- Seth, P.K.; Tara Devi, S.; Seth, S. Glucose-6-phosphate dehydrogenase deficiency and mental retardation. J. Inherit. Metab. Dis. 1981, 4, 93–94. [Google Scholar] [CrossRef]
- LaRue, N.; Kahn, M.; Murray, M.; Leader, B.T.; Bansil, P.; McGray, S.; Kalnoky, M.; Zhang, H.; Huang, H.; Jiang, H.; et al. Comparison of Quantitative and Qualitative Tests for Glucose-6-Phosphate Dehydrogenase Deficiency. Am. J. Trop. Med. Hyg. 2014, 91, 854–861. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? a Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Napolitano, A.; Schiavi, S.; La Rosa, P.; Rossi-Espagnet, M.C.; Petrillo, S.; Bottino, F.; Tagliente, E.; Longo, D.; Lupi, E.; Casula, L.; et al. Sex Differences in Autism Spectrum Disorder: Diagnostic, Neurobiological, and Behavioral Features. Front. Psychiatry 2022, 13, 889636. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, D.; Ulusu, N.N. The Possible Role of The Glucose-6-Phosphate Dehydrogenase Enzyme Deficiency in The Polyneuropathies. J. Basic Clin. Health Sci. 2020; preprint. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, D.; Chen, J.; Qin, Y.-Y.; Sheng, R.; Feng, X.; Chen, Z.; Ding, Y.; Li, M.; Qin, Z.-H. G6PD plays a neuroprotective role in brain ischemia through promoting pentose phosphate pathway. Free Radic. Biol. Med. 2017, 112, 433–444. [Google Scholar] [CrossRef]
- Nishimura, Y.; Kanda, Y.; Sone, H.; Aoyama, H.; Gasparovic, A.C. Oxidative Stress as a Common Key Event in Developmental Neurotoxicity. Oxidative Med. Cell. Longev. 2021, 2021, 6685204. [Google Scholar] [CrossRef]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative Stress in Autism Spectrum Disorder—Current Progress of Mechanisms and Biomarkers. Front. Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef] [PubMed]
- Irvine, N.; England-Mason, G.; Field, C.J.; Dewey, D.; Aghajafari, F. Prenatal Folate and Choline Levels and Brain and Cognitive Development in Children: A Critical Narrative Review. Nutrients 2022, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Pravenec, M.; Kožich, V.; Krijt, J.; Sokolová, J.; Zídek, V.; Landa, V.; Šimáková, M.; Mlejnek, P.; Šilhavý, J.; Oliyarnyk, O.; et al. Folate Deficiency Is Associated with Oxidative Stress, Increased Blood Pressure, and Insulin Resistance in Spontaneously Hypertensive Rats. Am. J. Hypertens. 2012, 26, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Balashova, O.A.; Visina, O.; Borodinsky, L.N. Folate action in nervous system development and disease. Dev. Neurobiol. 2018, 78, 391–402. [Google Scholar] [CrossRef]
- Hoxha, B.; Hoxha, M.; Domi, E.; Gervasoni, J.; Persichilli, S.; Malaj, V.; Zappacosta, B. Folic Acid and Autism: A Systematic Review of the Current State of Knowledge. Cells 2021, 10, 1976. [Google Scholar] [CrossRef]
- Wang, M.; Li, K.; Zhao, D.; Li, L. The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: A meta-analysis. Mol. Autism 2017, 8, 51. [Google Scholar] [CrossRef]
- Frye, R.E.; Slattery, J.; Delhey, L.; Furgerson, B.; Strickland, T.; Tippett, M.; Sailey, A.; Wynne, R.; Rose, S.; Melnyk, S.; et al. Folinic acid improves verbal communication in children with autism and language impairment: A randomized double-blind placebo-controlled trial. Mol. Psychiatry 2016, 23, 247–256. [Google Scholar] [CrossRef]
- Frye, R.E.; Rossignol, D.A.; Scahill, L.; McDougle, C.J.; Huberman, H.; Quadros, E.V. Treatment of Folate Metabolism Abnormalities in Autism Spectrum Disorder. Semin. Pediatr. Neurol. 2020, 35, 100835. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Cerebral Folate Deficiency. Folate Receptor Alpha Autoantibodies and Leucovorin (Folinic Acid) Treatment in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1141. [Google Scholar] [CrossRef]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol. Psychiatry 2012, 18, 369–381. [Google Scholar] [CrossRef]
- Hughes, H.; Oreno, R.; Ashwood, P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav. Immun. 2022; preprint. [Google Scholar] [CrossRef]
| Author and Publication Year | Study Design | Population Characteristics | Key Findings |
|---|---|---|---|
| Mondal et al., 2021 [28] | Literature review | Not mentioned | ASD is associated with a low level of NADPH, which suggests that the deficiency of G6PD is one of the major contributing factors for dysregulation of oxidative balance in pathogenesis of ASD. |
| Olusanya et al., 2014 [30] | Literature review | Not mentioned | Severe neonatal hyperbilirubinemia is frequently associated with neonatal mortality and neurodevelopmental disorders in countries with significant G6PD deficiency. |
| Lin et al., (2022) [31] | Systematic review and meta-analysis | Not mentioned | G6PD deficiency was identified as a risk factor for neonatal hyperbilirubinemia (OR = 1.62, 95% CI: 1.44, 1.81, Z = 8.39, p < 0.00001). Hyperbilirubinemia may lead to severe complications, including lifelong disability such as growth retardation, encephalopathy, autism and hearing impairment. |
| Al-Salehi et al., 2009 [29] | Case series | 49 subjects (37 males & 12 females) have a typical triad of autism symptoms; social deficits, communication impairment & rigid ritualistic interest. | There is a possible correlation between G6PD deficiency and autism. Potentially through bilirubin induced brain damage causing neuropathological changes. |
| Olusanya et al., 2015 [32] | Systematic Review and Meta-Analysis | Not mentioned | Infants with G6PD deficiency have an elevated risk of severe hyperbilirubinemia (OR, 8.01; 95% CI, 2.09–30.69, p = 0.002). Surviving infants may acquire long-term neurodevelopmental sequelae such as cerebral palsy, sensorineural hearing loss, intellectual difficulties or gross developmental delays |
| Alagoz et al., 2020 [33] | Case report | 8-year-old boy with hemizygous variation in G6PD gene and heterozygous mutation in CIC gene | The child suffers from focal epileptiform activity and hypsarrhythmia in electroencephalography (EEG), seizures, psychomotor retardation, speech impairment, intellectual disability, developmental regression, and learning difficulties. |
| B Dowd et al., 1980 [34] | Observational, screening-based study on a clinical population. | 100 patients who were classified as mentally retarded and who were hospitalized under state care. | The screening and subsequent statistical analysis of the data indicates that the incidence of G-6-PD is drastically higher among Caucasian males in the atypical population than is to be expected and it is somewhat higher among the atypical Negroes. indicates strongly that there may be a relationship between G-6-PD deficiency and limited mental capacity. |
| Toren et al., 1994 [35] | Case series | 6 children of one family who are deficient of adenylate kinase enzyme and in three of them a combined G6PD deficiency was found. | Five of these patients suffer from mild mental retardation and the sixth is severely retarded. The mildly retarded patients have limited learning abilities, poor performance at school and a limited vocabulary. |
| P K Seth, et al. 1981 [36] | Observational study | 152 mentally retarded individuals and control group 156 | Of the mentally retarded individuals, 11.18% were found to be deficient in glucose-6-phosphate dehydro-genase compared with 2.56% of the controls. This indicates a possible association between G6PD deficiency and mental subnormality. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshamsi, M.A.; Al Teneiji, M.T.; Kar, S.S.; Dube, R. Exploring the Association Between Glucose-6-Phosphate Dehydrogenase Deficiency and Autism Spectrum Disorder: A Narrative Review. Children 2025, 12, 1054. https://doi.org/10.3390/children12081054
Alshamsi MA, Al Teneiji MT, Kar SS, Dube R. Exploring the Association Between Glucose-6-Phosphate Dehydrogenase Deficiency and Autism Spectrum Disorder: A Narrative Review. Children. 2025; 12(8):1054. https://doi.org/10.3390/children12081054
Chicago/Turabian StyleAlshamsi, Maitha Abdulla, Maitha Tareq Al Teneiji, Subhranshu Sekhar Kar, and Rajani Dube. 2025. "Exploring the Association Between Glucose-6-Phosphate Dehydrogenase Deficiency and Autism Spectrum Disorder: A Narrative Review" Children 12, no. 8: 1054. https://doi.org/10.3390/children12081054
APA StyleAlshamsi, M. A., Al Teneiji, M. T., Kar, S. S., & Dube, R. (2025). Exploring the Association Between Glucose-6-Phosphate Dehydrogenase Deficiency and Autism Spectrum Disorder: A Narrative Review. Children, 12(8), 1054. https://doi.org/10.3390/children12081054







