Clinical and Phenotypic Characteristics of Early-Onset Inflammatory Bowel Disease: A Five-Year Observational Study
Abstract
1. Introduction
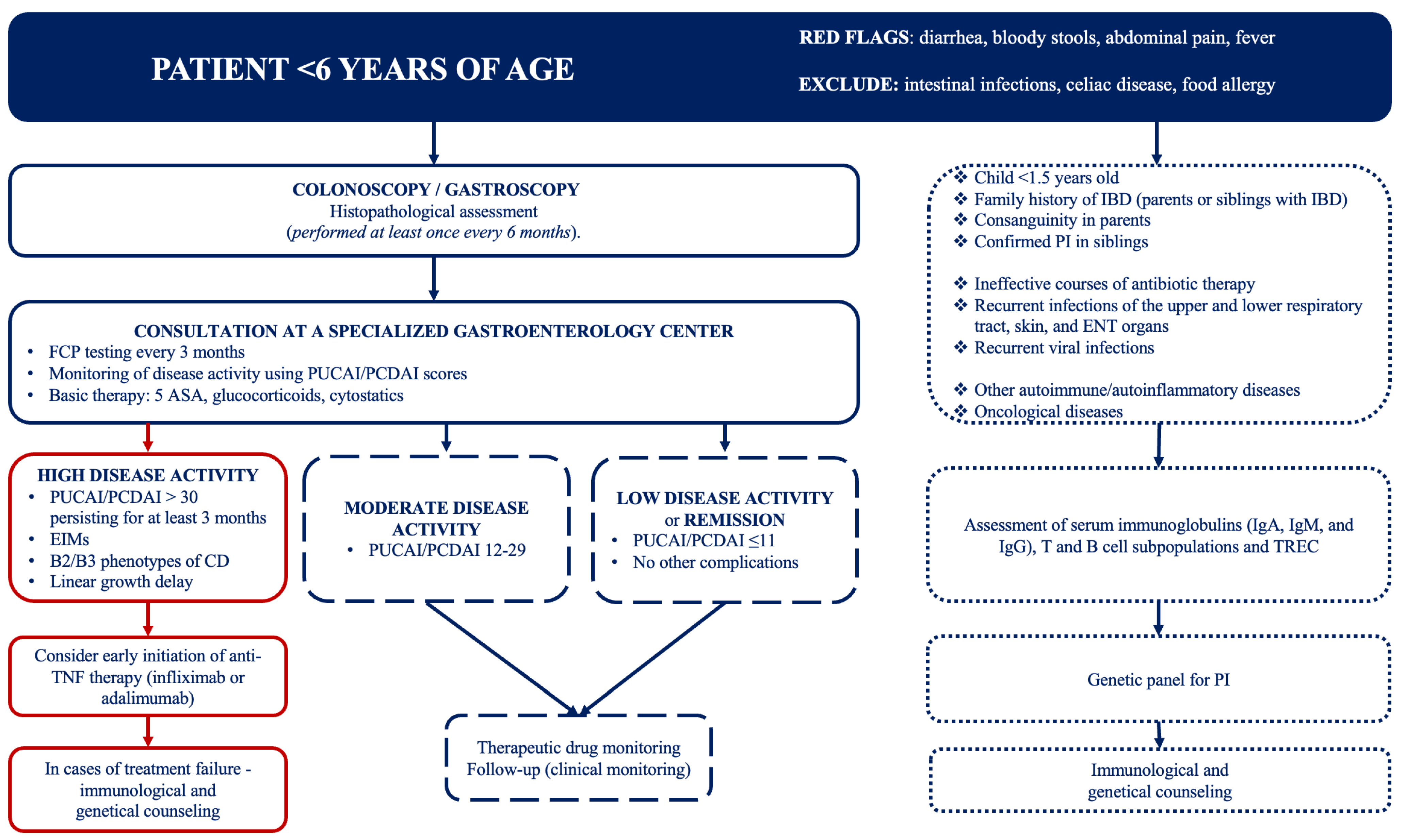
2. Materials and Methods
3. Results
3.1. Characteristics of Patients
3.2. Heredity
3.3. Body Weight and Height
3.4. Clinical and Laboratory Data
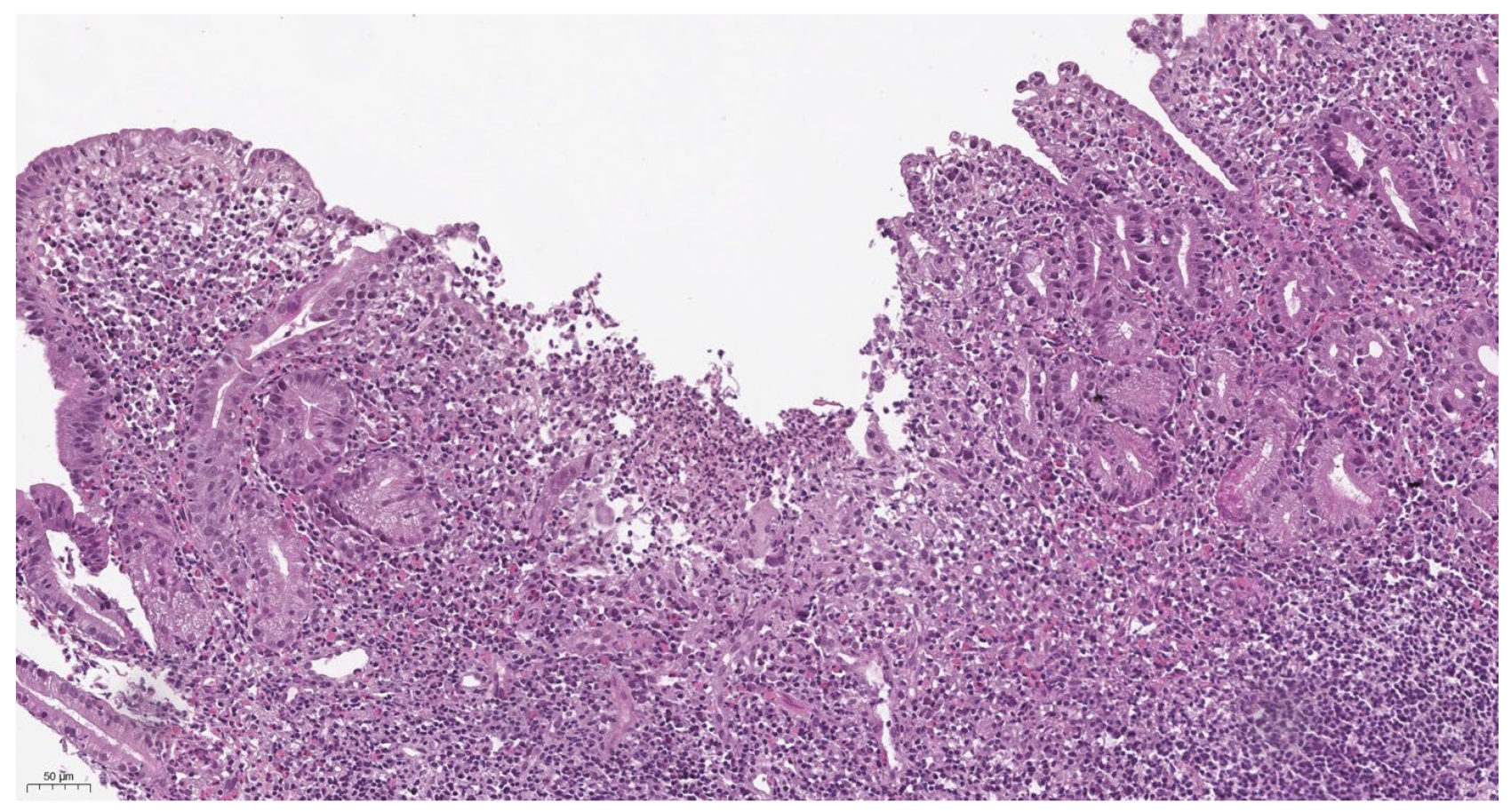
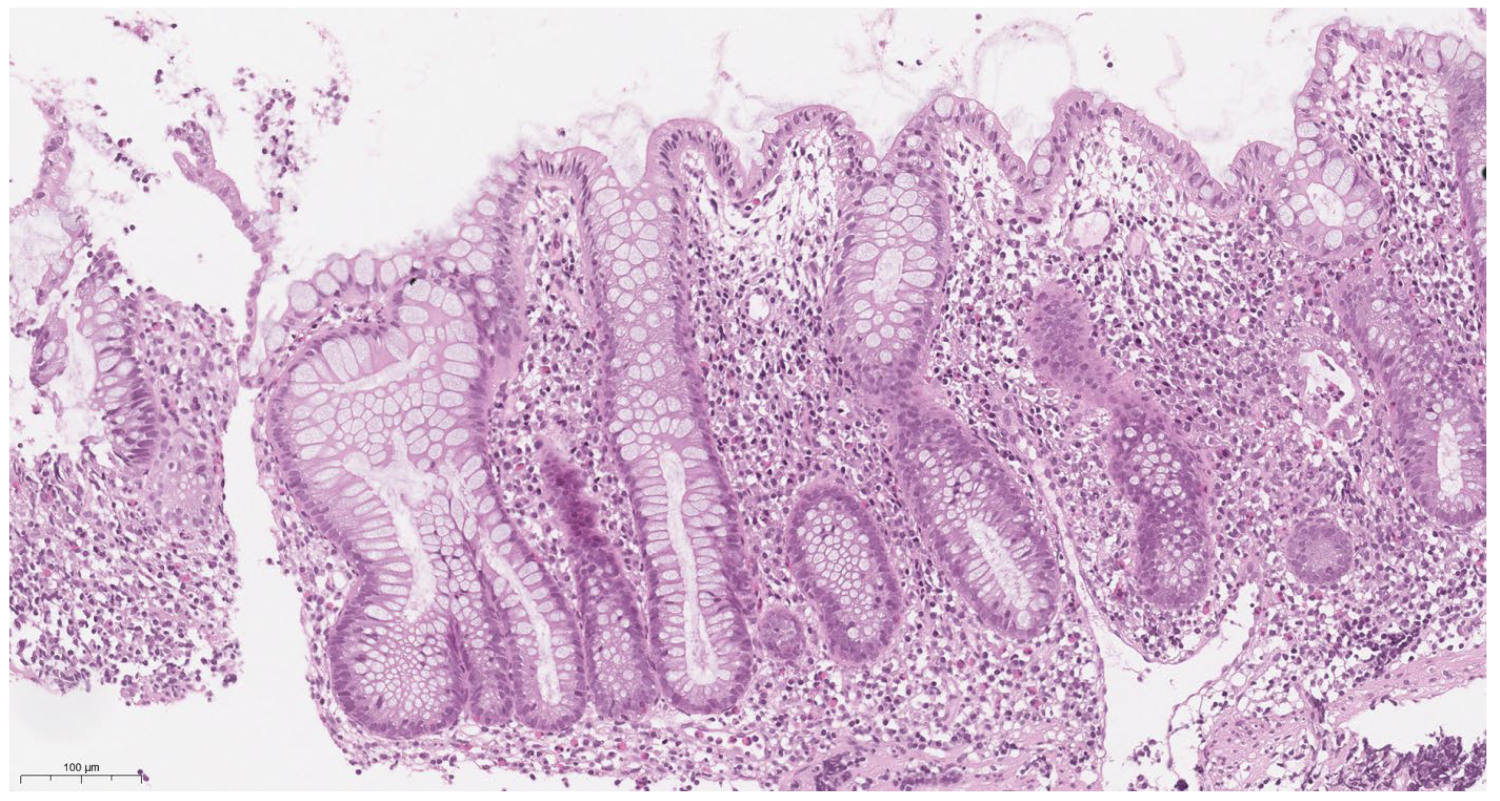
3.5. Morphological Characteristics
3.6. Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhaliwal, J.; Walters, T.D. Phenotypic Variation in Paediatric Inflammatory Bowel Disease by Age: A Multicentre Prospective Inception Cohort Study of the Canadian Children IBD Network. J. Crohn’s Colitis 2020, 14, 445–454. [Google Scholar] [CrossRef]
- Lu, C.L. Clinical presentations of inflammatory bowel disease: East meets West. J. Chin. Med. Assoc. 2017, 80, 51–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopez, R.N.; Appleton, L.; Gearry, R.B.; Day, A.S. Rising Incidence of Paediatric Inflammatory Bowel Disease in Canterbury, New Zealand, 1996–2015. J. Pediatr. Gastroenterol. Nutr. 2018, 66, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, J.R.; Baldassano, R.N. The role of monogenic disease in children with very early onset inflammatory bowel disease. Curr. Opin. Pediatr. 2017, 29, 566–571. [Google Scholar] [CrossRef]
- Parente, P.; Pastore, M.; Grillo, F.; Fassan, M.; Francalanci, P.; Dirodi, A.; Rossi, C.; Arpa, G.; De Angelis, P.; Gullo, I.; et al. Very Early Onset-IBD: Evidence for the need of a multidisciplinary approach. Pathologica 2022, 114, 3–11. [Google Scholar] [CrossRef]
- Bequet, E.; Sarter, H.; Fumery, M.; Vasseur, F.; Armengol-Debeir, L.; Pariente, B.; Ley, D.; Spyckerelle, C.; Coevoet, H.; Laberenne, J.E.; et al. Incidence and Phenotype at Diagnosis of Very-Early-Onset Compared with Later-Onset Paediatric Inflammatory Bowel Disease: A Population-Based Study [1988–2011]. J. Crohn’s Colitis 2017, 11, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Fortinsky, K.J.; Gozdyra, P.; Heuvel, M.V.D.; Van Limbergen, J.; Griffiths, A.M. Epidemiology of pediatric inflammatory bowel disease: A systematic review of international trends. Inflamm. Bowel Dis. 2011, 17, 423–439. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Schwerd, T.; Koletzko, S.; Shah, N.; Kammermeier, J.; Elkadri, A.; Ouahed, J.; Wilson, D.C.; Travis, S.P.; Turner, D.; et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014, 147, 990–1007.e3. [Google Scholar] [CrossRef]
- Tegtmeyer, D.; Seidl, M.; Gerner, P.; Baumann, U.; Klemann, C. Inflammatory bowel disease caused by primary immunodeficiencies-Clinical presentations, review of literature, and proposal of a rational diagnostic algorithm. Pediatr. Allergy Immunol. 2017, 28, 412–429. [Google Scholar] [CrossRef]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef]
- Lang-Schwarz, C.; Angeloni, M.; Agaimy, A.; Atreya, R.; Becker, C.; Dregelies, T.; Danese, S.; Fléjou, J.F.; Gaßler, N.; Grabsch, H.I.; et al. Validation of the ‘Inflammatory Bowel Disease-Distribution, Chronicity, Activity [IBD-DCA] Score’ for Ulcerative Colitis and Crohn’s Disease. J. Crohn’s Colitis 2021, 15, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, H.H.; Charbit-Henrion, F.; Kotlarz, D.; Shouval, D.S.; Schwerd, T.; Strisciuglio, C.; de Ridder, L.; van Limbergen, J.; Macchi, M.; Snapper, S.B.; et al. Clinical Genomics for the Diagnosis of Monogenic Forms of Inflammatory Bowel Disease: A Position Paper from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 456–473. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, C.R.; Donat, E.; Benninga, M.A.; Broekaert, I.J.; Gottrand, F.; Kolho, K.L.; Lionetti, P.; Miele, E.; Orel, R.; Papadopoulou, A.; et al. The Use of Fecal Calprotectin Testing in Paediatric Disorders: A Position Paper of the European Society for Paediatric Gastroenterology and Nutrition Gastroenterology Committee. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 617–640. [Google Scholar] [CrossRef] [PubMed]
- Holtman, G.A.; Lisman-van Leeuwen, Y.; Reitsma, J.B.; Berger, M.Y. Noninvasive Tests for Inflammatory Bowel Disease: A Meta-analysis. Pediatrics 2016, 137, e20152126. [Google Scholar] [CrossRef]
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Colitis 2020, 7, jjaa161. [Google Scholar] [CrossRef]
- Aloi, M.; Lionetti, P.; Barabino, A.; Guariso, G.; Costa, S.; Fontana, M.; Romano, C.; Lombardi, G.; Miele, E.; Alvisi, P.; et al. SIGENP IBD Group. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Hemker, M.; Hutfless, S.; Al Kazzi, E.S.; Lerer, T.; Mack, D.; LeLeiko, N.; Griffiths, A.; Cabrera, J.; Otley, A.; Rick, J.; et al. Clinical Presentation and Five-Year Therapeutic Management of Very Early-Onset Inflammatory Bowel Disease in a Large North American Cohort. J. Pediatr. 2015, 167, 527–532.e1. [Google Scholar] [CrossRef]
- Gupta, N.; Bostrom, A.G.; Kirschner, B.S.; Cohen, S.A.; Abramson, O.; Ferry, G.D.; Gold, B.D.; Winter, H.S.; Baldassano, R.N.; Smith, T.; et al. Presentation and disease course in early- compared to later-onset pediatric Crohn’s disease. Am. J. Gastroenterol. 2008, 103, 2092–2098. [Google Scholar] [CrossRef]
- Cioffi, M.; Rosa, A.D.; Serao, R.; Picone, I.; Vietri, M.T. Laboratory markers in ulcerative colitis: Current insights and future advances. World J. Gastrointest. Pathophysiol. 2015, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Kugathasan, S.; Annese, V.; Biank, V.; Leshinsky-Silver, E.; Davidovich, O.; Kimmel, G.; Shamir, R.; Palmieri, O.; Karban, A.; et al. Pediatric onset Crohn’s colitis is characterized by genotype-dependent age-related susceptibility. Inflamm. Bowel Dis. 2007, 13, 1509–1515. [Google Scholar] [CrossRef]
- Conrad, M.A.; Carreon, C.K.; Dawany, N.; Russo, P.; Kelsen, J.R. Distinct Histopathological Features at Diagnosis of Very Early Onset Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Mamula, P.; Telega, G.W.; Markowitz, J.E.; Brown, K.A.; Russo, P.A.; Piccoli, D.A.; Baldassano, R.N. Inflammatory bowel disease in children 5 years of age and younger. Am. J. Gastroenterol. 2002, 97, 2005–2010. [Google Scholar] [CrossRef]
- Nameirakpam, J.; Rikhi, R.; Rawat, S.S.; Sharma, J.; Suri, D. Genetics on early onset inflammatory bowel disease: An update. Genes Dis. 2019, 7, 93–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudbaek, J.J.; Borbye-Lorenzen, N.; Poulsen, G.J.; Koziol, A.; Skogstrand, K.; Jess, T. Inflammatory Markers at Birth and Risk of Early-Onset Inflammatory Bowel Disease. Gastroenterology 2024, 167, 1225–1227.e4. [Google Scholar] [CrossRef]
- Gasparetto, M.; Guariso, G.; Pozza, L.V.; Ross, A.; Heuschkel, R.; Zilbauer, M. Clinical course and outcomes of diagnosing Inflammatory Bowel Disease in children 10 years and under: Retrospective cohort study from two tertiary centres in the United Kingdom and in Italy. BMC Gastroenterol. 2016, 16, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breton, J.; Kastl, A.; Conrad, M.A.; Baldassano, R.N. Positioning Biologic Therapies in the Management of Pediatric Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2020, 16, 400–414. [Google Scholar] [PubMed] [PubMed Central]
- Kelsen, J.R.; Sullivan, K.E.; Rabizadeh, S.; Singh, N.; Snapper, S.; Elkadri, A.; Grossman, A.B. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper on the Evaluation and Management for Patients With Very Early-onset Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 389–403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cucinotta, U.; Arrigo, S.; Dipasquale, V.; Gramaglia, S.M.C.; Laganà, F.; Romano, C.; Gandullia, P. Clinical Course of Very Early-Onset Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Bramuzzo, M.; Giudici, F.; Arrigo, S.; Lionetti, P.; Zuin, G.; Romano, C.; Graziano, F.; Faraci, S.; Alvisi, P.; Signa, S.; et al. Efficacy and Tolerance of Thalidomide in Patients with Very Early Onset Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2024, 30, 20–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Category | n | UC | CD | OR | 95% Cl | p-Value |
|---|---|---|---|---|---|---|
| Number of patients with IBD | 37 | 20 | 17 | n/a | n/a | n/a |
| Gender | ||||||
| Male n (%) | 22 | 11 (55) | 11 (64.7) | n/a | n/a | 0.55 |
| Family history for IBD | 7 | 4 (20) | 3 (17.6) | n/a | n/a | 0.86 |
| Age at the time of diagnosis, Me (IQR), years | 37 | 5 (3–6) | 5 (4–6) | n/a | n/a | 0.81 |
| The age of the first symptoms (mean + SD), months | 37 | 43.9 ± 19.1 | 45.9 ± 17.7 | n/a | n/a | 0.74 |
| Mass-growth characteristics | ||||||
| Height, SDS < −2 | 4 | n/a | 4 (23.5) | n/a | n/a | n/a |
| Body weight | ||||||
| SDS < −3 | 4 | n/a | 4 | n/a | n/a | 0.02 * |
| SDS < −2 | 7 | 6 (30) | 1 (5.9) | n/a | n/a | |
| SDS < −2–−1 | 6 | 3 (15) | 3 (17.6) | n/a | n/a | |
| BMI (mean ± SD), kg/m2 | 37 | 13.8 ± 1.38 | 13.6 ± 1.32 | n/a | n/a | 0.62 |
| Clinical characteristics, n (%) | ||||||
| Abdominal pain | 23 | 12 (60) | 11 (64.7) | 0.82 | 0.22–3.12 | 0.77 |
| Diarrhea | 30 | 18 (90) | 12 (70.6) | 3.75 | 0.62–22.6 | 0.13 |
| Rectal bleeding | 28 | 17 (85) | 11 (64.7) | 0.32 | 0.067–1.57 | 0.15 |
| Defecation at night | 9 | 9 (45) | n/a | n/a | n/a | n/a |
| Fever | 6 | 0 | 6 (35.3) | n/a | n/a | n/a |
| Anemia | 1 | 1 (5) | 0 | n/a | n/a | n/a |
| Extraintestinal manifestations | 7 | 7 (35) | 7 (41.2) | n/a | n/a | 1 |
| Primary chronic onset | 28 | 16 (80) | 12 (70.6) | n/a | n/a | 0.44 |
| Subacute onset | 9 | 4 (20) | 5 (29.4) | n/a | n/a | |
| Laboratory | ||||||
| Fecal calprotectin (mean ± SD), mcg/g | 22 | 721.49 ± 365.33 | 1070.25 ± 559.09 | n/a | n/a | 0.11 |
| CRP Me (IQR) | 37 | 11.35 (0.1–26.23) | 5 (1.4–29) | n/a | n/a | 0.8 |
| Albumin (mean ± SD), g/L | 37 | 42.5 ± 4.18 | 43.5 ± 6.64 | n/a | n/a | 0.22 |
| Endoscopic characteristics, n (%) | ||||||
| CD, localization n (%) | 17 | n/a | n/a | n/a | n/a | n/a |
| L1 | 1 | n/a | 1 (5.9) | n/a | n/a | n/a |
| L2 | 11 | n/a | 11 (64.7) | n/a | n/a | n/a |
| L3 | 5 | n/a | 5 (29.4) | n/a | n/a | n/a |
| CD, phenotypes n (%) | ||||||
| E1 | 14 | n/a | 14 (82.4) | n/a | n/a | n/a |
| E2 | 2 | n/a | 2 (11.8) | n/a | n/a | n/a |
| E3 | 1 | n/a | 1 (5.8) | n/a | n/a | n/a |
| UC, localization n (%) | ||||||
| E2 | 4 | 4 (20) | n/a | n/a | n/a | n/a |
| E3 | 4 | 4 (20) | n/a | n/a | n/a | n/a |
| E4 | 12 | 12 (60) | n/a | n/a | n/a | n/a |
| Endoscopic findings | ||||||
| Ulcers | 18 | 6 (30) | 12 (70.6) | 5.60 | 1.36–23.1 | 0.02 * |
| Aphthae | 12 | 5 (25) | 7 (41.2) | 2.10 | 0.52–8.51 | 0.29 |
| Erosion | 22 | 15 (75) | 7 (41.2) | 4.29 | 1.06–17.4 | 0.04 * |
| Limited continuous inflammation of the intestinal mucosa | 8 | 6 (30) | 2 (11.8) | 0.31 | 0.054–1.81 | 0.18 |
| Microabscess | 5 | 4 (23.5) | 1 (5) | 0.25 | 0.025–2.49 | 0.21 |
| PCDAI (Me (IQR) | 17 | n/a | 25 (20–30) | n/a | n/a | n/a |
| PUCAI (Me (IQR) | 20 | 37.5 (20–46.3) | n/a | n/a | n/a | n/a |
| Duration of diagnosis IBD-U, Me (IQR), week | 16 | 24 (20–48) | 40 (30–45.5) | n/a | n/a | 0.56 |
| UC | CD | p-Value | |||||
|---|---|---|---|---|---|---|---|
| EIMs, n | Before the Appearance of Intestinal Manifestations, n | After the Appearance of Intestinal Manifestations, n | EIMs, n | Before the Appearance of Intestinal Manifestations, n | After the Appearance of Intestinal Manifestations, n | ||
| P/a | 2 | 1 | 2 | 5 | 1 | 4 | <0.001 * |
| Erythema nodosum | 2 | 0 | 2 | 1 | 0 | 1 | n/a |
| Uveit | 0 | 0 | 0 | 1 | 0 | 1 | n/a |
| A/s | 1 | 1 | 0 | 4 | 1 | 3 | 0.32 |
| PSC | 2 | 0 | 2 | 1 | 0 | 1 | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samolygo, I.S.; Manina, M.A.; Yablokova, E.A.; Stribul, P.A.; Novikov, A.V.; Antishin, A.S.; Pestova, A.S.; Tertychnyy, A.S.; Munblit, D.; Erdes, S.I. Clinical and Phenotypic Characteristics of Early-Onset Inflammatory Bowel Disease: A Five-Year Observational Study. Children 2025, 12, 952. https://doi.org/10.3390/children12070952
Samolygo IS, Manina MA, Yablokova EA, Stribul PA, Novikov AV, Antishin AS, Pestova AS, Tertychnyy AS, Munblit D, Erdes SI. Clinical and Phenotypic Characteristics of Early-Onset Inflammatory Bowel Disease: A Five-Year Observational Study. Children. 2025; 12(7):952. https://doi.org/10.3390/children12070952
Chicago/Turabian StyleSamolygo, Ivan S., Marina A. Manina, Ekaterina A. Yablokova, Pavel A. Stribul, Alexander V. Novikov, Anton S. Antishin, Albina S. Pestova, Alexander S. Tertychnyy, Daniel Munblit, and Svetlana I. Erdes. 2025. "Clinical and Phenotypic Characteristics of Early-Onset Inflammatory Bowel Disease: A Five-Year Observational Study" Children 12, no. 7: 952. https://doi.org/10.3390/children12070952
APA StyleSamolygo, I. S., Manina, M. A., Yablokova, E. A., Stribul, P. A., Novikov, A. V., Antishin, A. S., Pestova, A. S., Tertychnyy, A. S., Munblit, D., & Erdes, S. I. (2025). Clinical and Phenotypic Characteristics of Early-Onset Inflammatory Bowel Disease: A Five-Year Observational Study. Children, 12(7), 952. https://doi.org/10.3390/children12070952










