The Pathway Is Clear but the Road Remains Unpaved: A Scoping Review of Implementation of Tools for Early Detection of Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
- What is the reported frequency of use of GMA, HINE, and MRI in the early detection of CP across countries and healthcare systems?
- What contextual enablers and barriers influence the implementation of these tools in clinical practice?
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Participants: Studies involving healthcare professionals, clinical teams, or healthcare systems engaged in early detection of CP.
- Concept: Studies investigating the use, implementation, or integration of recommended early detection tools for CP—GMA, HINE, neuroimaging, and other tools cited in the international guidelines. This included research describing awareness, frequency of use, barriers and facilitators to implementation, or contextual factors influencing clinical practice.
- Context: Studies conducted in any healthcare or service setting, across all geographic and economic contexts (high-, middle-, and low-income countries), including clinical, community-based, and public or private health settings.
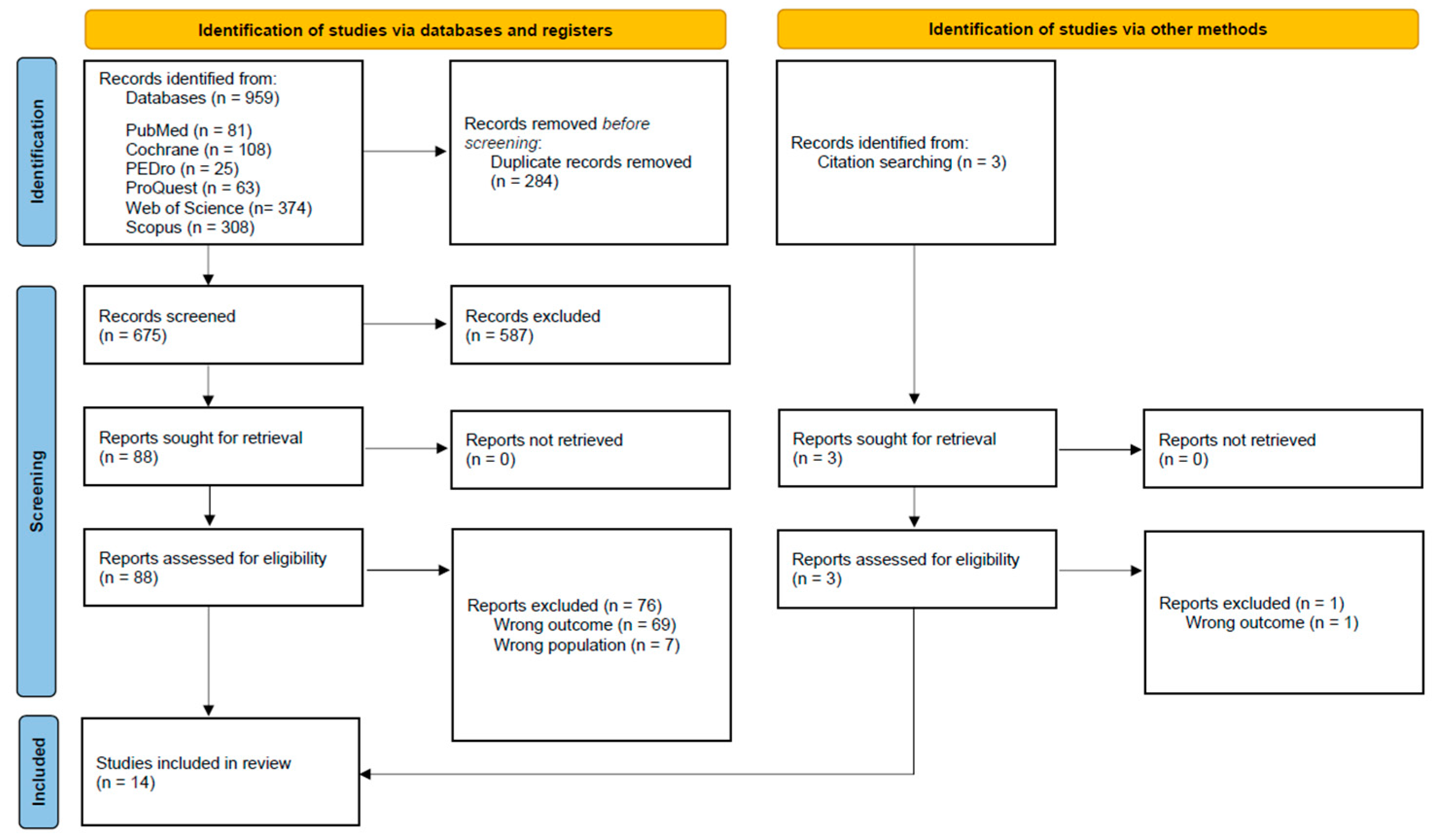
2.3. Screening Process
2.4. Data Extraction, Synthesis, and Appraisal
3. Results
3.1. Geographic and Professional Overview
3.2. Evidence (Research, Clinical Experience, and Families) and Awareness of Early CP Detection and Diagnosis
3.3. Referral Pathways for CP Diagnosis
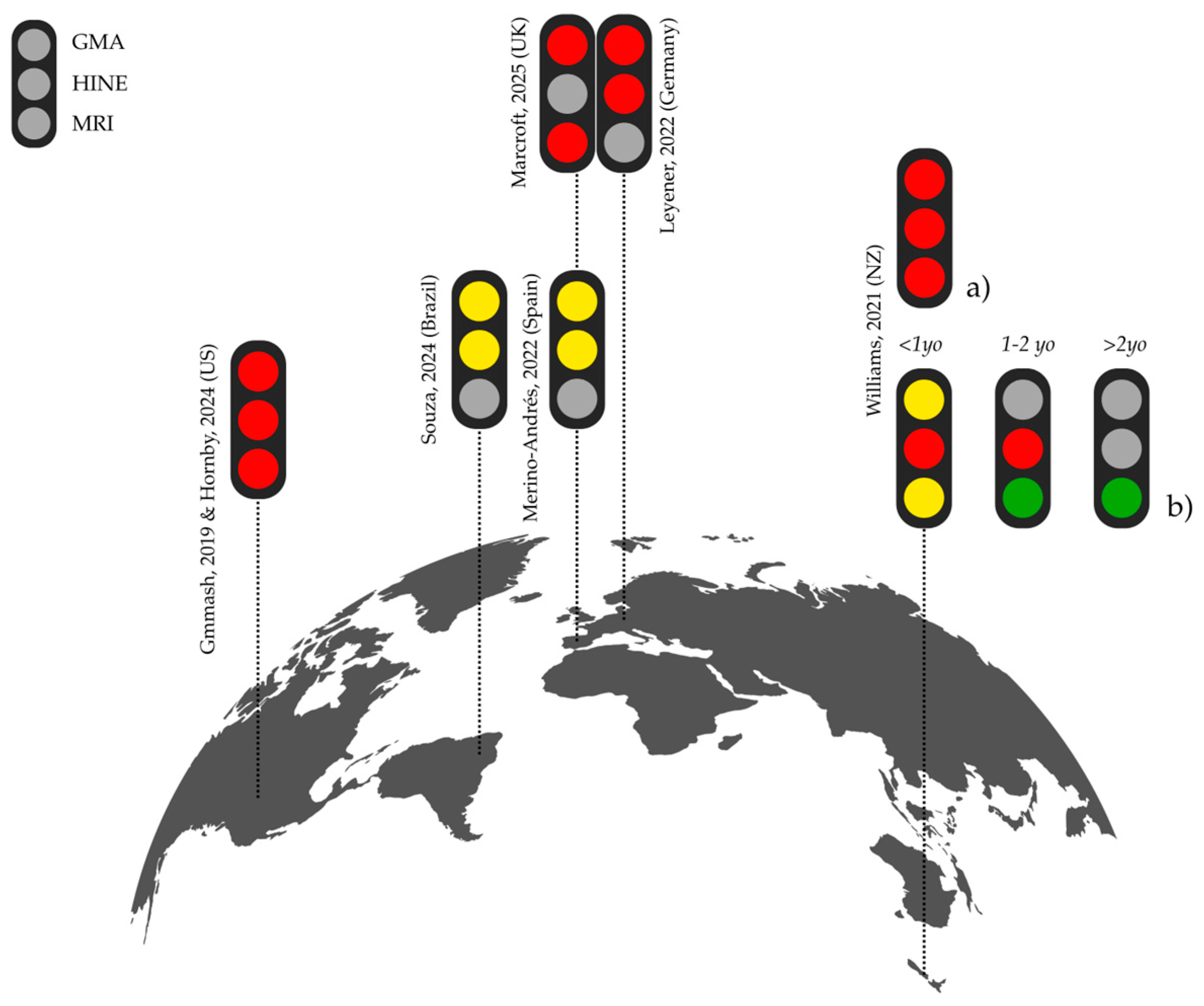
3.4. Use of GMA, HINE, and MRI
3.4.1. GMA
3.4.2. HINE
3.4.3. MRI
3.5. Use of Other Motor Assessments Tools with Strong or Conditional Recommendation
3.6. Use of Alternative Assessment Tools
3.7. Enablers and Barriers
- System-level factors were widely reported across studies, with most barriers (n = 190) related to staffing constraints, time allocation, financial resources, and/or lack of referral pathways.Staffing and workload issues were a central concern. Providers described high caseloads and limited personnel, making it difficult to integrate new assessments into routine care, even when trained. A clinician in New Zealand summarized this as “constantly in crisis mode and little time to prep for sessions or implement new tools” [13]. This was echoed in Spain [25], where “inflexible schedules” led providers to rely on clinical judgement over standardized tools.Funding limitations were also frequently cited. Spanish physical therapists reported “economic difficulties to access training”, while similar concerns arose in contexts like New Zealand [13] and Maryland or Delaware [28], where a provider mentioned costs of training, materials, and time required for courses [13,28].Infrastructure and operational limitations further constrained implementation. In Auckland, fewer than half considered that resources—e.g., information technology (10%), financial resources (20%), and human resources (15%)—were adequate to support new practices. One participant observed: “I do not see currently… that we have any of the resources (human and non-human) that are required for the implementation of this pathway […]” [34].Delays in referral and the lack of standardized pathways compounded these issues. A provider in New Zealand highlighted the need for “[…] clear guidelines on when and how to screen and refer” [13], concerns echoed in Spain and the US, where bureaucratic hurdles and frequent protocol changes slowed adoption [25,28]. In contrast, integrated follow-up programs or established referral frameworks were viewed as critical enablers, facilitating joint assessments and smoother coordination: “This makes referral, joint assessment and collaboration much easier for families and for us” [13].Additional system-level enablers (n = 49) included organizational support and protected time for training, as reported in Spain, Maryland, and Delaware [25,28]. In this context, a provider in New Zealand highlighted the need for broader access to education opportunities: “Whilst I have read about HINE and GMA, haven’t attended training on GMA—if this training could be made more available and accessible to therapists in NZ that would be amazing”.
- Social-level enablers (n = 89) and barriers (n = 73), though less frequently reported than system-level barriers, played a critical role. Leadership and administrative support, peer collaboration, and organizational culture shaped implementation outcomes.Leadership consistently emerged as a key driver of success, with 65% of participants in New Zealand feeling they had the power and authority to influence implementation. Support from supervisors and administrators keen to follow evidence-based practice was identified as a facilitator [13,28]. However, several participants emphasized the challenge of limited leadership involvement: “Staff not wanting to change the status quo from how it’s always been done” [13]. Findings from Mulqueeney et al. (2024) [34] further illustrated this tension: only half of participants felt clear on their implementation roles, and less than a third had been involved in planning. Focus groups described leadership both as a barrier and an enabler. One participant observed it worked best when “a really passionate person at the top [was] talking about brain care all the time” [34]. Equity also emerged as a leadership concern: “If we don’t make an effort to make this whole thing equitable then it won’t be” [34].Multidisciplinary collaboration and peer support were frequently reported as critical enablers operating at different levels. Providers described informal peer exchanges and shared experiences [13,25], alongside the need for structured collective action and operational alignment between teams (“Would need the team to work together to change practice—would need an algorithm and help with scheduling” [28]). Peer review and supervision reinforced good practice [25] In New Zealand. Survey findings supported this collaborative culture: most team members were open to change—with only 19% preferring to maintain current practices—and between 52% and 70% agreed that their organization valued open communication and dialogue, relationships, and teamwork [34].In contrast, lack of coordination across teams and limited indirect service time were identified as barriers [25]. As one Spanish provider summarized: “A change of mentality is needed”—toward fostering a culture of shared leadership, interprofessional collaboration, and peer-driven support.
- Health professional knowledge and perceptions consistently emerged as key factors of implementation—more like enablers (n = 80) than barriers (n = 57). In New Zealand, 60% of participants believed they had the skills and knowledge to implement the recommendations [34]. Access to professional development and knowledge sharing—e.g., through conferences, external courses attendance, or “use of different apps, social networks and being connect to the field of pediatrics” [25]—facilitated implementation. “Guidelines’ development” [25] and clinical pathways were also described as both relevant facilitators and barriers—and not just their absence in the workplace, but, for example, “the many steps one needs to go through to be OKed to use a specific assessment tool” [28].Importantly, both “clinical experience” and “family experience” were commonly cited as forms of evidence guiding decision-making, although with mixed consensus [34]. While most New Zealand providers valued family experience, there were 61 quotes identifying family experience as a barrier and 35 as an enabler, highlighting tensions around low knowledge and/or engagement (“Sharing information is the keystone to having parents integrated in care” [34] or “Lack of families’ involvement” [25]), myths, taboos, and misconceptions about CP that could complicate early detection discussions. Some expressed concern about causing undue stress in families who ultimately did not receive a CP diagnosis, describing this as potentially “paternalistic” [34].Professional perceptions of early diagnosis also intersected with broader cultural attitudes. There was no current consensus amongst doctors around the need/importance of early diagnosis of CP, feeling uncomfortable with giving it early and the avoidance of tough conversations (“Severe is fine, I’m very happy to make the call. But under six months, you know, unless they’re severe, then, I start to talk about, well, risk of or, you know, maybe or needing more information. Very uncomfortable around making a definite diagnosis at that stage”), or reliance on tools (“While the sensitivity and the specificity of the MRI and the HINE are high, their positive predictive value is not. So… there’s reasonable chance that if you say someone has CP they don’t actually”) [34]. Others, however, viewed early and transparent communication—including the use of family experience and clinical intuition—as key to building trust, tailoring care, and initiating appropriate intervention.
- Clinical considerations and individual drive, though less frequently reported (n = 19 enablers; n = 17 barriers), shaped decisions around tools use in meaningful ways. Providers often described self-driven motivation as a critical enabler for implementation efforts [13].In contrast, increasing case complexity was reported to reduce the capacity for closely monitoring infants at risk [13]. Others highlighted variability in clinical routines (“I have different patient groups” [25]). Finally, some providers expressed concern that complex or time-consuming assessment could disrupt the flow of clinical care without delivering tangible benefits to patients [29].
4. Discussion
- Building Capacity… And Then What?
- From Vision to Routine: Shaping Systems that Make Early Detection the Norm
- Aligning Pathways: A Shared Aim for Timely Diagnosis
- “If we don’t make an effort to make this whole thing equitable then it won’t be”: Implementation under the Equity Lens
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIMS | Alberta Infant Motor Scale |
| ASQ | Ages and Stages Questionnaires |
| CFIR | Consolidated Framework for Implementation Research |
| CP | Cerebral Palsy |
| DAYC | Developmental Assessment of Young Children |
| Ei3 | Early Identification and Intervention for Infants Network |
| GMA | General Movement Assessment |
| HINE | Hammersmith Infant Neurological Examination |
| JBI | Joanna Briggs Institute |
| MAI | Motor Assessment of Infants |
| MRI | Magnetic Resonance Imaging |
| NGO | Non-Governmental Organizations |
| NICU | Neonatal Intensive Care Unit |
| NSMDA | Neuro-Sensory Motor Developmental Assessment |
| PCC | Participant–Concept–Context |
| PDMS-2 | Peabody Developmental Motor Scales |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
| TIMP | Test of Infant Motor Performance |
| US | United States |
Appendix A
| First Author, Year | Country | Method | n | Setting (n, %) | Providers (n, %) | Experience |
|---|---|---|---|---|---|---|
| Maitre, 2016 [29] | US | Survey and electronic medical record audit | 12 (survey) and 50 electronic medical records | Nationwide Children’s Hospital (Neonatal follow-up program) | Advance practice nurses, general pediatricians, developmental/behavioral pediatricians, pediatric neurologist, neonatologists, and specialty fellows in neonatology and developmental medicine | Not described |
| Byrne, 2017 [30] | US | Survey | 11–26 | Nationwide Children’s Hospital (Neonatal follow-up program) | Physical therapist, occupational therapist, advanced nurse practitioners, general pediatricians, developmental pediatricians, pediatric neurologists, nurses, dietitians, social workers, and speech–language therapist | Not described |
| Gmmash, 2019 [23] | US | Survey | 269 | Early intervention | Physical therapists (n = 180) and occupational therapists (n = 89) | Not described |
| Williams, 2021 [13] | New Zealand | Survey | 159 | Hospital/health services (n = 116, 77%), private practice (n = 7, 5%), hospital/private practice (n = 9, 6%), university (n = 5, 3%), non-governmental organization (n = 4, 3%) | Physical therapists (n = 66), occupational therapists (n = 23), general pediatricians (n = 22), neurodevelopmental therapists (n = 11), neonatologists (n = 10), orthopedic surgeons (n = 7), developmental pediatricians (n = 5), trainee doctors (n = 4), speech–language therapists (n = 4), general practitioners (n = 2), pediatric rehabilitation consultant (n = 1), nurse specialist (n = 1), and early childhood nurse (n = 1) | 1–5 years (n = 22, 15%), 6–14 years (n = 52, 35%), 15+ years (n = 75, 50%) |
| Davidson, 2022 [33] | Australia | Pre/post-implementation data | Not reported | Statewide tertiary pediatric early intervention service (Western Australia) | Not described | Not described |
| Leyener, 2022 [24] | Germany | Survey | Not reported | NICUs | Medical doctors, physical therapists, nurses, and others | Not described |
| Merino-Andrés, 2022 [25] | Spain | Survey | 109 | Early intervention (infants < 1 year): clinical centers (62.9%) | Physical therapists (n = 109, 100%) | <10 years (58.6%) |
| Butera, 2024 [32] | US | Implementation (awareness and capacity-building), pre/post-training survey | 70 (symposium), 211 (HINE training), 46 (GMA training), Implementation conference | Early Identification and Intervention for Infants Network, Los Angeles (participants of HINE training): children’s services medical therapy program (51.1%), children’s services general program (1.4%), NICU (7.2%), inpatient pediatrics (10.1%), outpatient therapy practice (16.5%), outpatient medical practice (2.9%), early intervention (6.5%) | Participants of HINE training: physical therapists (n = 114, 64.4%), occupational therapists (n = 44, 24.9%), developmental pediatrician (n = 4, 2.3%), pediatrics (n = 4, 2.3%), nurse practitioner (n = 4, 2.3%), speech–language therapists (n = 2, 1.1%), and neonatology (n = 2, 1.1%) | Not described |
| Hidalgo-Robles, 2024 [35] | Spain | Pre/post-training survey | 11 | Early intervention | Physical therapists (n = 3, 27.3%), speech–language therapists (n = 3, 27.3%), psychologists (n = 3, 27.3%), and occupational therapists (n = 2, 18.2%) | <5 years (54%), 5–15 years (36%), 16–20 years (9%) |
| Hornby, 2024 [28] | US | Survey | 72 | Maryland and Delaware Early Intervention: Health or education settings for children < 3 years, with risk factors for CP | Physical therapists (n = 36, 48.6%), occupational therapists (n = 18, 24.3%), developmental pediatrician (n = 1, 1.4%), general pediatrician (n = 3, 4.1%), orthopedic surgeon (n = 1, 1.4%), pediatric neurologist (n = 3, 4.1%), physiatrist (n = 1, 1.4%), medically complex pediatrician (n = 2, 2.7%), nurse practitioner/physician assistant (n = 1, 1.4%), early childhood nurse (n = 2, 2.7%), researcher (n = 1, 1.4%), early intervention administrator (n = 1, 1.4%), social worker (n = 1, 1.4%), special educator (n = 2, 2.7%), and speech–language therapist (n = 1, 1.4%) | 6–14 years (n = 21, 29.6%), 15+ years (n = 50, 70.4%) |
| Mulqueeney, 2024 [34] | New Zealand | Survey, focus groups | 27 (survey), 20 (focus groups/interviews) | Te Toka Tumai Auckland NICU, Starship Children’s Hospital community, developmental pediatrics services, Auckland | Nurses (n = 13, 48%), neonatologists (n = 7, 26%), therapists (occupational therapist, physical therapist, speech therapist) (n = 4, 15%), developmental pediatricians (n = 2, 7%), and neonatal registrar (n = 1, <1%) | 1–3 years (n = 3, 11%), 4–10 (n = 3, 11%), +10 years (n = 21, 78%) |
| Souza, 2024 [26] | Brazil | Survey | 205 | Early intervention (< 3 years): public health services (77, 37.6%), private healthcare providers (71, 34.6%), universities and other educational institutions (28, 13.7%), self-employed (21, 10.2%), NGO (5, 2.4%), other services (3, 1.5%) | Physical therapists (n = 168, 82%) and occupational therapists (n = 37, 18%) | 0–5 years (n = 21, 10.2%), 5–10 years (n = 46, 22.4%), 10–15 years (n = 47, 22.9%), 15–20 years (n = 34, 16.6%), >20 years (n = 53, 25.9%) |
| Sutter, 2024 [31] | US | Pre/postguidelines-publication retrospective medical record review | Not reported | University of Wisconsin Waisman Center Newborn Follow-Up Clinic, Madison (Newborn Follow-Up clinic < 3 years) | Developmental pediatricians, pediatric neurologists, physical medicine rehabilitation consultants, physical therapists, occupational therapists, and speech–language therapists | Not described |
| Marcroft, 2025 [27] | UK (England, Scotland, and Wales) | Survey (preprint) | 154 | Neonatal units (NICUs, Local Neonatal Units, Special Care Baby Units) | Medical doctors (n = 124, 80.5%), physical therapists (n = 22, 14.3%), occupational therapists (n = 4, 2.6%), and nurses/other (n = 4, 2.6%) | Not described |
References
- Byrne, R.; Duncan, A.; Pickar, T.; Burkhardt, S.; Boyd, R.N.; Neel, M.L.; Maitre, N.L. Comparing Parent and Provider Priorities in Discussions of Early Detection and Intervention for Infants with and at Risk of Cerebral Palsy. Child. Care Health Dev. 2019, 45, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.A.; Alzaher, W.; Mackey, A.; Hogan, A.; Battin, M.; Sorhage, A.; Stott, N.S. “It Should Have Been Given Sooner, and We Should Not Have to Fight for It”: A Mixed-Methods Study of the Experience of Diagnosis and Early Management of Cerebral Palsy. J. Clin. Med. 2021, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Guttmann, K.; Flibotte, J.; DeMauro, S.B. Parental Perspectives on Diagnosis and Prognosis of Neonatal Intensive Care Unit Graduates with Cerebral Palsy. J. Pediatr. 2018, 203, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Friel, K.M.; Chakrabarty, S.; Martin, J.H. Pathophysiological Mechanisms of Impaired Limb Use and Repair Strategies for Motor Systems after Unilateral Injury of the Developing Brain. Dev. Med. Child. Neurol. 2013, 55, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Greaves, S.; Hoare, B. Upper Limb Therapy for Infants and Young Children with Unilateral Cerebral Palsy: A Clinical Framework. J. Clin. Med. 2024, 13, 6873. [Google Scholar] [CrossRef] [PubMed]
- Leite, H.R.; de Sousa Junior, R.R.; Souto, D.O.; Medeiros e Silva, J.M.; de Lima, A.F.B.; de Miranda Drumond, C.; Policiano, E.B.C.; Marques, A.C.; de Carvalho Chagas, P.S.; Longo, E. F-Words Ingredients of Non-Invasive Interventions for Young Ambulant Children with Cerebral Palsy: A Scoping Review. Dev. Med. Child. Neurol. 2024, 67, 150–164. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.C.; Hidalgo-Robles, Á.; Longo, E.; Shrader, C.; Paleg, G. F-Words and Early Intervention Ingredients for Non-Ambulant Children with Cerebral Palsy: A Scoping Review. Dev. Med. Child. Neurol. 2024, 66, e43. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.-C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Prechtl, H.; Bos, A.; Ferrari, F.; Cioni, G. Prechtl’s Method on the Qualitative Assessment of General Movements in Preterm, Term and Young Infants, 1st ed.; Mac Keith Press: London, UK, 2008. [Google Scholar]
- Haataja, L.; Mercuri, E.; Regev, R.; Cowan, F.; Rutherford, M.; Dubowitz, V.; Dubowitz, L. Optimality Score for the Neurologic Examination of the Infant at 12 and 18 Months of Age. J. Pediatr. 1999, 135, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Romeo, D.M.; Chorna, O.; Novak, I.; Galea, C.; Del Secco, S.; Guzzetta, A. The Pooled Diagnostic Accuracy of Neuroimaging, General Movements, and Neurological Examination for Diagnosing Cerebral Palsy Early in High-Risk Infants: A Case Control Study. J. Clin. Med. 2019, 8, 1879. [Google Scholar] [CrossRef] [PubMed]
- Aravamuthan, B.R.; Fehlings, D.; Shetty, S.; Fahey, M.; Gilbert, L.; Tilton, A.; Kruer, M.C. Variability in Cerebral Palsy Diagnosis. Pediatrics 2021, 147, e2020010066. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.A.; Mackey, A.; Sorhage, A.; Battin, M.; Wilson, N.; Spittle, A.; Stott, N.S. Clinical Practice of Health Professionals Working in Early Detection for Infants with or at Risk of Cerebral Palsy across New Zealand. J. Paediatr. Child. Health 2021, 57, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Aravamuthan, B.R.; Fahey, M.C.; Fehlings, D.L.; Novak, I.; Kruer, M.C. The Need to Standardize the Diagnosis of Cerebral Palsy. Pediatrics 2025, 155, e2024069666. [Google Scholar] [CrossRef] [PubMed]
- Te Velde, A.; Morgan, C.; Novak, I.; Tantsis, E.; Badawi, N. Early Diagnosis and Classification of Cerebral Palsy: An Historical Perspective and Barriers to an Early Diagnosis. J. Clin. Med. 2019, 8, 1599. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Lange, K.; Klose, K.; Greiner, W.; Kraemer, A. Barriers and Strategies in Guideline Implementation—A Scoping Review. Healthcare 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Fetters, L.; Adde, L.; Badawi, N.; Bancale, A.; Boyd, R.N.; Chorna, O.; Cioni, G.; Damiano, D.L.; Darrah, J.; et al. Early Intervention for Children Aged 0 to 2 Years with or at High Risk of Cerebral Palsy: International Clinical Practice Guideline Based on Systematic Reviews. JAMA Pediatr. 2021, 175, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Maitre, N.L.; Damiano, D.; Byrne, R. Implementation of Early Detection and Intervention for Cerebral Palsy in High-Risk Infant Follow-Up Programs: U.S. and Global Considerations. Clin. Perinatol. 2023, 50, 269–279. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.; Scott, K.; Boyd, R.; Webb, A.; Taifalos, C.; Novak, I. Effectiveness of Early Diagnosis of Cerebral Palsy Guideline Implementation: A Systematic Review. Minerva Pediatr. 2024, 76, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Scoping Reviews. In JBI Manual for Evidence Synthesis; JBI: Tokyo, Japan, 2024. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Gmmash, A.S.; Effgen, S.K. Early Intervention Therapy Services for Infants with or at Risk for Cerebral Palsy. Pediatr. Phys. Ther. 2019, 31, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Leyener, U.; Kraushaar, C.; Dathe, A.K.; Felderhoff-Müser, U.; Marschik, P.B.; Zhang, D.; Hüning, B.M. Physiotherapy in German Neonatal Intensive Care Units: Indication and Clinical Application of the General Movements Assessments. Z. Geburtshilfe Neonatol. 2022, 226, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Merino-Andrés, J.; Hidalgo-Robles, Á.; Pérez-Nombela, S.; Williams, S.A.; Paleg, G.; Fernández-Rego, F.J. Tool Use for Early Detection of Cerebral Palsy: A Survey of Spanish Pediatric Physical Therapists. Pediatr. Phys. Ther. 2022, 34, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.A.; Leite, H.R.; Lucena, R.; Carvalho, A. Early Detection and Intervention for Children with High Risk of Cerebral Palsy: A Survey of Physical Therapists and Occupational Therapists in Brazil. Phys. Occup. Ther. Pediatr. 2024, 44, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Marcroft, C.; Cruickshank, H.; Johnson, S.; Exley, C.; Kolehmainen, N.; Thomson, R.; Basu, A. Neonatal Neurodevelopmental Follow-Up in the UK: A Survey of Current Practice and Future Recommendations. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Hornby, B.; Paleg, G.S.; Williams, S.A.; Hidalgo-Robles, Á.; Livingstone, R.W.; Montufar Wright, P.E.; Taylor, A.; Shrader, M.W. Identifying Opportunities for Early Detection of Cerebral Palsy. Children 2024, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Maitre, N.L.; Chorna, O.; Romeo, D.M.; Guzzetta, A. Implementation of the Hammersmith Infant Neurological Examination in a High-Risk Infant Follow-Up Program. Pediatr. Neurol. 2016, 65, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.; Noritz, G.; Maitre, N.L. Implementation of Early Diagnosis and Intervention Guidelines for Cerebral Palsy in a High-Risk Infant Follow-Up Clinic. Pediatr. Neurol. 2017, 76, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.N.; Legare, J.M.; Villegas, M.A.; Collins, K.M.; Eickhoff, J.; Gillick, B.T. Evidence-Based Infant Assessment for Cerebral Palsy: Diagnosis Timelines and Intervention Access in a Newborn Follow-up Setting. J. Child. Neurol. 2024, 39, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Butera, C.D.; Yeh, A.; Biniwale, M.; Bloch, E.; Craddock, D.; Doyle, M.; Iyer, S.N.; Kretch, K.S.; Liu, N.; Mirzaian, C.B.; et al. Development and Initial Outcomes of the Interdisciplinary ‘Early Identification and Intervention for Infants Network’ (Ei3) in Los Angeles. J. Clin. Med. 2024, 13, 7442. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.A.; Ward, R.; Elliott, C.; Harris, C.; Bear, N.; Thornton, A.; Salt, A.; Valentine, J. From Guidelines to Practice: A Retrospective Clinical Cohort Study Investigating Implementation of the Early Detection Guidelines for Cerebral Palsy in a State-Wide Early Intervention Service. BMJ Open 2022, 12, e063296. [Google Scholar] [CrossRef] [PubMed]
- Mulqueeney, A.; Battin, M.; McKillop, A.; Stott, N.S.; Allermo-Fletcher, A.; Williams, S.A. A Prospective Assessment of Readiness to Implement an Early Detection of Cerebral Palsy Pathway in a Neonatal Intensive Care Setting Using the PARIHS Framework. Implement. Sci. Commun. 2024, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Robles, Á.; Merino-Andrés, J.; Rodríguez-Fernández, Á.L.; Gutiérrez-Ortega, M.; León-Estrada, I.; Ródenas-Martínez, M. Reliability, Knowledge Translation, and Implementability of the Spanish Version of the Hammersmith Infant Neurological Examination. Healthcare 2024, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- te Velde, A.; Tantsis, E.; Novak, I.; Badawi, N.; Berry, J.; Golland, P.; Korkalainen, J.; McMurdo, R.; Shehata, R.; Morgan, C. Age of Diagnosis, Fidelity and Acceptability of an Early Diagnosis Clinic for Cerebral Palsy: A Single Site Implementation Study. Brain Sci. 2021, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; van Stralen, M.M.; West, R. The Behaviour Change Wheel: A New Method for Characterising and Designing Behaviour Change Interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Damschroder, L.J.; Reardon, C.M.; Widerquist, M.A.O.; Lowery, J. The Updated Consolidated Framework for Implementation Research Based on User Feedback. Implement. Sci. 2022, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Crowle, C.; Goyen, T.A.; Hardman, C.; Jackman, M.; Novak, I.; Badawi, N. Sensitivity and Specificity of General Movements Assessment for Diagnostic Accuracy of Detecting Cerebral Palsy Early in an Australian Context. J. Paediatr. Child. Health 2016, 52, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Morales-Monforte, E.; Bagur-Calafat, C.; Suc-Lerin, N.; Fornaguera-Martí, M.; Cazorla-Sánchez, E.; Girabent-Farrés, M. The Spanish Version of the Alberta Infant Motor Scale: Validity and Reliability Analysis. Dev. Neurorehabil. 2017, 20, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Vurrabindi, D.; Hilderley, A.J.; Kirton, A.; Andersen, J.; Cassidy, C.; Kingsnorth, S.; Munce, S.; Agnew, B.; Cambridge, L.; Herrero, M.; et al. Facilitators and Barriers to Implementation of Early Intensive Manual Therapies for Young Children with Cerebral Palsy across Canada. BMC Health Serv. Res. 2025, 25, 503. [Google Scholar] [CrossRef] [PubMed]
- Balas, E.A.; Boren, S.A. Managing Clinical Knowledge for Health Care Improvement. Yearb. Med. Inf. 2000, 09, 65–70. [Google Scholar] [CrossRef]
- Morris, Z.S.; Wooding, S.; Grant, J. The Answer Is 17 Years, What Is the Question: Understanding Time Lags in Translational Research. J. R. Soc. Med. 2011, 104, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Lazarowitz, R.; Taqi, D.; Lee, C.; Boruff, J.; McBain, K.; Majnemer, A.; Bussières, A.; Dahan-Oliel, N. Knowledge Translation Interventions to Increase the Uptake of Evidence-Based Practice Among Pediatric Rehabilitation Professionals: A Systematic Review. Phys. Occup. Ther. Pediatr. 2024, 45, 119–152. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.D.; Albrecht, L.; O’Leary, K.; Ball, G.D.C.; Hartling, L.; Hofmeyer, A.; Jones, C.A.; Klassen, T.P.; Burns, K.K.; Newton, A.S.; et al. Systematic Review of Knowledge Translation Strategies in the Allied Health Professions. Implement. Sci. 2012, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; McNamara, L.; te Velde, A. Best Practice Guidelines for Communicating to Parents the Diagnosis of Disability. Early Hum. Dev. 2019, 139, 104841. [Google Scholar] [CrossRef] [PubMed]
- Maitre, N.L.; Benninger, K.L.; Neel, M.L.; Haase, J.A.; Pietruszewski, L.; Levengood, K.; Adderley, K.; Batterson, N.; Hague, K.; Lightfoot, M.; et al. Standardized Neurodevelopmental Surveillance of High-Risk Infants Using Telehealth: Implementation Study during COVID-19. Pediatr. Qual. Saf. 2021, 6, E439. [Google Scholar] [CrossRef] [PubMed]
- Maitre, N.L.; Byrne, R.; Duncan, A.; Dusing, S.; Gaebler-Spira, D.; Rosenbaum, P.; Winter, S. “High-Risk for Cerebral Palsy” Designation: A Clinical Consensus Statement. J. Pediatr. Rehabil. Med. 2022, 15, 165–174. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.; Morgan, C.; Walker, K.; Novak, I. Cerebral Palsy—Don’t Delay. Dev. Disabil. Res. Rev. 2011, 17, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Hubermann, L.; Boychuck, Z.; Shevell, M.; Majnemer, A. Age at Referral of Children for Initial Diagnosis of Cerebral Palsy and Rehabilitation: Current Practices. J. Child. Neurol. 2015, 31, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Rouabhi, A.; Husein, N.; Dewey, D.; Letourneau, N.; Daboval, T.; Oskoui, M.; Kirton, A.; Shevell, M.; Dunbar, M.J.; Registry, C.C.P. Development of a Bedside Tool to Predict the Diagnosis of Cerebral Palsy in Term-Born Neonates. JAMA Pediatr. 2023, 177, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yao, S.; Tian, Y.; Zhang, C.; Zhao, T.; Wu, D.; Yu, G.; Lu, H. Automating General Movements Assessment with Quantitative Deep Learning to Facilitate Early Screening of Cerebral Palsy. Nat. Commun. 2023, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Romeo, D.M.; Velli, C.; Sini, F.; Pede, E.; Cicala, G.; Cowan, F.M.; Ricci, D.; Brogna, C.; Mercuri, E. Neurological Assessment Tool for Screening Infants during the First Year after Birth: The Brief-Hammersmith Infant Neurological Examination. Dev. Med. Child. Neurol. 2024, 66, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- García Ron, A.; Arriola Pereda, G.; Machado Casas, I.S.; Pascual Pascual, I.; Garriz Luis, M.; García Ribes, A.; Paredes Mercado, C.; Aguilera Albesa, S.; Peña Segura, J.L. Parálisis Cerebral. Protoc. Diagn. Ter. En. Neurol. Pediatr. 2022, 1, 103–114. [Google Scholar]
- Pallás Alonso, C.; García González, P.; Jimenez Moya, A.; Loureiro González, B.; Martín Peinador, Y.; Soriano Faura, J.; Torres Valdivieso, M.J.; Ginovart Galiana, G. Follow-up Protocol for Newborns of Birthweight Less than 1500g or Less than 32 Weeks Gestation. An. Pediatr. (Engl. Ed.) 2018, 88, e1–e229. [Google Scholar] [CrossRef]
- Sánchez de Muniain Sabater, P. (Ed.) Libro Blanco Sobre La Rehabilitación Infantil En España; Real Patronato Sobre Discapacidad, Ministerio de Sanidad, Consumo y Bienestar Social: Madrid, Spain, 2017.
- King, A.R.; Al Imam, M.H.; McIntyre, S.; Morgan, C.; Khandaker, G.; Badawi, N.; Malhotra, A. Early Diagnosis of Cerebral Palsy in Low- and Middle-Income Countries. Brain Sci. 2022, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.; Muhit, M.; Karim, T.; Smithers-Sheedy, H.; Novak, I.; Jones, C.; Badawi, N. Epidemiology of Cerebral Palsy in Bangladesh: A Population-Based Surveillance Study. Dev. Med. Child. Neurol. 2019, 61, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Karim, T.; Te Velde, A.; Ahmed, S.; Islam, M.; Morgan, C.; Novak, I.; Khandaker, G.; Badawi, N. Early Detection of Cerebral Palsy among a High-Risk Cohort in Low Resource Settings. Dev. Med. Child. Neurol. 2022, 64, 5–79. [Google Scholar] [CrossRef]
- Huang, H.-B.; Watt, M.J.; Hicks, M.; Zhang, Q.-S.; Lin, F.; Wan, X.-Q.; Chow, C.-B.; Cheung, P.-Y. A Family-Centered, Multidisciplinary Clinic for Early Diagnosis of Neurodevelopmental Impairment and Cerebral Palsy in China—A Pilot Observation. Front. Pediatr. 2022, 10, 840190. [Google Scholar] [CrossRef] [PubMed]
- Hewawitharana, G.; Darshana Ila, N.; Madhushani Ui, A.; Chathuranga Dp, S.; Priyangika Di, N.; Hewawitharana, B.; Wijesinghe, C.; Kodituwakku, P.; Phillips, J.; Yashoda Madumadhavie, R.G.; et al. Prediction of Cerebral Palsy and Cognitive Delay among High-Risk Children in a Developing Nation: A Successful Early Detection Programme. Dev. Med. Child. Neurol. 2025, 67, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Bosanquet, M.; Copeland, L.; Ware, R.; Boyd, R. A Systematic Review of Tests to Predict Cerebral Palsy in Young Children. Dev. Med. Child. Neurol. 2013, 55, 418–426. [Google Scholar] [CrossRef] [PubMed]
- BornToGetThere Project Consortium. Implementation of Early Detection and Early Intervention Service Delivery in Infants at Risk for Cerebral Palsy to Promote Infants’ Psychomotor Development and Maternal Health. BornToGetThere|Project|Fact Sheet|H2020|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/848201 (accessed on 3 July 2025).
- Karim, T.; Muhit, M.; Jahan, I.; Galea, C.; Morgan, C.; Smithers-Sheedy, H.; Badawi, N.; Khandaker, G. Outcome of Community-Based Early Intervention and Rehabilitation for Children with Cerebral Palsy in Rural Bangladesh: A Quasi-Experimental Study. Brain Sci. 2021, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Marschik, P.B.; Kwong, A.K.L.; Silva, N.; Olsen, J.E.; Schulte-Rüther, M.; Bölte, S.; Örtqvist, M.; Eeles, A.; Poustka, L.; Einspieler, C.; et al. Mobile Solutions for Clinical Surveillance and Evaluation in Infancy—General Movement Apps. J. Clin. Med. 2023, 12, 3576. [Google Scholar] [CrossRef] [PubMed]
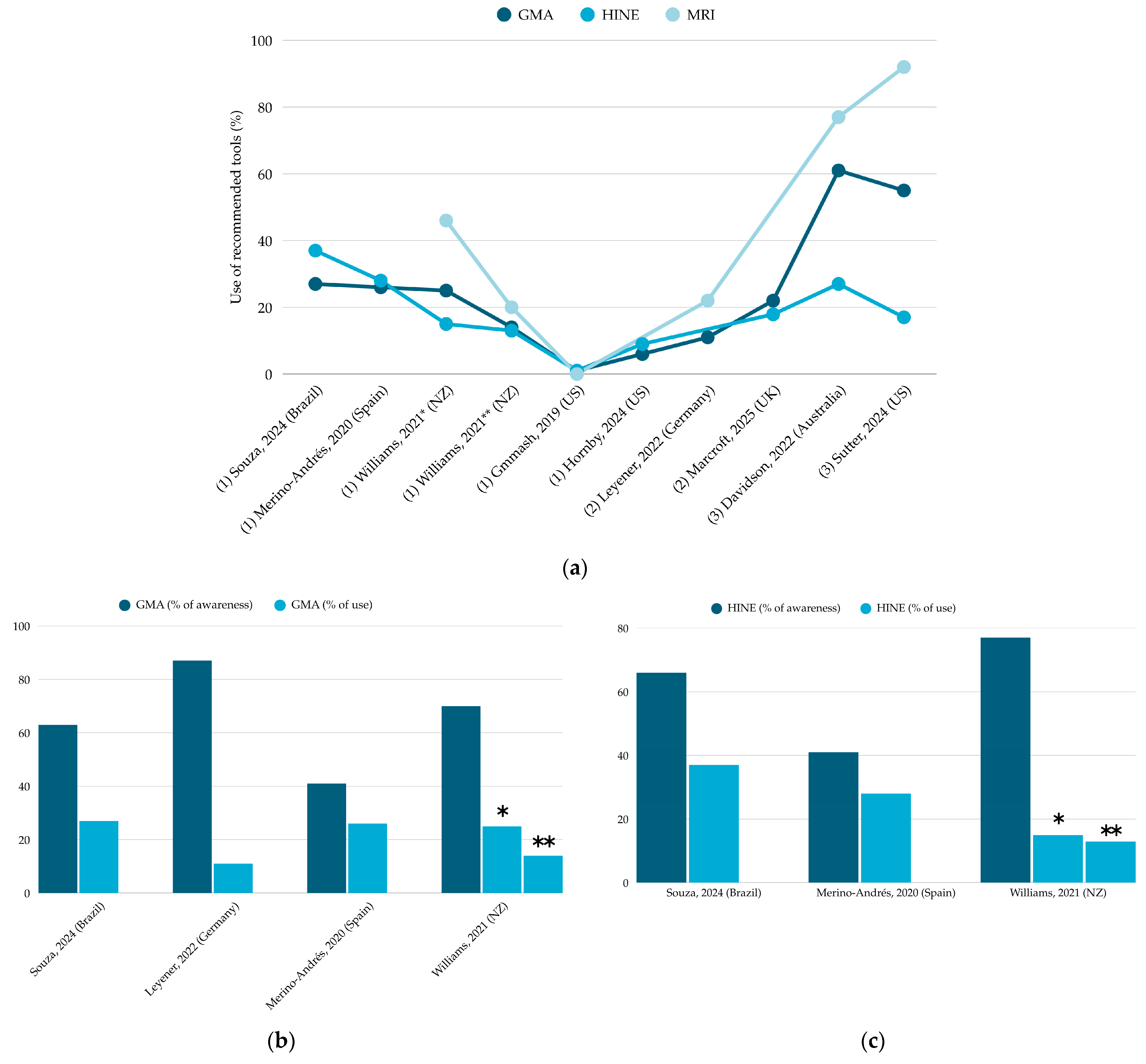
| Metric Type | First Author, Year | Providers (n) | Use of Recommended Tools (n, %) | Use of Alternative Tools (n, %) | |||
|---|---|---|---|---|---|---|---|
| GMA | HINE | MRI (Used/Referred) | Other Recommended Assessments | ||||
| Individual provider reports | Gmmash, 2019 [23] | 269 | 4 (1% 🔴) | 2 (0.7% 🔴) | 1 (0.4% 🔴) | DAYC: 33 (12% 🔴) TIMP: 9 (3% 🔴) | Abnormal Involuntary Movement Scale: 23 (8%) AEPS: 6 (2%) Bayley-III: 12 (4%) Battelle: 23 (8%) GMFM 88 and 66: 45 (17%) HELP: 13 (5%) IMP: 12 (4%) PDMS: 68 (25%) Do not use standardized tools: 15 (6%) |
| Williams, 2021 [13] | 54 (providing diagnosis) | Children < 1 year | |||||
| ++ 12 (25% 🟡) + 13 (27%) − 23 (48%) | ++ 7 (15% 🔴) + 18 (38%) − 23 (48%) | ++ 22 (46% 🟡) + 23 (48%) − 3 (6%) | AIMS: ++ 6 (13% 🔴), + 11 (23%), − 31 (65%) DAYC: ++ 17 (35% 🟡), + 3 (6%), − 28 (58%) MAI: ++ 8, (17% 🔴), + 7, (15%), − 33, (69%) NMSDA: ++ 3 (6% 🔴), + 6 (13%), − 39 (81%) TIMP: ++ 3 (6% 🔴), + 6 (13%), − 39 (81%) | Bayley: ++ 3 (6%), + 15 (38%), − 27 (56%) Clinical signs and symptoms: ++ 47 (98%), − 1 (2%) CUS: ++ 9 (19%), + 26 (54%), − 13 (27%) Dubowitz: ++ 2 (4%), + 16 (33%), − 30, (63%) Touwen: ++ 1 (2%), + 3 (6%), − 44 (92%) | |||
| Children between 1 and 2 years | |||||||
| NA | ++ 7 (15% 🔴) + 13 (28%) − 26 (57%) | ++ 27 (59% 🟢) + 15 (33%) − 4 (9%) | AIMS: ++ 7 (15% 🔴), + 19 (41%), − 20 (43%) DAYC: ++ 16 (35% 🟡), + 5 (11%), − 25 (54%) MAI: ++ 6 (13% 🔴), + 7 (15%), − 33 (72%) NMSDA: ++ 3 (7% 🔴), + 7 (15%), − 36 (78%) TIMP: NA | Bayley: ++ 7 (15%), + 19 (41%), − 20 (43%) Clinical signs and symptoms: ++ 42 (91%), + 3 (7%), − 1 (2%) CUS: ++ 4 (9%), + 11 (24%), − 31 (67%) Touwen: + 3 (7%), − 43 (93%) Dubowitz: NA | |||
| Children > 2 years | |||||||
| NA | NA | ++ 21 (55% 🟢) + 15 (39%) − 2 (5%) | AIMS: + 3 (8% 🔴), − 35 (92%) DAYC: ++ 14 (37% 🟡), + 5 (13%), − 19 (50%) MAI, NSMDA, TIMP: NA | Bayley: ++ 3 (8%), + 18 (47%), − 17 (45%) Clinical signs and symptoms: ++ 34, (89%), + 2 (5%), − 2 (5%) CUS: ++ 1 (3%), + 2 (5%), − 35 (92%) Touwen: ++ 1 (3%), + 1 (3%), − 36 (95%) Dubowitz: NA | |||
| 104 (not providing diagnosis) | ++ 15 (14% 🔴) + 15, (14%) − 77 (74%) | ++ 14 (13% 🔴) + 37, (36%) − 53 (51%) | ++ 21 (20% 🔴) + 24 (23%) − 59 (57%) | AIMS: ++ 20 (19% 🔴), + 29 (28%), − 55, (53%) DAYC: ++ 17 (16% 🔴), + 9 (9%), − 78, (75%) MAI: ++ 13 (13% 🔴), + 15 (14%), − 76 (73%) NSDA: ++ 15 (14% 🔴), + 15 (14%), − 84 (81%) TIMP: ++ 4 (4% 🔴), + 4 (4%), − 100 (96%) | Bayley: ++ 13 (13%), + 30 (29%), − 61 (59%) Clinical signs and symptoms: ++ 90 (87%), + 12 (12%), − 2 (2%) CUS: ++ 6 (6%), + 6 (6%), − 92 (88%) Dubowitz: ++ 3 (3%), + 11 (11%), − 90, (87%) Touwen: − 104 (100%) | ||
| Merino-Andrés, 2022 [25] | 109 | ++ (25.7% 🟡) + (11.9%) − (62.4%) | ++ (28.4% 🟡) + (11.9%) − (59.6%) | AIMS: ++ (41.3% 🟡), + (29.3%), − (29.3%) DAYC: ++ (0.9% 🔴), + (3.7%), − (95.4%) MAI: ++ (0.9% 🔴), + (11.9%), − (87.2%) NSMDA: ++ (0.9% 🔴), + (3.7%), − (95.4%) TIMP: ++ (2.8% 🔴), + (11%), − (86.2%) | ASQ: ++ (16.5%), + (13.8%), − (69.7%) Bayley: ++ (12.8%), + (19.3%), − (67.9%) Clinical history: ++ (88.1%), + (8.3%), − (3.7%) Dubowitz: ++ (0%), + (2.8%), − (97.2%) Touwen: ++ (0.9%), + (2.8%), − (96.3%) Vojta: ++ (32.1%), + (27.5%), − (40.4%) | ||
| Hornby, 2024 [28] | 72 | ++ (6% 🔴) + (4%) − (87%) NA (2%) | ++ (9% 🔴) + (20%) − (70%) NA (2%) | − (n = 40, 70.2%) | AIMS: ++ (9% 🔴), + (19%), − (71%), NA (2%) DAYC: ++ (59% 🟢), + (14%). − (27%) MAI: ++ (12% 🔴), + (14%), − (72%), NA (2%) NSMDA: ++ (6% 🔴), + (4%), − (89%), NA (2%) TIMP: ++ (2% 🔴), + (12%), − (83%), NA (3%) | Bayley: ++ (4%), + (14%), − (82%) Dubowitz: ++ (2%), + (4%), − (91%), NA (4%) PDMS: ++ (13%), + (46%), − (39%), NA (2%) | |
| Souza, 2024 [26] | 205 | 55 (26.8% 🟡) | 76 (37.1% 🟡) | AIMS: 128 (62.4% 🟢) TIMP: 51 (24.9% 🟡) DAYC: 6 (2.9% 🔴) NSMDA: 19 (9.3% 🔴) None of the options: 51 (24.9%) | |||
| Metric Type | First author, Year | Services (n) | Use of Recommended Tools (n, %) | Use of Alternative Tools (n, %) | |||
|---|---|---|---|---|---|---|---|
| GMA | HINE | MRI (Used/Referred) | Other Recommended Assessments | ||||
| Neonatal units report | Leyener, 2022 [24] | 63 | ++ 7 (11% 🔴) + 11 (17%) +/– 9 (14%) – 36 (57%) | NA | 14 (22% 🔴) | BNBAS: 3 (5%) CUS: 26 (41%) HNNE: 4 (6%) Miscellaneous: 6 (10%) Neurological examination according to Michaelis: 10 (16%) | |
| Marcroft, 2025 (preprint) [27] | 145 | 32 (22% 🔴) | 26 (17.9% 🔴) | AIMS: 24 (16.6% 🔴) | Bayley Screening Test: 15 (10.3%) Bayley-II: 5 (3.4%) Bayley-III: 80 (55.2%) Denver II: 3 (2.1%) Griffiths-III: 6 (4.1%) Informal assessment only: 23 (15.9%) NBO: 12 (8.3%) PARCA-R: 52 (35.9%) Schedule of Growing Skills: 38 (26.2%) SDQ: 12 (8.3%) Badger 2-year Questionnaire: 64 (44.1%) Other (including Wechsler and ASQ): 11 (7.6%) | ||
| Metric Type | First Author, Year | Infants (n) | Use of Recommended Tools (n, %) | |||
|---|---|---|---|---|---|---|
| GMA | HINE | MRI (Used/Referred) | Other Recommended Assessments | |||
| Patient-level data | Maitre, 2016 [29] | 50 | Before training (37% 🟡) | |||
| After training (90% 🟢) | ||||||
| Sutter, 2024 [31] | 44 | Before guidelines publication (~5% 🔴) | Before guidelines publication (0% 🔴) | Before guidelines publication (90% 🟢) | Before guidelines publication AIMS (~40% 🟡) TIMP (~10% 🔴) DAYC (0% 🔴) | |
| 47 | After guidelines publication (~55% 🟢) | After guidelines publication (~17% 🔴) | After guidelines publication (~92% 🟢) | After guidelines publication AIMS (~30% 🟡) TIMP (~50% 🟢) DAYC (~5% 🔴) | ||
| Davidson, 2022 [33] | 6 | Pre-implementation: Writhing/fidgety: 1 (16.7% 🔴) No GMA: 5 (83.3%) | Pre-implementation (infants referred ≤ 5 months): ≤5 months: 0 (0% 🔴) >5 months: 0 (0% 🔴) No HINE: 6 (100%) | Pre-implementation (infants referred ≤ 5 months): ≤5 months: 2 (33.3% 🟡) >5 months: 1 (16.7% 🔴) No MRI: 3 (50%) | ||
| 209 | Implementation phases: Writhing/fidgety: 127 (60.8% 🟢) No GMA: 82 (39.2%) | Implementation phases (infants referred < 5 months): ≤5 months: 57 (27.3% 🟡) >5 months: 44 (21.1% 🔴) No HINE: 108 (51.7%) | Implementation phases (infants referred ≤ 5 months): ≤5 months: 161 (77% 🟢) >5 months: 14 (16.7% 🔴) No MRI: 34 (16.3%) | |||
| 43 | NA | Pre-implementation (infants referred > 5 months): 0 (0% 🔴) Missing: 27 (62.7%) Not eligible: 16 (37.2%) | Pre-implementation (infants referred > 5 months): ≤5 months: 0 (0% 🔴) >5 months: 2 (4.7% 🔴) No MRI: 41 (95.3%) | |||
| 236 | NA | Implementation phases (infants referred > 5 months): 12 (5.1% 🔴) Missing: 167 (70.8%) Not eligible: 57 (24.2%) | Implementation phases (infants referred > 5 months): ≤5 months: 24 (10.2% 🔴) >5 months: 124 (52.5% 🟢) No MRI: 88 (37.3%) | |||
| Factors | Enablers (n) | Barriers (n) |
|---|---|---|
| System factors | Time and funding (n = 13) [13], (n = 7) [25]; System and personnel resources (53 quotes) [34] | Time, workload, and staffing (n = 25) [13], (n = 46) [25], (n not specified, ≤12) [29]; System and personnel resources (149 quotes) [34]; Funding (n = 19) [13], (n = 28) [25] |
| Funding; tool availability and use; time, workload, staffing; organizational structure/processes (n = 13) [28] | Funding; tool availability and use; time, workload, staffing; organizational structure/processes (n = 44) [28] | |
| Referral and health pathways (n = 12) [13], (n = 16) [25] | ||
| Quality improvement, peer review, and audit (n = 16) [13] | ||
| 👤👤👤👤👤👤👤👤👤👤 (n = 49) | 👤👤👤👤👤👤👤👤👤👤 👤👤👤👤👤👤👤👤👤👤 👤👤👤👤👤👤👤👤👤👤 👤👤👤👤👤👤👤👤 (n = 190) | |
| Social factors | Management, staff, and administration (n = 19) [13], (n = 9) [25] | Management, staff, and administration (n = 14) [13], (n = 12) [25] |
| Multidisciplinary teamwork (n = 8) [13], (n = 28) [25] | Multidisciplinary teamwork (n = 8) [13], (n = 18) [25] | |
| Administration/leadership and peer support/multidisciplinary working/clinical champions (n = 25) [28] Role of leadership (12 quotes) [34] | Administration/leadership, peer support/multidisciplinary working/clinical champions (n = 21) [28]; Role of leadership (10 quotes) [34] | |
| 👤👤👤👤👤👤👤👤👤👤 👤👤👤👤👤👤👤👤 (n = 89) | 👤👤👤👤👤👤👤👤👤👤 👤👤👤👤👤 (n = 73) | |
| Health professional knowledge and perceptions | Education/professional development and knowledge sharing (n = 16) [13], (n = 22) [25] | Knowledge/confidence in using tools (n = 10) [13], (n = 21) [25]; Inconsistent knowledge base about time and specifics of the neurological exam because of provider-type diversity (n not specified, ≤12) [29] |
| Guidelines and clinical pathways (n = 6) [13], (n = 9) [25]; Consensus about research evidence (11 quotes) [34] | Guidelines and clinical pathways (n = 6) [13]; Consensus about research evidence (15 quotes) [34] | |
| Health professional communication (n = 4) [25] | Health professional communication (n = 1) [25] | |
| Patient-tailored care (n = 1) [25] | ||
| Clinical experience (23 quotes) [34] | Clinical experience (52 quotes) [34] | |
| Family experience as evidence (35 quotes) [34] | Family experience as evidence (61 quotes) [34] | |
| Evaluation practices (1 quote) [34] | Evaluation practices (14 quotes) [34] | |
| Access to education; knowledge sharing/confidence/practice opportunities; guidelines and pathways (n = 22) [28] | Access to education; knowledge sharing/confidence/practice opportunities; guidelines and pathways (n = 19) [28] | |
| 👤👤👤👤👤👤👤👤👤👤 👤👤👤👤👤👤 (n = 80) | 👤👤👤👤👤👤👤👤👤👤 👤 (n = 57) | |
| Clinical considerations and internal drive | Self-driven/self-initiated (n = 13) [25] | |
| Case complexity and inconsistency in practice (n = 5) [13], (n = 1) [25]; Concerns about a complex neurological exam decreasing the clinical flow without tangible benefits to patients (n not specified, ≤12) [29] | ||
| Physical possibilities (n = 7) [25] | ||
| Clinical considerations and internal drive (n = 6) [28] | Clinical considerations and internal drive (n = 4) [28] | |
| 👤👤👤👤 (n = 19) | 👤👤👤 (n = 17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Robles, Á.; Merino-Andrés, J.; Cisse, M.R.S.; Pacheco-Molero, M.; León-Estrada, I.; Gutiérrez-Ortega, M. The Pathway Is Clear but the Road Remains Unpaved: A Scoping Review of Implementation of Tools for Early Detection of Cerebral Palsy. Children 2025, 12, 941. https://doi.org/10.3390/children12070941
Hidalgo-Robles Á, Merino-Andrés J, Cisse MRS, Pacheco-Molero M, León-Estrada I, Gutiérrez-Ortega M. The Pathway Is Clear but the Road Remains Unpaved: A Scoping Review of Implementation of Tools for Early Detection of Cerebral Palsy. Children. 2025; 12(7):941. https://doi.org/10.3390/children12070941
Chicago/Turabian StyleHidalgo-Robles, Álvaro, Javier Merino-Andrés, Mareme Rose Samb Cisse, Manuel Pacheco-Molero, Irene León-Estrada, and Mónica Gutiérrez-Ortega. 2025. "The Pathway Is Clear but the Road Remains Unpaved: A Scoping Review of Implementation of Tools for Early Detection of Cerebral Palsy" Children 12, no. 7: 941. https://doi.org/10.3390/children12070941
APA StyleHidalgo-Robles, Á., Merino-Andrés, J., Cisse, M. R. S., Pacheco-Molero, M., León-Estrada, I., & Gutiérrez-Ortega, M. (2025). The Pathway Is Clear but the Road Remains Unpaved: A Scoping Review of Implementation of Tools for Early Detection of Cerebral Palsy. Children, 12(7), 941. https://doi.org/10.3390/children12070941







