Effect of Malnutrition on Femoral Cartilage Thickness in Pediatric Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Participants
- •
- Patients diagnosed with primary malnutrition
- •
- Age- and sex-matched healthy controls (because femoral cartilage thickness varies with age and sex [11], the control group was matched accordingly to reduce confounding).
2.3. Inclusion and Exclusion Criteria
2.4. Anthropometric Measurements
2.5. Diagnostic Criteria for Primary Malnutrition
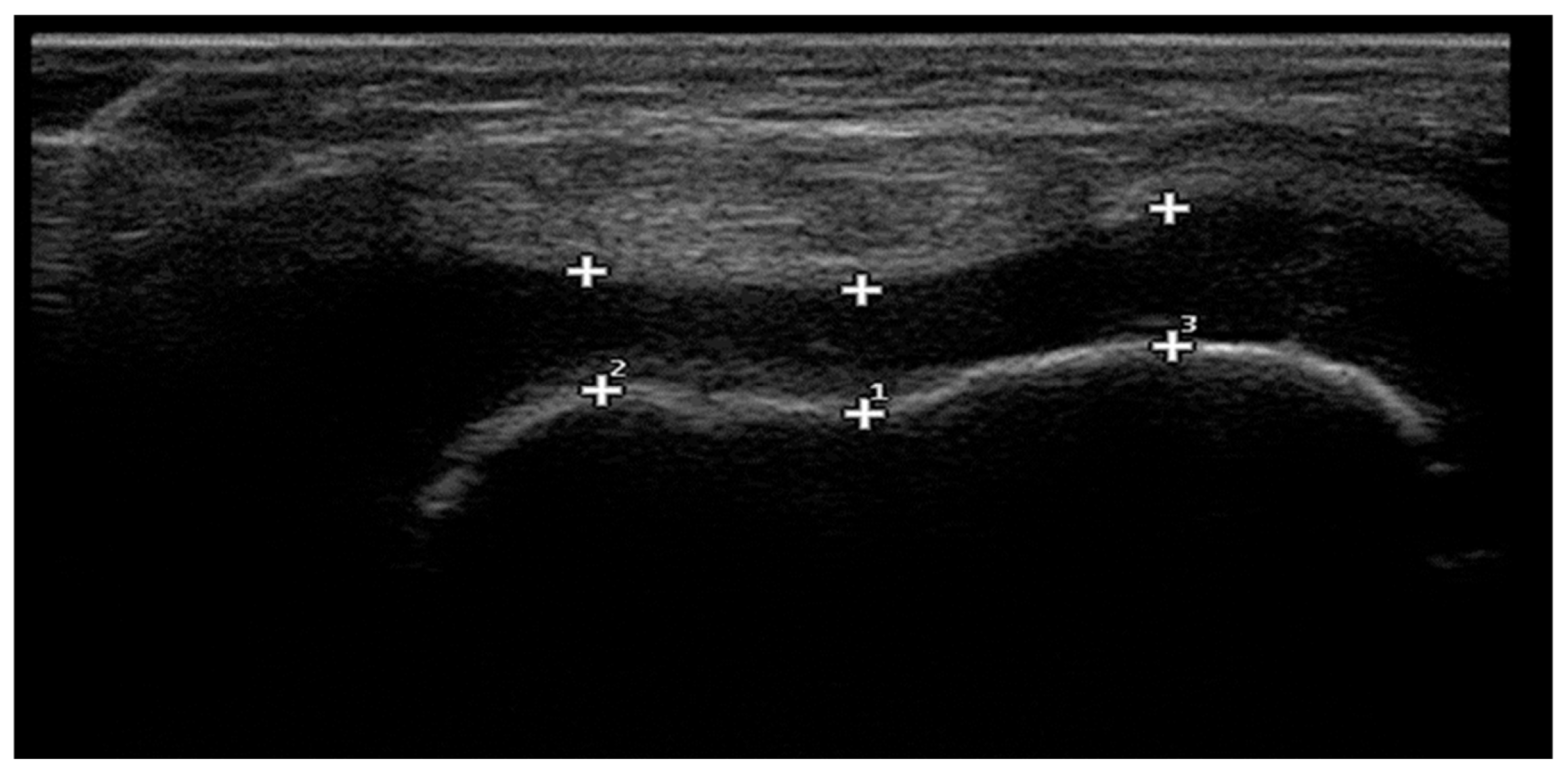
2.6. Ultrasonographic Measurement of Femoral Cartilage Thickness
2.7. Power Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Study Implications and Future Directions
4.2. Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| USG | Ultrasonographic |
| BMI | Body mass index |
| MAC | Mid-arm circumference |
| TST | Triceps skinfold thickness |
| FLC | Femoral lateral condyle |
| ICA | Intercondylar area |
| MLC | Femoral medial condyle |
References
- Branca, F.; Demaio, A.; Udomkesmalee, E.; Baker, P.; Aguayo, V.M.; Barquera, S.; Dain, K.; Keir, L.; Lartey, A.; Mugambi, G.; et al. A New Nutrition Manifesto for a New Nutrition Reality. Lancet 2020, 395, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Shahrin, L.; Chisti, M.J.; Ahmed, T. Primary and Secondary Malnutrition. In Pediatric Nutrition in Practice; Koletzko, B., Ed.; Karger: Basel, Switzerland, 2015; pp. 139–146. [Google Scholar] [CrossRef]
- Dipasquale, V.; Cucinotta, U.; Romano, C. Acute Malnutrition in Children: Pathophysiology, Clinical Effects and Treatment. Nutrients 2020, 12, 2413. [Google Scholar] [CrossRef]
- Ozturk, M.E.; Yabanci Ayhan, N. Evaluation of Malnutrition and Screening Tools in Hospitalized Children. Clin. Nutr. ESPEN 2023, 57, 770–778. [Google Scholar] [CrossRef]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Malas, F.U.; Kara, M.; Aktekin, L.; Ersöz, M.; Ozcakar, L. Does Vitamin D Affect Femoral Cartilage Thickness? An Ultrasonographic Study. Clin. Rheumatol. 2014, 33, 1331–1334. [Google Scholar] [CrossRef]
- Buyukavci, R.; Buyukavci, M.A.; Akturk, S.; Arslan, F.N.; Dogan, D.; Canaloglu, S.K. The Relationship between Motor Performance and Femoral Cartilage Thickness in Children with Down Syndrome. Acta Neurol. Belg. 2021, 121, 351–355. [Google Scholar] [CrossRef]
- Ozcakar, L.; Tunc, H.; Oken, O.; Unlu, Z.; Durmus, B.; Baysal, O.; Altay, Z.; Tok, F.; Akkaya, N.; Doğu, B.; et al. Femoral Cartilage Thickness Measurements in Healthy Individuals: Learning, Practicing and Publishing with TURK-MUSCULUS. J. Back Musculoskelet. Rehabil. 2014, 27, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gat-Yablonski, G.; De Luca, F. Effect of Nutrition on Statural Growth. Horm. Res. Paediatr. 2017, 88, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Spannow, A.H.; Pfeiffer-Jensen, M.; Andersen, N.T.; Herlin, T.; Stenbog, E. Ultrasonographic Measurements of Joint Cartilage Thickness in Healthy Children: Age- and Sex-Related Standard Reference Values. J. Rheumatol. 2010, 37, 2595–2601. [Google Scholar] [CrossRef]
- Puntis, J.W.L. Clinical Evaluation and Anthropometry. In Pediatric Nutrition in Practice; Koletzko, B., Ed.; Karger: Basel, Switzerland, 2008; pp. 6–12. [Google Scholar]
- Khaliq, A. Coexisting Forms of Malnutrition in Children of Pakistan. Ph.D. Thesis, Queensland University of Technology, School of Public Health and Social Work, Faculty of Health, Brisbane, Australia, 2023. Available online: https://eprints.qut.edu.au/241461/1/Asif_Khaliq_thesis.pdf (accessed on 22 July 2025).
- Windschall, D.; Trauzeddel, R.; Haller, M.; Krumrey-Langkammerer, M.; Nimtz-Talaska, A.; Berendes, R.; Ganser, G.; Nirschl, C.; Schoof, P.; Trauzeddel, R.F.; et al. Pediatric Musculoskeletal Ultrasound: Age- and Sex-Related Normal B-Mode Findings of the Knee. Rheumatol. Int. 2016, 36, 1569–1577. [Google Scholar] [CrossRef]
- Pradsgaard, D.O.; Fiirgaard, B.; Spannow, A.H.; Heuck, C.; Herlin, T. Cartilage Thickness of the Knee Joint in Juvenile Idiopathic Arthritis: Comparative Assessment by Ultrasonography and Magnetic Resonance Imaging. J. Rheumatol. 2015, 42, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Adiguzel, E.; Tok, F.; Ata, E.; Yasar, E.; Yilmaz, B. Ultrasonographic Assessment of Femoral Cartilage Thickness in Patients with Cerebral Palsy. PM R 2018, 10, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Weber, R.; Nourkami-Tutdibi, N.; Bous, M.; Goedicke-Fritz, S.; Hans, M.C.; Hein, S.; Wolf, M.A.; Landgraeber, S.; Zemlin, M.; et al. Ultrasound-Guided Determination Demonstrates Influence of Age, Sex and Type of Sport on Medial Femoral Condyle Cartilage Thickness in Children and Adolescents. Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 1423–1433. [Google Scholar] [CrossRef]
- Faber, S.C.; Eckstein, F.; Lukasz, S.; Mühlbauer, R.; Hohe, J.; Englmeier, K.H.; Reiser, M. Gender Differences in Knee Joint Cartilage Thickness, Volume and Articular Surface Areas: Assessment with Quantitative Three-Dimensional MR Imaging. Skelet. Radiol. 2001, 30, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Ding, C.; Glisson, M.; Hynes, K.; Ma, D.; Cicuttini, F. Knee Articular Cartilage Development in Children: A Longitudinal Study of the Effect of Sex, Growth, Body Composition, and Physical Activity. Pediatr. Res. 2003, 54, 230–236. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Ba, D.M.; Ericson, J.E.; Na, M.; Gao, X.; Fronterre, C.; Chinchilli, V.M.; Schiff, S.J. Global, Regional and National Epidemiology and Prevalence of Child Stunting, Wasting and Underweight in Low- and Middle-Income Countries, 2006–2018. Sci. Rep. 2021, 11, 5204. [Google Scholar] [CrossRef]
- Sidharthan, S.; Yau, A.; Almeida, B.A.; Shea, K.G.; Greditzer, H.G.; Jones, K.J.; Fabricant, P.D. Patterns of Articular Cartilage Thickness in Pediatric and Adolescent Knees: A Magnetic Resonance Imaging-Based Study. Arthrosc. Sports Med. Rehabil. 2021, 3, e381–e390. [Google Scholar] [CrossRef]
- Gau, C.C.; Yao, T.C.; Gan, S.T.; Lin, S.J.; Yeh, K.W.; Chen, L.C.; Ou, L.S.; Lee, W.I.; Wu, C.Y.; Huang, J.L. Age, Gender, Height and Weight in Relation to Joint Cartilage Thickness among School-Aged Children from Ultrasonographic Measurement. Pediatr. Rheumatol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Yaméogo, C.W.; Cichon, B.; Fabiansen, C.; Iuel-Brockdorf, A.S.; Shepherd, S.; Filteau, S.; Traoré, A.S.; Christensen, V.B.; Michaelson, K.F.; Brage, S.; et al. Correlates of Physical Activity among Young Children with Moderate Acute Malnutrition. J. Pediatr. 2017, 181, 235–241. [Google Scholar] [CrossRef]
- Herrera, H.G.A.; Llinas, P.J.; Florez, L.; Montes, C.B.; Obando, D.V.; Solorzano, A.D.; Loaiza, D.; Guillen Astete, C. Ultrasound Measurement of Femoral Cartilage Thickness in the Knee of Healthy Young University Students. Rev. Esp. Cir. Ortop. Traumatol. 2020, 64, 244–250. [Google Scholar] [CrossRef]
- Sinanoglu, M.S.; Gungor, S.; Dag, N.; Varol, F.I.; Kenc, S.; Gok, E. Ultrasound and Shear Wave Elastography Assessment of Diaphragm Thickness and Stiffness in Malnourished Pediatric Patients. Eur. J. Pediatr. 2024, 184, 35. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Bennell, K.; Cicuttini, F.M. Effect of Physical Activity on Cartilage Development in Healthy Kids. Br. J. Sports Med. 2003, 37, 382–383. [Google Scholar] [CrossRef] [PubMed]
| Normal | Malnutrition | |||
|---|---|---|---|---|
n-% | Mild n-% | Moderate n-% | Severe n-% | |
| Weight Z score | −1 < Weight Z score ≤ 2 | −2 < Weight Z score ≤ −1 | −3 < Weight Z score ≤ −2 | ≤−3 |
| 61–42.4 | 16–11.1 | 43–29.9 | 24–16.7 | |
| Height Z score | −1 < Height Z score ≤ 2 | −2 < Height Z score ≤ −1 | −3 < Height Z score ≤ −2 | ≤−3 |
| 79–54.9 | 29–20.1 | 25–17.4 | 11–7.6 | |
| MAC Z score | −1 < MAC Z score ≤ 2 | −2 < MAC Z score ≤ −1 | −3 < MAC Z score ≤ −2 | ≤−3 |
| 50–34.7 | 45–31.3 | 28–19.4 | 21–14.6 | |
| Healthy Group (62) n-% | Malnutrition (83) n-% | p | |
|---|---|---|---|
| Gender | 0.516 | ||
| Female | 32–52.5 | 39–47 | |
| Male | 29–47.5 | 44–53 | |
| Mean ± SD | Mean ± SD | ||
| Age (months) | 7.84 ± 4.62 | 7.86 ± 5.36 | 0.976 |
| R-FLC (mm) | 3.02 ± 0.84 | 3.64 ± 1.41 | 0.002 |
| R-ICA (mm) | 3.07 ± 0.79 | 3.26 ± 0.91 | 0.174 |
| R-MLC (mm) | 3.19 ± 0.78 | 3.73 ± 1.38 | 0.004 |
| L-FLC (mm) | 2.92 ± 0.73 | 3.59 ± 1.39 | <0.001 |
| L-ICA (mm) | 3.07 ± 0.76 | 3.19 ± 0.73 | 0.345 |
| L-MLC (mm) | 3.14 ± 0.77 | 3.84 ± 1.41 | <0.001 |
| Healthy Group (61) Mean ± SD | Mild Malnutrition (16) Mean ± SD | Moderate Malnutrition (43) Mean ± SD | Severe Malnutrition (24) Mean ± SD | p | |
|---|---|---|---|---|---|
| Age (months) | 7.84 ± 4.62 | 4.48 ± 1.08 | 5.54 ± 0.85 | 8.14 ± 5.82 | 0.992 |
| R-FLC (mm) | 3.02 ± 0.84 α | 3.59 ± 1.12 | 3.63 ± 1.53 β | 3.68 ± 1.40 β | 0.034 |
| R-ICA (mm) | 3.07 ± 0.79 | 3.19 ± 0.93 | 3.24 ± 0.94 | 3.35 ± 0.56 | 0.527 |
| R-MLC (mm) | 3.19 ± 0.78 α | 3.43 ± 1.22 | 3.77 ± 1.46 β | 3.87 ± 1.37 β | 0.036 |
| L-FLC (mm) | 2.92 ± 0.73 α | 3.56 ± 1.16 | 3.60 ± 1.41 β | 3.60 ± 1.55 β | 0.010 |
| L-ICA (mm) | 3.07 ± 0.76 | 3.11 ± 0.61 | 3.15 ± 0.78 | 3.34 ± 0.73 | 0.520 |
| L-MLC (mm) | 3.14 ± 0.77 α | 3.69 ± 1.34 | 3.87 ± 1.48 β | 3.90 ± 1.38 β | 0.007 |
| Age | BMI Z-Score | Weight Z-Score | Height Z-Score | MAC Z-Score | TST Z-Score | ||
|---|---|---|---|---|---|---|---|
| R-FLC | r | −0.647 ** | −0.102 | −0.168 ** | −0.239 ** | −0.071 | −0.280 ** |
| p | <0.001 | 0.230 | 0.045 | 0.004 | 0.398 | 0.001 | |
| R-ICA | r | −0.521 ** | 0.089 | −0.132 | −0.134 | −0.025 | −0.277 ** |
| p | <0.001 | 0.292 | 0.115 | 0.109 | 0.794 | 0.001 | |
| R-MLC | r | −0.673 ** | −0.150 | −0.223 ** | −0.257 ** | −0.055 | −0.267 ** |
| p | <0.001 | 0.076 | 0.007 | 0.002 | 0.516 | 0.002 | |
| L-FLC | r | −0.625 ** | −0.116 | −0.209 ** | −0.286 ** | −0.097 | −0.284 ** |
| p | <0.001 | 0.171 | 0.012 | 0.001 | 0.248 | 0.001 | |
| L-ICA | r | −0.531 ** | −0.093 | −0.121 | −0.145 | −0.036 | −0.268 ** |
| p | <0.001 | 0.274 | 0.152 | 0.083 | 0.672 | 0.002 | |
| L-MLC | r | −0.650 ** | −0.156 | −0.238 ** | −0.284 ** | −0.100 | −0.271 ** |
| p | <0.001 | 0.064 | 0.004 | 0.001 | 0.232 | 0.002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güngör, Ş.; Büyükavcı, R.; Varol, F.İ.; Gök, E.; Aktürk, S. Effect of Malnutrition on Femoral Cartilage Thickness in Pediatric Patients. Children 2025, 12, 1021. https://doi.org/10.3390/children12081021
Güngör Ş, Büyükavcı R, Varol Fİ, Gök E, Aktürk S. Effect of Malnutrition on Femoral Cartilage Thickness in Pediatric Patients. Children. 2025; 12(8):1021. https://doi.org/10.3390/children12081021
Chicago/Turabian StyleGüngör, Şükrü, Raikan Büyükavcı, Fatma İlknur Varol, Emre Gök, and Semra Aktürk. 2025. "Effect of Malnutrition on Femoral Cartilage Thickness in Pediatric Patients" Children 12, no. 8: 1021. https://doi.org/10.3390/children12081021
APA StyleGüngör, Ş., Büyükavcı, R., Varol, F. İ., Gök, E., & Aktürk, S. (2025). Effect of Malnutrition on Femoral Cartilage Thickness in Pediatric Patients. Children, 12(8), 1021. https://doi.org/10.3390/children12081021






