Perceptions of Infant Cry Sounds Among Tobacco and Cannabis Using Mothers and Their Association with Tobacco and Cannabis Cravings
Abstract
1. Introduction
1.1. Tobacco and Cannabis Cravings
1.2. Maternal Tobacco and Cannabis Use and Maternal Negative Affect
1.3. Maternal Tobacco and Cannabis Use, Negative Affect and Responsiveness to Infant Signals
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Measures
2.3.1. Depressive Symptoms
2.3.2. Anger/Hostility
2.3.3. Substance Use
2.3.4. Maternal Perceptions of Infant Cries
2.3.5. Tobacco and Cannabis Craving
2.4. Missing Data
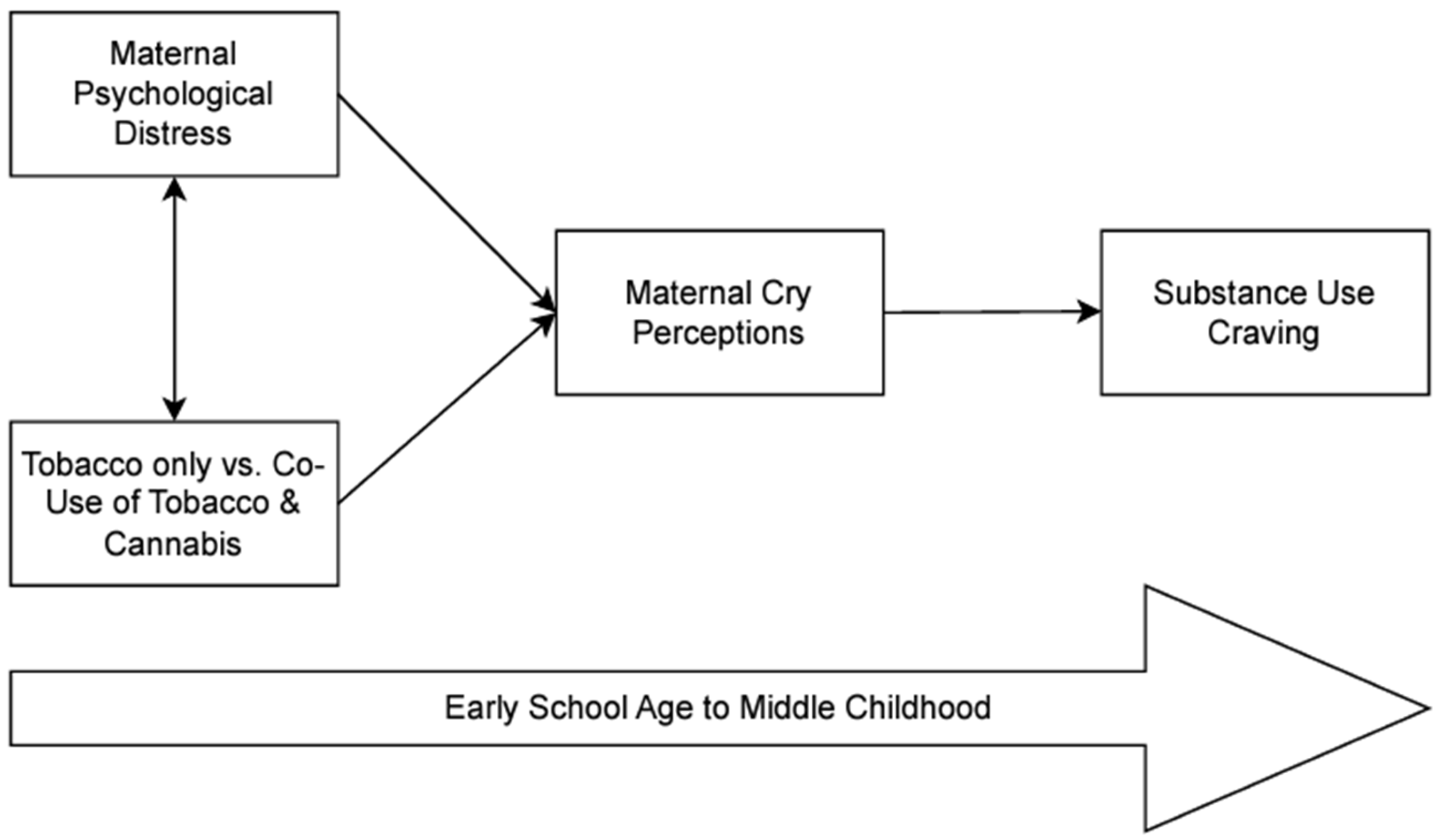
2.5. Analytic Plan
3. Results
3.1. Preliminary Analyses
3.2. Primary Analyses
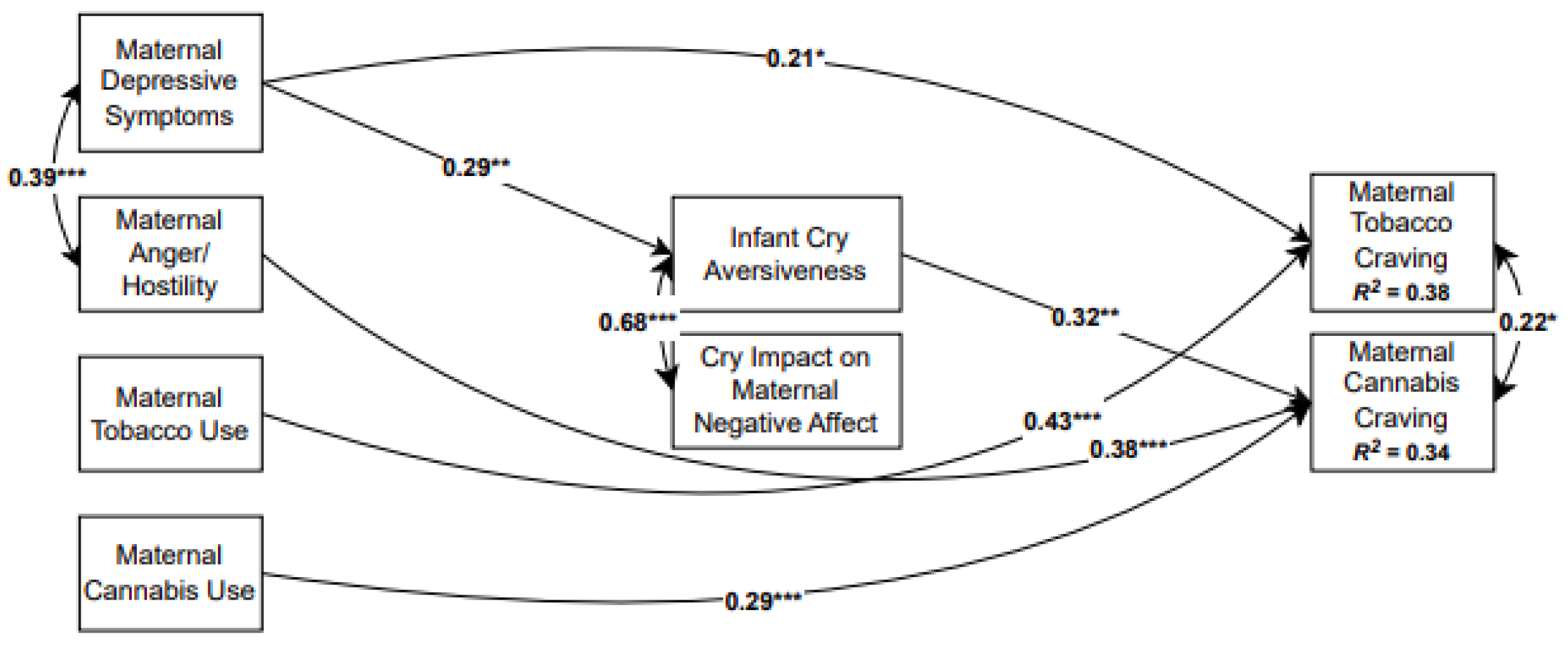
3.3. Overall Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TLFB | Timeline Followback Interview |
| BSI | Brief Symptom Inventory |
| BPAQ | Buss-Perry Aggression Questionnaire |
| QSU-4 | Questionnaire of Smoking Urges-4 |
| RMSEA | Root mean square error or approximation |
| SRMR | Standardized root mean square residual |
| CFI | Comparative fit index |
References
- Cornelius, M.E.; Loretan, C.G.; Jamal, A.; Lynn, B.C.D.; Mayer, M.; Alcantara, I.C.; Neff, L. Tobacco product use among adults–United States, 2021. Morb. Mortal. Wkly. Rep. 2023, 72, 475–483. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2021 National Survey on Drug Use and Health (HHS Publication No. PEP22-07-01-005, NSDUH Series H-57). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. 2022. Available online: https://library.samhsa.gov/product/results-2021-national-survey-drug-use-and-health-nsduh-key-substance-use-and-mental-health (accessed on 8 June 2025).
- Monte, A.A.; Zane, R.D.; Heard, K.J. The implications of marijuana legalization in Colorado. JAMA 2015, 313, 241–242. [Google Scholar] [CrossRef]
- Calvigioni, D.; Hurd, Y.L.; Harkany, T.; Keimpema, E. Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur. Child Adolesc. Psychiatry 2014, 23, 931–941. [Google Scholar] [CrossRef]
- Mehmedic, Z.; Chandra, S.; Slade, D.; Denham, H.; Foster, S.; Patel, A.S.; Ross, S.A.; Khan, I.A.; ElSohly, M.A. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci. 2010, 55, 1209–1217. [Google Scholar] [CrossRef]
- El Marroun, H.; Tiemeier, H.; Jaddoe, V.W.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.; Verhulst, F.C.; van den Brink, W.; Huizink, A.C. Demographic, emotional and social determinants of cannabis use in early pregnancy: The Generation R study. Drug Alcohol Depend. 2008, 98, 218–226. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2014. [Google Scholar]
- Passey, M.E.; Sanson-Fisher, R.W.; D’Este, C.A.; Stirling, J.M. Tobacco, alcohol and cannabis use during pregnancy: Clustering of risks. Drug Alcohol Depend. 2014, 134, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Serre, F.; Fatseas, M.; Swendsen, J.; Auriacombe, M. Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug Alcohol Depend. 2015, 148, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Serre, F.; Fatseas, M.; Denis, C.; Swendsen, J.; Auriacombe, M. Predictors of craving and substance use among patients with alcohol, tobacco, cannabis or opiate addictions: Commonalities and specificities across substances. Addict. Behav. 2018, 83, 123–129. [Google Scholar] [CrossRef]
- Shiyko, M.; Naab, P.; Shiffman, S.; Li, R. Modeling complexity of EMA data: Time-varying lagged effects of negative affect on smoking urges for subgroups of nicotine addiction. Nicotine Tob. Res. 2014, 16 (Suppl. S2), 144–150. [Google Scholar] [CrossRef]
- Epstein, D.H.; Willner-Reid, J.; Vahabzadeh, M.; Mezghanni, M.; Lin, J.L.; Preston, K.L. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch. Gen. Psychiatry 2009, 66, 88–94. [Google Scholar] [CrossRef]
- Krahn, D.D.; Bohn, M.J.; Henk, H.J.; Gorssman, J.L.; Gosnell, B. Patterns of urges during early abstinence in alcohol-dependent subjects. Am. J. Addict. 2005, 14, 248–255. [Google Scholar] [CrossRef]
- Trujillo, M.A.; Khoddam, R.; Greenberg, J.B.; Dyal, S.R.; Ameringer, K.J.; Zvolensky, M.J.; Leventhal, A.M. Distress tolerance as a correlate of tobacco dependence and motivation: Incremental relations over and above anxiety and depressive symptoms. Behav. Med. 2017, 43, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.A.; Karelitz, J.L.; Giedgowd, C.E.; Conklin, C. A Negative mood effects on craving to smoke in women versus men. Addict. Behav. 2013, 38, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Heishman, S.J.; Evans, R.J.; Singleton, E.G.; Levin, K.H.; Copersino, M.L.; Gorelick, D. A Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 2009, 102, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Swendsen, J.; Ben-Zeev, D.; Granholm, E. Real-time electronic ambulatory monitoring of substance use and symptom expression in schizophrenia. Am. J. Psychiatry 2011, 168, 202–209. [Google Scholar] [CrossRef]
- Ludman, E.J.; McBride, C.M.; Nelson, J.C.; Curry, S.J.; Grothaus, L.C.; Lando, H.A.; Pirie, P.L. Stress, depressive symptoms, and smoking cessation among pregnant women. Health Psychol. 2000, 13, 149–155. [Google Scholar] [CrossRef]
- Munafò, M.R.; Heron, J.; Araya, R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine Tob. Res. 2008, 10, 1620. [Google Scholar] [CrossRef]
- Goodwin, R.D.; Zhu, J.; Heisler, Z.; Metz, T.D.; Wyka, K.; Wu, M.; Das Eiden, R. Cannabis use during pregnancy in the United States: The role of depression. Drug Alcohol Depend. 2020, 210, 107881. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Sarovar, V.; Tucker, L.Y.; Goler, N.C.; Alexeeff, S.E.; Ridout, K.K.; Avalos, L.A. Association of Depression, Anxiety, and Trauma With Cannabis Use During Pregnancy. JAMA Netw. Open 2020, 3, e1921333. [Google Scholar] [CrossRef]
- Level, R.; Shisler, S.; Seay, D.; Ivanova, M.; Kelm, M.; Eiden, R.D.; Schuetze, P. Within- and between-family transactions of maternal depression, parenting, and child engagement in the first two years of life: Role of prenatal maternal risk and tobacco use. Depress. Anxiety 2021, 38, 1279–1288. [Google Scholar] [CrossRef]
- Eiden, R.D.; Leonard, K.E.; Colder, C.R.; Homish, G.G.; Schuetze, P.; Gray, T.R.; Huestis, M.A. Anger, hostility, and aggression as predictors of persistent smoking during pregnancy. J. Stud. Alcohol Drugs 2011, 72, 926–932. [Google Scholar] [CrossRef]
- Schuetze, P.; Eiden, R.D.; Colder, C.R.; Huestis, M.; Leonard, K. Prenatal risk and infant regulation: Indirect Pathways via Fetal Growth, Prenatal Stress and Maternal Aggression/Hostility. Child Dev. 2018, 89, e123–e137. [Google Scholar] [CrossRef]
- Ostlund, B.; Perez-Edgar, K.E.; Shisler, S.; Terrell, S.; Godleski, S.; Schuetze, P.; Eiden, R.D. Prenatal substance exposure and maternal hostility from pregnancy to toddlerhood: Associations with temperament profiles at 16-months of age. Dev. Psychopathol. 2021, 33, 1566–1583. [Google Scholar] [CrossRef]
- Suchman, N.E.; DeCoste, C.; Castiglion, N.; McMahon, T.J.; Rounsaville, B.; Mayes, L. The Mothers and Toddlers Program, an attachment-based parenting intervention for substance using women: Post-treatment results from a randomized clinical pilot. Attach. Hum. Dev. 2010, 12, 483–504. [Google Scholar] [CrossRef]
- Punamäki, R.L.; Flykt, M.; Belt, R.; Lindblom, J. Maternal substance use disorder predicting children’s emotion regulation in middle childhood: The role of early mother-infant interaction. Heliyon 2021, 7, e06728. [Google Scholar] [CrossRef]
- Landi, N.; Montoya, J.; Kober, H.; Rutherford, H.J.; Mencl, W.E.; Worhunsky, P.D.; Potenza, M.N.; Mayes, L.C. Maternal neural responses to infant cries and faces: Relationships with substance use. Front. Psychiatry 2011, 2, 32. [Google Scholar] [CrossRef]
- Schuetze, P.; Zeskind, P.S.; Eiden, R.D. The perceptions of infant distress signals varying in pitch by cocaine-using mothers. Infancy 2003, 4, 65–83. [Google Scholar] [CrossRef]
- Rutherford, H.J.; Mayes, L.C. Parenting and addiction: Neurobiological insights. Curr. Opin. Psychol. 2017, 15, 55–60. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A. Endocrine induced changes in brain function during pregnancy. Brain Res. 2010, 1364, 198–215. [Google Scholar] [CrossRef]
- Mackie, K. Signaling via CNS cannabinoid receptors. Mol. Cell. Endocrinol. 2008, 286 (Suppl. S1), S60–S65. [Google Scholar] [CrossRef]
- Schuetze, P.; Zeskind, P.S. Relations between women’s depressive symptoms and perceptions of infant distress signals varying in pitch. Infancy 2001, 2, 483–499. [Google Scholar] [CrossRef]
- Esposito, G.; Manian, N.; Truzzi, A.; Bornstein, M. H Response to infant cry in clinically depressed and non-depressed mothers. PLoS ONE 2017, 12, e0169066. [Google Scholar] [CrossRef]
- Donovan, W.L.; Leavitt, L.A.; Walsh, R.O. Conflict and depression predict maternal sensitivity to infant cries. Infant Behav. Dev. 1998, 21, 505–517. [Google Scholar] [CrossRef]
- Kelm, M.R.; Schuetze, P.; Eiden, R.D. Prenatal tobacco and tobacco-Cannabis co-exposure and unpredictability in maternal anger/hostility: Implications for toddler reactivity. Neurotoxicol. Teratol. 2024, 106, 107399. [Google Scholar] [CrossRef]
- Eiden, R.D.; Shisler, S.; Granger, D.A.; Schuetze, P.; Colangelo, J.; Huestis, M.A. Prenatal Tobacco and Cannabis Exposure: Associations with Cortisol Reactivity in Early School Age Children. Int. J. Behav. Med. 2020, 27, 343–356. [Google Scholar] [CrossRef]
- Perry, K.J.; Level, R.A.; Schuetze, P.; Eiden, R.D. Prenatal tobacco, tobacco–cannabis co-exposure, and child emotion regulation: The role of child autonomic functioning and sensitive parenting. Dev. Psychol. 2024, 60, 1544–1561. [Google Scholar] [CrossRef]
- Schuetze, P.; Zhao, J.; Eiden, R.D.; Shisler, S. Prenatal Exposure to Tobacco and Marijuana and Child Autonomic Regulation and Reactivity: An Analysis of Indirect Pathways via Maternal Psychopathology and Parenting. Dev. Psychobiol. 2019, 6, 1022–1034. [Google Scholar] [CrossRef]
- Gray, T.R.; Eiden, R.D.; Leonard, K.E.; Connors, G.J.; Shisler, S.; Huestis, M.A. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin. Chem. 2010, 56, 1442–1450. [Google Scholar] [CrossRef]
- Eiden, R.D.; Zhao, J.; Casey, M.; Shisler, S.; Schuetze, P.; Colder, C.R. Pre-and postnatal tobacco and cannabis exposure and child behavior problems: Bidirectional associations, joint effects, and sex differences. Drug Alcohol Depend. 2018, 185, 82–92. [Google Scholar] [CrossRef]
- Gray, T.R.; Eiden, R.D.; Leonard, K.E.; Connors, G.; Shisler, S.; Huestis, M.A. Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine Tob. Res. 2010, 12, 658–664. [Google Scholar] [CrossRef]
- Derogatis, L.R. BSI Brief Symptom Inventory: Administration, Scoring, and Procedures Manual, 4th ed.; National Computer Systems: Minneapolis, MN, USA, 1993. [Google Scholar]
- Buss, A.H.; Perry, M. The aggression questionnaire. J. Personal. Soc. Psychol. 1992, 63, 452–459. [Google Scholar] [CrossRef]
- Sobell, L.C.; Sobell, M.B. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In Measuring Alcohol Consumption: Psychosocial and Biochemical Methods; Litten, R.Z., Allen, J.P., Eds.; Humana Press: Totowa, NJ, USA, 1992; pp. 41–72. [Google Scholar] [CrossRef]
- Robinson, S.M.; Sobell, L.C.; Sobell, M.B.; Leo, G.I. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav. 2014, 28, 154–162. [Google Scholar] [CrossRef]
- Brown, R.A.; Burgess, E.S.; Sales, S.D.; Whiteley, J.A.; Evans, D.M.; Miller, I.W. Reliability and validity of a smoking timeline follow-back interview. Psychol. Addict. Behav. 1998, 12, 101–112. [Google Scholar] [CrossRef]
- Tiffany, S.T.; Drobes, D.J. The development and initial validation of a questionnaire on smoking urges. Br. J. Addict. 1991, 86, 1467–1476. [Google Scholar] [CrossRef]
- Little, R.J.A. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 1988, 83, 1198–1202. [Google Scholar] [CrossRef]
- Arbuckle, J.L. Full Information Estimation in the Presence of Incomplete Data, 1st ed.; Psychology Press: London, UK, 1996. [Google Scholar]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide, 8th ed.; Muthén Muthén: Los Angeles, CA, USA, 2022. [Google Scholar]
- MacKinnon, D.P.; Lockwood, C.M.; Williams, J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivar. Behav. Res. 2004, 39, 99–128. [Google Scholar] [CrossRef]
- Tibbe, T.D.; Montoya, A.K. Correcting the bias correction for the bootstrap confidence interval in mediation analysis. Front. Psychol. 2022, 13, 810258. [Google Scholar] [CrossRef]
- Suchman, N.E.; DeCoste, C.; Leigh, D.; Borelli, J. Reflective functioning in mothers with drug use disorders: Implications for dyadic interactions with infants and toddlers. Am. J. Bioeth. 2010, 12, 567–585. [Google Scholar] [CrossRef]
- Ashare, R.L.; Sinha, R.; Lampert, R.; Weinberger, A.H.; Anderson, G.M.; Lavery, M.E.; Yanagisawa, K.; McKee, S.A. Blunted vagal reactivity predicts stress-precipitated tobacco smoking. Psychopharmacology 2012, 220, 259–268. [Google Scholar] [CrossRef]
- Speck, B.; Isenhour, J.L.; Gao, M.M.; Conradt, E.; Crowell, S.E.; Raby, K.L. Pregnant women’s autonomic responses to an infant cry predict young infants’ behavioral avoidance during the still-face paradigm. Dev. Psychol. 2023, 59, 2237–2247. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Telang, F. Addiction: Beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, 15037–15042. [Google Scholar] [CrossRef]
- Marsden, D.G.; Loukas, A.; Chen, B.; Perry, C.L.; Wilkinson, A.V. Associations between frequency of cigarette and alternative tobacco product use and depressive symptoms: A longitudinal study of young adults. Addict. Behav. 2019, 99, 106078. [Google Scholar] [CrossRef]
- Nguyen, N.; Peyser, N.D.; Olgin, J.E.; Pletcher, M.J.; Beatty, A.L.; Modrow, M.F.; Carton, T.W.; Khatib, R.; Djibo, D.A.; Ling, P.M.; et al. Associations between tobacco and cannabis use and anxiety and depression among adults in the United States: Findings from the COVID-19 citizen science study. PLoS ONE 2023, 18, e0289058. [Google Scholar] [CrossRef]
- Obisesan, O.H.; Mirbolouk, M.; Osei, A.D.; Orimoloye, O.A.; Uddin, S.M.I.; Dzaye, O.; El Shahawy, O.; Al Rifai, M.; Bhatnagar, A.; Stokes, A.; et al. Association Between e-Cigarette Use and Depression in the Behavioral Risk Factor Surveillance System, 2016–2017. JAMA Netw. Open 2019, 2, e1916800. [Google Scholar] [CrossRef]
- Weinberger, A.H.; Chaiton, M.O.; Zhu, J.; Wall, M.M.; Hasin, D.S.; Goodwin, R.D. Trends in the prevalence of current, daily, and nondaily cigarette smoking and quit ratios by depression status in the U.S.: 2005–2017. Am. J. Prev. Med. 2020, 58, 691–698. [Google Scholar] [CrossRef]
- Ansell, E.B.; Laws, H.B.; Roche, M.J.; Sinha, R. Effects of marijuana use on impulsivity and hostility in daily life. Drug Alcohol Depend. 2015, 148, 136–142. [Google Scholar] [CrossRef]
- Dillon, K.H.; Van Voorhees, E.E.; Elbogen, E.B.; Beckham, J.C.; Calhoun, P.S.; Brancu, M.; Yoash-Gantz, R.E. Cannabis use disorder, anger, and violence in Iraq/Afghanistan-era veterans. J. Psychiatr. Res. 2021, 138, 375–379. [Google Scholar] [CrossRef]
- Pahl, K.; Brook, J.S.; Koppel, J. Trajectories of marijuana use and psychological adjustment among urban African American and Puerto Rican women. Psychol. Med. 2011, 41, 1775–1783. [Google Scholar] [CrossRef]
- Wycoff, A.M.; Metrik, J.; Trull, T.J. Affect and cannabis use in daily life: A review and recommendations for future research. Drug Alcohol Depend. 2018, 191, 223–233. [Google Scholar] [CrossRef]
- Chase, H.W.; Moses-Kolko, E.L.; Zevallos, C.; Wisner, K.L.; Phillips, M.L. Disrupted posterior cingulate—Amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Soc. Cogn. Affect. Neurosci. 2014, 9, 1069–1075. [Google Scholar] [CrossRef]
- Laurent, H.K.; Ablow, J.C. A cry in the dark: Depressed mothers show reduced neural activation to their own infant’s cry. Soc. Cogn. Affect. Neurosci. 2012, 7, 125–134. [Google Scholar] [CrossRef]
- Cohn, J.F.; Campbell, S.B.; Matias, R.; Hopkins, J. Face-to-face interactions of postpartum depressed and nondepressed mother-infant pairs at 2 months. Dev. Psychol. 1990, 26, 15–23. [Google Scholar] [CrossRef]
- Graham, C.A.; Easterbrooks, M.A. School-aged children’s vulnerability to depressive symptomatology: The role of attachment security, maternal depressive symptomatology, and economic risk. Dev. Psychopathol. 2000, 12, 201–213. [Google Scholar] [CrossRef]
- Cornelius, M.D.; Day, N. Developmental consequences of prenatal tobacco exposure. Curr. Opin. Neurol. 2009, 22, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Gerbing, D.W. The Effect of Sampling Error on Convergence, Improper Solutions, and Goodness-of-Fit Indices for Maximum Likelihood Confirmatory Factor Analysis. Psychometrika 1984, 49, 155–173. [Google Scholar] [CrossRef]
- Loman, M.M.; Gunnar, M.R. Early experience and the development of stress reactivity and regulation in children. Neurosci. Biobehav. Rev. 2010, 34, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Stams, G.J.; Juffer, F.; van IJzendoorn, M.H. Maternal sensitivity, infant attachment, and temperament in arly childhood predict adjustment in middle childhood: The case of adopted children and their biologically unrelated parents. Dev. Psychol. 2002, 38, 806–821. [Google Scholar] [CrossRef]
- Tronick, E.; Beeghly, M. Infants’ meaning-making and the development of mental health problems. Am. Psychol. 2011, 66, 107–119. [Google Scholar] [CrossRef]
| Tobacco | Co-Exposed | F/χ2 (p) | Partial η2 | |
|---|---|---|---|---|
| Maternal Substance Use | ||||
| Cigarettes Per Day | 6.09 (5.89) | 7.11 (5.86) | 0.64 (0.43) | 0.03 |
| Joints Per Day | 0 (0) | 2.05 (3.92) | 7.89 (0.006) | 0.18 |
| Maternal Psychological Distress | ||||
| BPAQ Total Score (Anger/Hostility) | 2.29 (0.61) | 2.52 (0.75) | 3.50 (0.07) | 0.14 |
| BSI Subscale Score (Depressive Symptoms) | 0.57 (0.71) | 0.65 (0.72) | 0.27 (0.61) | 0.06 |
| Maternal Cry Perception | ||||
| Impact on Negative Affect | 2.98 (0.98) | 2.89 (1.09) | 0.13 (0.73) | 0.04 |
| Cry Aversiveness | 3.23 (0.89) | 3.37 (1.02) | 0.41 (0.52) | 0.05 |
| Craving Scale Score | ||||
| Tobacco | 0.67 (1.59) | 0.58 (3.08) | 0.13 (0.72) | 0.01 |
| Cannabis | 0 (0) | 0.33 (1.18) | 10.12 (0.002) | 0.16 |
| Demographics | ||||
| Maternal Age | 24.94 (5.56) | 24.14 (5.22) | 0.48 (0.49) | 0.04 |
| Maternal Education (years) | 12.21 (1.86) | 12.23 (1.9) | 0.77 (0.58) | 0.03 |
| Race (% nonWhite) | 59.28% | 68.04% | 9.64 (0.003) |
| Assessment Point and Construct | Sample Size |
|---|---|
| Prenatal/Birth | |
| Maternal Substance Use–Self-report | n = 96 |
| Salivary Assays for Substance Use | n = 96 |
| Infant Meconium | n = 76 |
| Early School-age–Kindergarten | |
| Maternal Depression | n = 91 |
| Maternal Anger/Hostility | n = 89 |
| Middle Childhood | |
| Maternal Tobacco/Cannabis Use | n = 96 |
| Maternal Cry Perceptions | n = 94 |
| Maternal Cravings for Tobacco/Cannabis | n = 94 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. Co-Use vs. Tobacco Use | - | |||||
| 2. Maternal BPAQ Total Score | - | |||||
| 3. Maternal Depression | 0.39 *** | - | ||||
| 4. Cry Impact on Negative Affect | 0.07 | 0.19 | - | |||
| 5. Cry Aversiveness | 0.13 | 0.28 ** | 0.69 *** | - | ||
| 6. Tobacco Craving | 0.42 *** | 0.23 * | 0.01 | 20 | - | |
| 7. Cannabis Craving | −0.03 | 0.35 *** | 0.16 | 0.20 | 0.20 * |
| Direct Pathway | Direct Effect |
|---|---|
| Maternal Depressive Symptoms → Tobacco Craving | 0.21 * |
| Maternal Tobacco Use → Tobacco Craving | 0.43 ** |
| Maternal Anger/ → Cannabis Craving | 0.38 ** |
| Maternal Cannabis Use → Cannabis Craving | 0.29 ** |
| Maternal Depressive Symptoms → Infant Cry Aversiveness | 0.29 ** |
| Maternal Anger/Hostility → Infant Cry Aversiveness | 0.03 |
| Maternal Tobacco Use → Infant Cry Aversiveness | −0.07 |
| Maternal Cannabis Use → Infant Cry Aversiveness | −0.08 |
| Maternal Depressive Symptoms → Cry Impact on Maternal Negative Affect | 0.19 |
| Maternal Anger/Hostility → Cry Impact on Maternal Negative Affect | −0.02 |
| Maternal Tobacco Use → Cry Impact on Maternal Negative Affect | −0.05 |
| Maternal Cannabis Use → Cry Impact on Maternal Negative Affect | −0.03 |
| Indirect Path | Lower 5% | Estimate | Upper 5% |
|---|---|---|---|
| Maternal Depressive Symptoms → Infant Cry Aversiveness → Maternal Tobacco Craving | −0.004 | 0.047 | 0.125 |
| Maternal Depressive Symptoms → Cry Impact on Maternal Negative Affect → Maternal Tobacco Craving | −0.044 | 0.001 | 0.034 |
| Maternal Depressive Symptoms → Infant Cry Aversiveness → Maternal Cannabis Craving | −0.001 | 0.091 | 0.190 |
| Maternal Depressive Symptoms → Cry Impact on Maternal Negative Affect → Maternal Cannabis Craving | −0.129 | −0.042 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuetze, P.; Kelm, M.R.; Bell, O.; Eiden, R.D. Perceptions of Infant Cry Sounds Among Tobacco and Cannabis Using Mothers and Their Association with Tobacco and Cannabis Cravings. Children 2025, 12, 1006. https://doi.org/10.3390/children12081006
Schuetze P, Kelm MR, Bell O, Eiden RD. Perceptions of Infant Cry Sounds Among Tobacco and Cannabis Using Mothers and Their Association with Tobacco and Cannabis Cravings. Children. 2025; 12(8):1006. https://doi.org/10.3390/children12081006
Chicago/Turabian StyleSchuetze, Pamela, Madison R. Kelm, Olivia Bell, and Rina D. Eiden. 2025. "Perceptions of Infant Cry Sounds Among Tobacco and Cannabis Using Mothers and Their Association with Tobacco and Cannabis Cravings" Children 12, no. 8: 1006. https://doi.org/10.3390/children12081006
APA StyleSchuetze, P., Kelm, M. R., Bell, O., & Eiden, R. D. (2025). Perceptions of Infant Cry Sounds Among Tobacco and Cannabis Using Mothers and Their Association with Tobacco and Cannabis Cravings. Children, 12(8), 1006. https://doi.org/10.3390/children12081006






