Trends in Term-Equivalent Age Brain Volumes in Infants Born Across the Gestational Age Spectrum
Abstract
Highlights
- •
- Lower gestational age is significantly related to larger cerebrospinal fluid volume.
- •
- Trends of higher gestational age with larger volume of brain tissues are seen.
- •
- Gestational age may drive early brain volumes with other neonatal clinical factors.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Acquisition
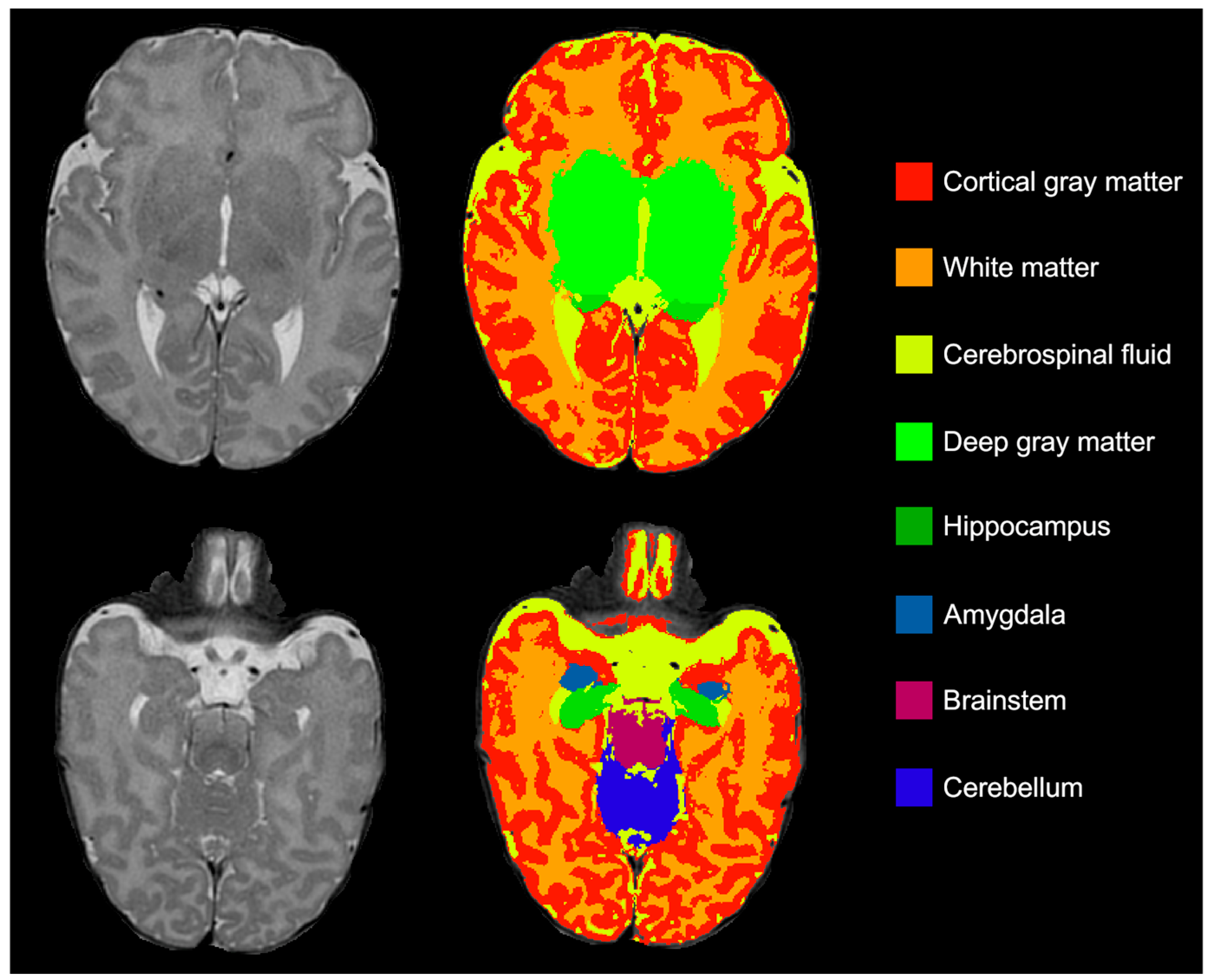
2.3. Brain Volumes
2.4. Statistics
3. Results
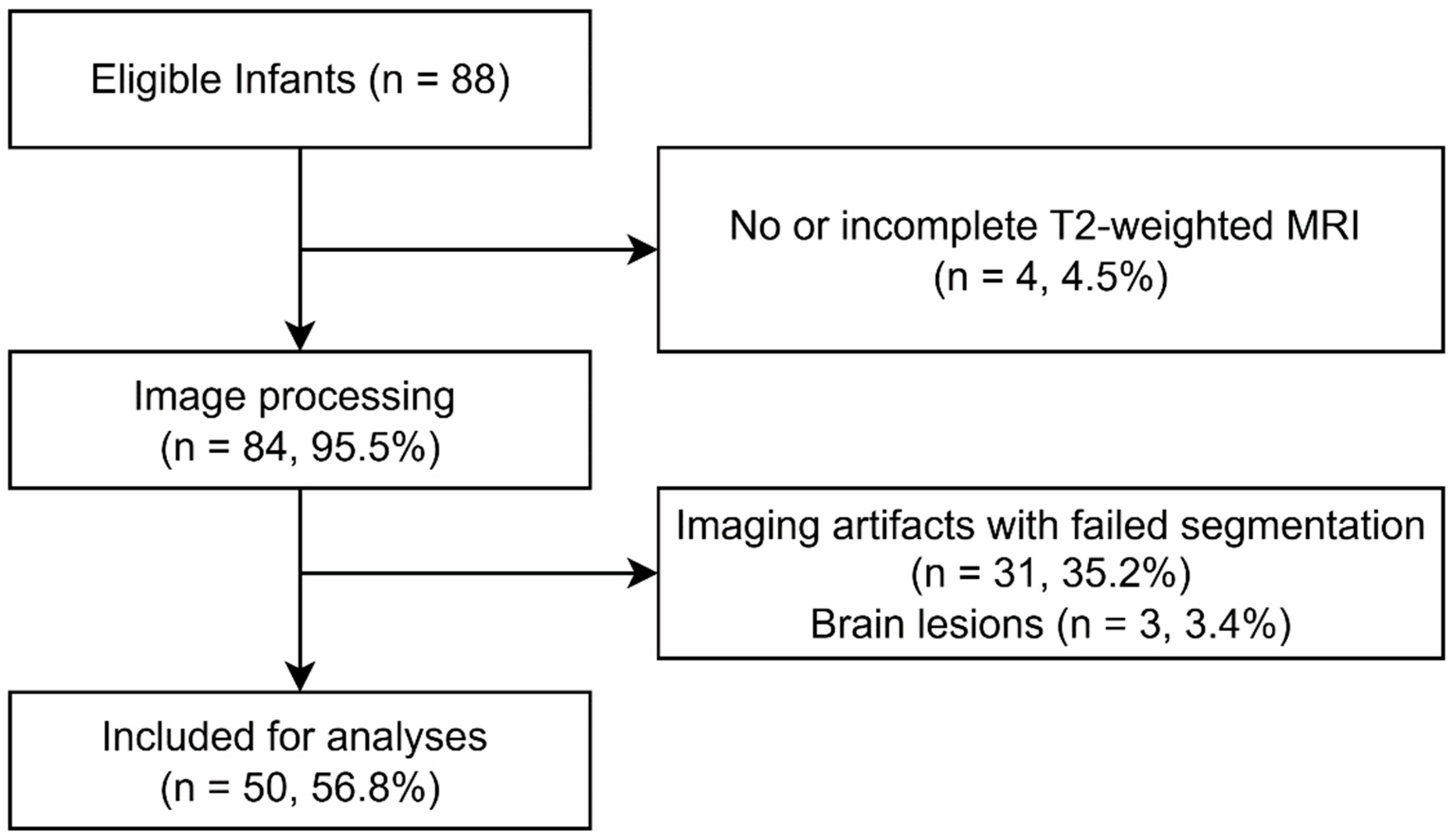
3.1. Infant Characteristics
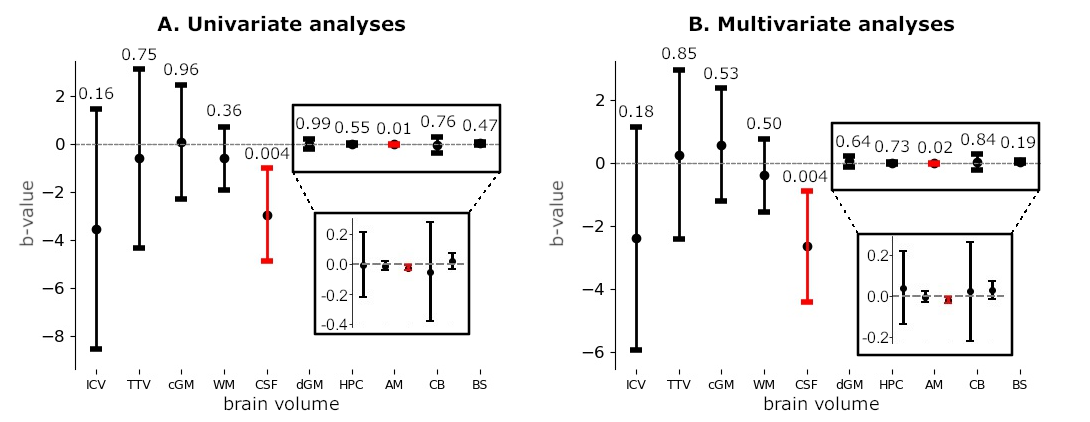
3.2. Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 14 September 2024).
- Bhutta, A.T.; Cleves, M.A.; Casey, P.H.; Cradock, M.M.; Anand, K.J.S. Cognitive and Behavioral Outcomes of School-Aged Children Who Were Born Preterm: A Meta-analysis. JAMA 2002, 288, 728–737. [Google Scholar] [CrossRef]
- Hüppi, P.S.; Warfield, S.; Kikinis, R.; Barnes, P.D.; Zientara, G.P.; Jolesz, F.A.; Tsuji, M.K.; Volpe, J.J. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 1998, 43, 224–235. [Google Scholar] [CrossRef]
- Kinney, H.C. The Near-Term (Late Preterm) Human Brain and Risk for Periventricular Leukomalacia: A Review. Semin. Perinatol. 2006, 30, 81–88. [Google Scholar] [CrossRef]
- Yates, N.; Gunn, A.J.; Bennet, L.; Dhillon, S.K.; Davidson, J.O. Preventing Brain Injury in the Preterm Infant—Current Controversies and Potential Therapies. Int. J. Mol. Sci. 2021, 22, 1671. [Google Scholar] [CrossRef]
- Romberg, J.; Wilke, M.; Allgaier, C.; Nägele, T.; Engel, C.; Poets, C.F.; Franz, A. MRI-based brain volumes of preterm infants at term: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, F520–F526. [Google Scholar] [CrossRef]
- Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Isgum, I.; de Vries, L.S.; Benders, M.J.N.L. Brain tissue volumes in preterm infants: Prematurity, perinatal risk factors and neurodevelopmental outcome: A systematic review. J. Matern.-Fetal Neonatal Med. 2012, 25 (Suppl. S1), 89–100. [Google Scholar] [CrossRef]
- Boswinkel, V.; Verschuur, A.S.; Nijholt, I.M.; van Osch, J.A.; Oosterveld, J.N.; Beare, R.J.; Slump, C.H.; de Vries, L.S.; Boomsma, M.F.; Meijler, G.v.W. Mild brain lesions do not affect brain volumes in moderate-late preterm infants. Eur. J. Paediatr. Neurol. 2021, 34, 91–98. [Google Scholar] [CrossRef]
- Niwa, T.; Suzuki, K.; Sugiyama, N.; Imai, Y. Regional volumetric assessment of the brain in moderately preterm infants (30–35 gestational weeks) scanned at term-equivalent age on magnetic resonance imaging. Early Hum. Dev. 2017, 111, 36–41. [Google Scholar] [CrossRef]
- Thompson, D.K.; Kelly, C.E.; Chen, J.; Beare, R.; Alexander, B.; Seal, M.L.; Lee, K.J.; Matthews, L.G.; Anderson, P.J.; Doyle, L.W.; et al. Characterisation of brain volume and microstructure at term-equivalent age in infants born across the gestational age spectrum. NeuroImage Clin. 2019, 21, 101630. [Google Scholar] [CrossRef]
- Munakata, S.; Okada, T.; Okahashi, A.; Yoshikawa, K.; Usukura, Y.; Makimoto, M.; Hosono, S.; Takahashi, S.; Mugishima, H.; Okuhata, Y. Gray matter volumetric MRI differences late-preterm and term infants. Brain Dev. 2013, 35, 10–16. [Google Scholar] [CrossRef]
- Alexander, B.; Kelly, C.E.; Adamson, C.; Beare, R.; Zannino, D.; Chen, J.; Murray, A.L.; Loh, W.Y.; Matthews, L.G.; Warfield, S.K.; et al. Changes in neonatal regional brain volume associated with preterm birth and perinatal factors. NeuroImage 2019, 185, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Kersbergen, K.J.; Makropoulos, A.; Aljabar, P.; Groenendaal, F.; de Vries, L.S.; Counsell, S.J.; Benders, M.J. Longitudinal Regional Brain Development and Clinical Risk Factors in Extremely Preterm Infants. J. Pediatr. 2016, 178, 93–100.e6. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.K.; Warfield, S.K.; Carlin, J.B.; Pavlovic, M.; Wang, H.X.; Bear, M.; Kean, M.J.; Doyle, L.W.; Egan, G.F.; Inder, T.E. Perinatal risk factors altering regional brain structure in the preterm infant. Brain 2007, 130, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Limperopoulos, C.; Soul, J.S.; Gauvreau, K.; Huppi, P.S.; Warfield, S.K.; Bassan, H.; Robertson, R.L.; Volpe, J.J.; du Plessis, A.J. Late Gestation Cerebellar Growth Is Rapid and Impeded by Premature Birth. Pediatrics 2005, 115, 688–695. [Google Scholar] [CrossRef]
- Boswinkel, V.; Krüse-Ruijter, M.F.; Nijboer-Oosterveld, J.; Nijholt, I.M.; Edens, M.A.; de Tollenaer, S.M.M.; Wu, M.-N.S.; Boomsma, M.F.; de Vries, L.S.; Meijler, G.v.W. Incidence of brain lesions in moderate-late preterm infants assessed by cranial ultrasound and MRI: The BIMP-study. Eur. J. Radiol. 2021, 136, 109500. [Google Scholar] [CrossRef]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Beare, R.J.; Chen, J.; Kelly, C.E.; Alexopoulos, D.; Smyser, C.D.; Rogers, C.E.; Loh, W.Y.; Matthews, L.G.; Cheong, J.L.Y.; Spittle, A.J.; et al. Neonatal Brain Tissue Classification with Morphological Adaptation and Unified Segmentation. Front. Neuroinform. 2016, 10, 143–155. [Google Scholar] [CrossRef]
- Boardman, J.P.; Counsell, S.J.; Rueckert, D.; Hajnal, J.V.; Bhatia, K.K.; Srinivasan, L.; Kapellou, O.; Aljabar, P.; Dyet, L.E.; Rutherford, M.A.; et al. Early growth in brain volume is preserved in the majority of preterm infants. Ann. Neurol. 2007, 62, 185–192. [Google Scholar] [CrossRef]
- Inder, T.E.; Warfield, S.K.; Wang, H.; Hüppi, P.S.; Volpe, J.J. Abnormal Cerebral Structure Is Present at Term in Premature Infants. Pediatrics 2005, 115, 286–294. [Google Scholar] [CrossRef]
- Barry, T.J.; Murray, L.; Fearon, P.; Moutsiana, C.; Johnstone, T.; Halligan, S.L. Amygdala volume and hypothalamic-pituitary-adrenal axis reactivity to social stress. Psychoneuroendocrinology 2017, 85, 96–99. [Google Scholar] [CrossRef]
- Fowler, C.H.; Bogdan, R.; Gaffrey, M.S. Stress-induced cortisol response is associated with right amygdala volume in early childhood. Neurobiol. Stress 2021, 14, 100329. [Google Scholar] [CrossRef]
- Mueller, M.E.; Graz, M.B.; Truttmann, A.C.; Schneider, J.; Duerden, E.G. Neonatal amygdala volumes, procedural pain and the association with social-emotional development in children born very preterm. Brain Struct. Funct. 2024, 229, 2369–2378. [Google Scholar] [CrossRef]
- Ortinau, C.; Neil, J. The neuroanatomy of prematurity: Normal brain development and the impact of preterm birth. Clin. Anat. 2015, 28, 168–183. [Google Scholar] [CrossRef]
- Vasileiadis, G.T.; Gelman, N.; Han, V.K.M.; Williams, L.-A.; Mann, R.; Bureau, Y.; Thompson, R.T. Uncomplicated Intraventricular Hemorrhage Is Followed by Reduced Cortical Volume at Near-Term Age. Pediatrics 2004, 114, e367. [Google Scholar] [CrossRef]
- Back, S.A.; Miller, S.P. Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Ann. Neurol. 2018, 75, 469–486. [Google Scholar] [CrossRef]
- Back, S.A. White matter injury in the preterm infant: Pathology and mechanisms. Acta Neuropathol. 2018, 134, 331–349. [Google Scholar] [CrossRef]
- Clouchoux, C.; Guizard, N.; Evans, A.C.; Du Plessis, A.J.; Limperopoulos, C. Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am. J. Obstet. Gynecol. 2012, 206, 173. [Google Scholar] [CrossRef]
- Tam, E.W.Y.; Miller, S.P.; Studholme, C.; Chau, V.; Glidden, D.; Poskitt, K.J.; Ferriero, D.M.; Barkovich, A.J. Differential Effects of Intraventricular Hemorrhage and White Matter Injury on Preterm Cerebellar Growth. J. Pediatr. 2011, 158, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Padilla, N.; Alexandrou, G.; Blennow, M.; Lagercrantz, H.; Ådén, U. Brain Growth Gains and Losses in Extremely Preterm Infants at Term. Cereb. Cortex 2014, 25, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.K.; Kelly, C.E.; Chen, J.; Beare, R.; Alexander, B.; Seal, M.L.; Lee, K.; Matthews, L.G.; Anderson, P.J.; Doyle, L.W.; et al. Early life predictors of brain development at term-equivalent age in infants born across the gestational age spectrum. NeuroImage 2019, 185, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Bouyssi-Kobar, M.; Du Plessis, A.J.; Mccarter, R.; Brossard-Racine, M.; Murnick, J.; Tinkleman, L.; Robertson, R.L.; Limperopoulos, C. Third Trimester Brain Growth in Preterm Infants Compared With In Utero Healthy Fetuses. Pediatrics 2016, 138, e20161640. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Beltrano, W.; Haynes, L.; Dewey, D.; Bray, S. Long-Term Effects of Preterm Birth on Children’s Brain Structure: An Analysis of the Adolescent Brain Cognitive Development (ABCD) Study. eNeuro 2023, 10, ENEURO.0196-22.2023. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef] [PubMed]

| Included Infants | Excluded Infants | p-Value | Preterm Infants | Term Born Infants | p-Value | |
|---|---|---|---|---|---|---|
| n = 50 | n = 38 | n = 42 | n = 8 | |||
| Neonatal period | ||||||
| Gestational age in weeks, median (range) | 36.0 (25.7–40.1) | 36.1 (26.3–40.3) | 0.923 | 35.5 (25.7–36.9) | 39.4 (38.7–40.1) | <0.001 |
| Birth weight in grams, median (range) | 2315 (915–4280) | 2388 (900–4060) | 0.723 | 2133 (915–4280) | 3346 (2880–3970) | <0.001 |
| Birth weight percentile, median (range) | 45.0 (1.0–94.0) | 52.5 (4.0–96.0) | 0.242 | 45 (1.0–94.0) | 47.0 (16.0–91.0) | 0.990 |
| Small for GA <10th percentile, n (%) | 6 (12) | 2 (5.3) | 0.276 | 6 (14.3) | 0 (0) | 0.254 |
| Small for GA <3rd percentile, n (%) | 1 (2) | 0 (0) | 0.381 | 1 (2.4) | 0 (0) | 0.659 |
| z-scores of body weight | 0.02 (−2.35–3.26) | 0.065 (−1.74–1.81) | 0.539 | 0.075 (−2.35–3.26) | −0.075 (−0.99–1.33) | 0.708 |
| Head circumference in cm, median (range) | 31.8(24.5–37.0) a | 31.7 (24.5–37.0) b | 0.470 | 31.5 (24.5–37.0) c | 34.5 (34.0–35.0) | <0.001 |
| Sex | 0.119 | 0.368 | ||||
| Male, n (%) | 32 (64) | 18 (47) | 28 (67) | 4 (50) | ||
| Female, n (%) | 18 (36) | 20 (53) | 14 (33) | 4 (50) | ||
| Admission level | 0.01 | <0.001 | ||||
| No admission, n (%) | 17 (34) | 18 (47) | 9 (21) | 8 (100) | ||
| Admission to high care, n (%) | 23 (46) | 6 (16) | 23 (55) | 0 (0) | ||
| Admission to intensive care, n (%) | 10 (20) | 14 (37) | 10 (24) | 0 (0) | ||
| Characteristics at MRI | ||||||
| PMA at MRI in weeks, median (range) | 42.2 (40.0–46.9) | 42.1 (40.0–46.9) | 0.730 | 42.0 (40.0–46.9) | 42.4 (41.0–45.0) | 0.649 |
| PNA at MRI in weeks, median (range) | 6.9 (1.4–20.7) | 6.1 (1.4–19.1) | 0.846 | 7.4 (3.4–20.7) | 3.1 (1.4–6.3) | <0.001 |
| Weight in grams, median (range) | 3775 (2210–5330) | 3850 (2690–5470) | 0.601 | 3708 (2210–5330) | 3985 (3060–4240) | 0.507 |
| Head circumference in cm, median (range) | 36.0 (33.5–39.5) a | 36.5 (34.5–40.5) b | 0.810 | 36.0 (33.5–39.5) d | 36.3 (35.0–37.5) e | 0.606 |
| Mean Volume (SD), in cm3 | Min–Max, in cm3 | EP (n = 2), Volume (SD) in cm3 | VP (n = 7), Volume (SD) in cm3 | MP (n = 5), Volume (SD) in cm3 | LP (n = 28), Volume (SD) in cm3 | FT (n = 8), Volume (SD) in cm3 | |
|---|---|---|---|---|---|---|---|
| ICV | 513.81 (58.31) | 439.26–666.92 | 539.22 (26.25) | 544.59 (75.59) | 519.48 (74.39) | 507.16 (53.78) | 500.24 (54.22) |
| TTV | 422.22 (42.89) | 362.13–531.22 | 428.62 (41.38) | 425.67 (59.42) | 425.53 (64.61) | 421.97 (35.38) | 416.41 (48.17) |
| cGM | 190.55 (27.16) | 139.32–267.88 | 183.51 (27.03) | 193.85 (41.61) | 191.77 (43.12) | 190.19 (22.44) | 189.92 (23.29) |
| WM | 162.01 (15.19) | 133.04–193.36 | 175.03 (8.51) | 161.51 (20.30) | 163.92 (18.61) | 161.76 (11.90) | 158.85 (21.08) |
| CSF | 91.59 (24.46) | 55.60–169.54 | 110.61 (15.13) | 118.92 (26.55) | 93.95 (18.83) | 85.19 (23.74) | 83.83 (9.35) |
| dGM | 28.43 (2.51) | 24.64–35.33 | 27.50 (1.79) | 28.82 (2.67) | 28.79 (4.13) | 28.48 (2.34) | 27.93 (2.42) |
| HPC | 2.86 (0.33) | 2.18–3.64 | 2.98 (0.18) | 2.94 (0.38) | 2.82 (0.33) | 2.84 (0.36) | 2.83 (0.30) |
| AM | 1.57 (0.21) | 1.25–2.10 | 1.65 (0.09) | 1.72 (0.25) | 1.62 (0.24) | 1.55 (0.19) | 1.43 (0.18) |
| CB | 29.61 (3.80) | 23.40–38.14 | 30.78 (3.35) | 29.79 (4.57) | 29.55 (5.23) | 29.83 (3.72) | 28.42 (3.21) |
| BS | 7.20 (0.63) | 6.03–8.59 | 7.16 (0.42) | 7.04 (0.62) | 7.06 (0.98) | 7.31 (0.61) | 7.03 (0.57) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verschuur, A.S.; van Wezel-Meijler, G.; Low, S.; Nijholt, I.M.; Metcalfe, A.; Skiffington, J.; Slater, D.M.; Bergeron, A.; Fiedrich, E.; Boomsma, M.F.; et al. Trends in Term-Equivalent Age Brain Volumes in Infants Born Across the Gestational Age Spectrum. Children 2025, 12, 1026. https://doi.org/10.3390/children12081026
Verschuur AS, van Wezel-Meijler G, Low S, Nijholt IM, Metcalfe A, Skiffington J, Slater DM, Bergeron A, Fiedrich E, Boomsma MF, et al. Trends in Term-Equivalent Age Brain Volumes in Infants Born Across the Gestational Age Spectrum. Children. 2025; 12(8):1026. https://doi.org/10.3390/children12081026
Chicago/Turabian StyleVerschuur, Anouk S., Gerda van Wezel-Meijler, Selma Low, Ingrid M. Nijholt, Amy Metcalfe, Janice Skiffington, Donna M. Slater, Amy Bergeron, Elsa Fiedrich, Martijn F. Boomsma, and et al. 2025. "Trends in Term-Equivalent Age Brain Volumes in Infants Born Across the Gestational Age Spectrum" Children 12, no. 8: 1026. https://doi.org/10.3390/children12081026
APA StyleVerschuur, A. S., van Wezel-Meijler, G., Low, S., Nijholt, I. M., Metcalfe, A., Skiffington, J., Slater, D. M., Bergeron, A., Fiedrich, E., Boomsma, M. F., Tax, C. M. W., Leemans, A., & Leijser, L. M. (2025). Trends in Term-Equivalent Age Brain Volumes in Infants Born Across the Gestational Age Spectrum. Children, 12(8), 1026. https://doi.org/10.3390/children12081026








