Ambulatory Blood Pressure Monitoring in Children: A Cross-Sectional Study of Blood Pressure Indices
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants and Data Collection
- (1)
- Aged between 5 and 18 years;
- (2)
- The presence ofCKD or elevated office blood pressure readings;
- (3)
- Previously diagnosed hypertension based on office BP, or follow-up for hypertension risk;
- (4)
- Availability of valid 24 h ABPM data.
- (1)
- Children who were acutely hospitalized at the time of ABPM;
- (2)
- Incomplete ABPM records with fewer than 75% valid readings;
- (3)
- Missing clinical or laboratory data required for analysis.
2.3. Blood Pressure Assessment and Definition of Hypertension
2.4. Outcomes and Definitions
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABPM | Ambulatory Blood Pressure Monitoring |
| AASI | Ambulatory Arterial Stiffness Index |
| ARV | Average Real Variability |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| CKD | Chronic Kidney Disease |
| DBP | Diastolic Blood Pressure |
| GFR | Glomerular Filtration Rate |
| PP | Pulse Pressure |
| PPI | Pulse Pressure Index |
| RPP | Rate Pressure Product |
| SBP | Systolic Blood Pressure |
| ACEI | Angiotensin-Converting Enzyme Inhibitor |
| ARB | Angiotensin II Receptor Blocker |
| CCB | Calcium Channel Blocker |
| BB | Beta-Blocker |
| SD | Standard Deviation |
| WCH | White Coat Hypertension |
References
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global Prevalence of Hypertension in Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Urbina, E.M.; Brady, T.M.; Baker-Smith, C.M.; Daniels, S.R.; Hayman, L.L.; Mitsnefes, M.; Tran, A.; Zachariah, J.P. Ambulatory Blood Pressure Monitoring in Children and Adolescents: 2022 Update: A Scientific Statement from the American Heart Association. Hypertension 2022, 79, e179–e188. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, G.; Mitsnefes, M.M.; Flynn, J.T.; Becker, R.C.; Daniels, S.; Falkner, B.E.; Ferguson, M.; Urbina, E.M. Pediatric and Adult Ambulatory Blood Pressure Thresholds and Blood Pressure Load as Predictors of Left Ventricular Hypertrophy in Adolescents. J. Clin. Hypertens. 2021, 23, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Tanaka, K.; Ohkubo, T.; Kondo, T.; Kikuya, M.; Metoki, H.; Hashimoto, T.; Satoh, M.; Inoue, R.; Asayama, K.; et al. Ambulatory versus home versus clinic blood pressure: The association with subclinical cerebrovascular diseases: The Ohasama Study. Hypertension 2012, 59, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T. Ambulatory Blood Pressure Monitoring in Children: Imperfect Yet Essential. Pediatr. Nephrol. 2011, 26, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Khan, S.A.; Wong, N.D.; Larson, M.G.; Levy, D. Is Pulse Pressure Useful in Predicting Risk for Coronary Heart Disease? The Framingham Heart Study. Circulation 1999, 100, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; Kollias, A.; Giovas, P.P.; Papagiannis, J.; Roussias, L.G. Ambulatory Arterial Stiffness Index, Pulse Pressure, and Pulse Wave Velocity in Children and Adolescents. Hypertens. Res. 2010, 33, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Mulè, G.; Calcaterra, I.; Costanzo, M.; Morreale, M.; D’Ignoto, F.; Castiglia, A.; Geraci, G.; Rabbiolo, G.; Vaccaro, F.; Cottone, S. Average Real Variability of 24-h Systolic Blood Pressure is Associated with Microalbuminuria in Patients with Primary Hypertension. J. Hum. Hypertens. 2016, 30, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Basalely, A.; Hill-Horowitz, T.; Sethna, C.B. Ambulatory Blood Pressure Monitoring in Pediatrics: An Update on Interpretation and Classification of Hypertension Phenotypes. Curr. Hypertens. Rep. 2022, 24, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Azukaitis, K.; Kirchner, M.; Doyon, A.; Litwin, M.; Bayazit, A.; Duzova, A.; Canpolat, N.; Jankauskiene, A.; Shroff, R.; Melk, A.; et al. Arterial Stiffness and Chronic Kidney Disease Progression in Children. Clin. J. Am. Soc. Nephrol. 2022, 17, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Am. J. Kidney Dis. 2002, 39 (Suppl. S1), S1–S266. [Google Scholar]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Fernández, J.R.; Ayala, D.E.; Mojón, A.; Alonso, I.; Smolensky, M. Circadian Rhythm of Double (Rate-Pressure) Product in Healthy Normotensive Young Subjects. Chronobiol. Int. 2001, 18, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Li, Y.; Thijs, L.; McCormack, P.; Staessen, J.A.; O’Brien, E.; Stanton, A. Ambulatory Arterial Stiffness Index: Rationale and Methodology. Blood Press. Monit. 2006, 11, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Mena, L.; Pintos, S.; Queipo, N.V.; Aizpúrua, J.A.; Maestre, G.; Sulbarán, T. A Reliable Index for the Prognostic Significance of Blood Pressure Variability. J. Hypertens. 2005, 23, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Lona, G.; Hauser, C.; Köchli, S.; Infanger, D.; Endes, K.; Schmidt-Trucksäss, A.; Hanssen, H. Association of Blood Pressure, Obesity, and Physical Activity with Arterial Stiffness in Children: A Systematic Review and Meta-Analysis. Pediatr. Res. 2022, 91, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Lentferink, Y.E.; Kromwijk, L.A.J.; van der Aa, M.P.; Knibbe, C.A.J.; van der Vorst, M.M.J. Increased Arterial Stiffness in Adolescents with Obesity. Glob. Pediatr. Health 2019, 6, 2333794X19831297. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.; Meyers, M.; Schnall, J.; Chorny, N.; Frank, R.; Infante, L.; Sethna, C.B. Blood Pressure Variability in Children with Primary vs. Secondary Hypertension. J. Clin. Hypertens. 2014, 16, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Myette, R.L.; Feber, J.; Blinder, H.; Bendiak, G.N.; Foster, B.J.; MacLean, J.E.; Constantin, E.; Katz, S.L. Blood Pressure Variability in Children with Obesity and Sleep-Disordered Breathing Following Positive Airway Pressure Treatment. Pediatr. Res. 2022, 92, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Peng, K.; Du, R.; Huang, X.; Sun, W.; Ding, L.; Wang, P.; Huang, Y.; Xu, Y.; Xu, M.; et al. High glomerular filtration rate is associated with arterial stiffness in Chinese population. J. Hypertens. 2017, 35, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Polaconda, S.; Nair, N.; Chakraborty, R.; Sethi, S.; Krishnappa, V.; Kapur, G.; Mhanna, M.; Kusumi, K. Association of Pulse Pressure, Pulse Pressure Index, and Ambulatory Arterial Stiffness Index with Kidney Function in a Cross-Sectional Pediatric Chronic Kidney Disease Cohort from the CKiD Study. J. Clin. Hypertens. 2020, 22, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.D.; Keehn, L.; Milne, L.; Sofocleous, P.; Chowienczyk, P.J. Decreased Arterial Elasticity in Children with Nondialysis Chronic Kidney Disease is Related to Blood Pressure and Not to Glomerular Filtration Rate. Hypertension 2015, 66, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Lurbe, E.; Torro, I.; Alvarez, V.; Nawrot, T.; Paya, R.; Redon, J.; Staessen, J.A. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension 2005, 45, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ding, X.; Zhang, X.; Su, S.; Treiber, F.A.; Vlietinck, R.; Fagard, R.; Derom, C.; Gielen, M.; Loos, R.J.F.; et al. Genetic and Environmental Influences on Blood Pressure Variability: A Study in Twins. J. Hypertens. 2013, 31, 690–697. [Google Scholar] [CrossRef] [PubMed]



| Total | CKD | Non-CKD | p-Value | |
|---|---|---|---|---|
| Number (%) | 70 | 41 (58.6) | 29 (41.4) | |
| Gender (male, n [%]) | 38 (54.3) | 23 (56.1) | 15 (51.7) | 0.81 |
| Age (years, mean ± SD) | 11.6 ± 3.5 | 11.2 ± 3.7 | 12.0 ± 3.0 | 0.34 |
| Height (cm, mean ± SD) | 136.5 ± 19.8 | 132.7 ± 18.6 | 141.9 ± 20.6 | 0.07 |
| Weight kg (median [IQR]) | 30 [22–58.3] | 25.7 [20.7–40.8] | 48.5 [25.2–73.8] | 0.004 |
| BMI (median [IQR]) | 16.7 [14.7–25.9] | 15.4 [14–19.3] | 22.1 [16.6–28.8] | <0.001 |
| Initial GFR (mL/min/1.73 m2 [IQR]) | 69 [23.36–120] | 41.30 [12.87–95] | 120 [84.75–120] | <0.001 |
| Last GFR (mL/min/1.73 m2 [IQR]) | 50.3 [7.81–120] | 17 [7.02–62.4] | 120 [120–120] | <0.001 |
| Hypertension studies | ||||
| Office SBP (mmHg, mean ± SD) | 127.1 ± 16.4 | 124.8 ± 16.5 | 127.0 ±14.6 | 0.47 |
| Office DBP (mmHg, median [IQR]) | 77 [66–86] | 77 [67–88] | 74 [65–83] | 0.24 |
| Office PPI (mean ± SD) | 0.39 ± 0.08 | 0.36 ± 0.07 | 0.4 ± 0.1 | 0.001 |
| Office PP (mean ± SD) | 50.0 ± 12.7 | 45.6 ± 9.5 | 56.2 ± 14.2 | 0.002 |
| ABPM Overall SBP (mmHg, mean ± SD) | 118.6 ± 13.9 | 117.8 ± 15.1 | 119.8 ±12.2 | 0.99 |
| ABPM Overall DBP (mmHg, median [IQR]) | 71 [63–76.1] | 72 [63–79] | 69 [61–74] | 0.25 |
| Office BP percentile (>95th, n [%]) | 33 (70.2) | 25 (67.6) | 8 (80) | 0.70 |
| ABPM PPI (median [IQR]) | 0.4 [0.36–0.45] | 0.39 [0.3–0.42] | 0.42 [0.39–0.45] | 0.03 |
| ABPM PP (mean ± SD) | 46.7 ± 8.9 | 44.0 ± 8.3 | 50.5 ± 8.4 | 0.002 |
| AASI (mean ± SD) | 0.34 ± 0.17 | 0.34 ± 0.17 | 0.35 ± 0.16 | 0.37 |
| RPP (median [IQR]) | 10,787 [9538–11,639] | 10,650 [9265–11,562] | 10,958 [9746–11,823] | 0.60 |
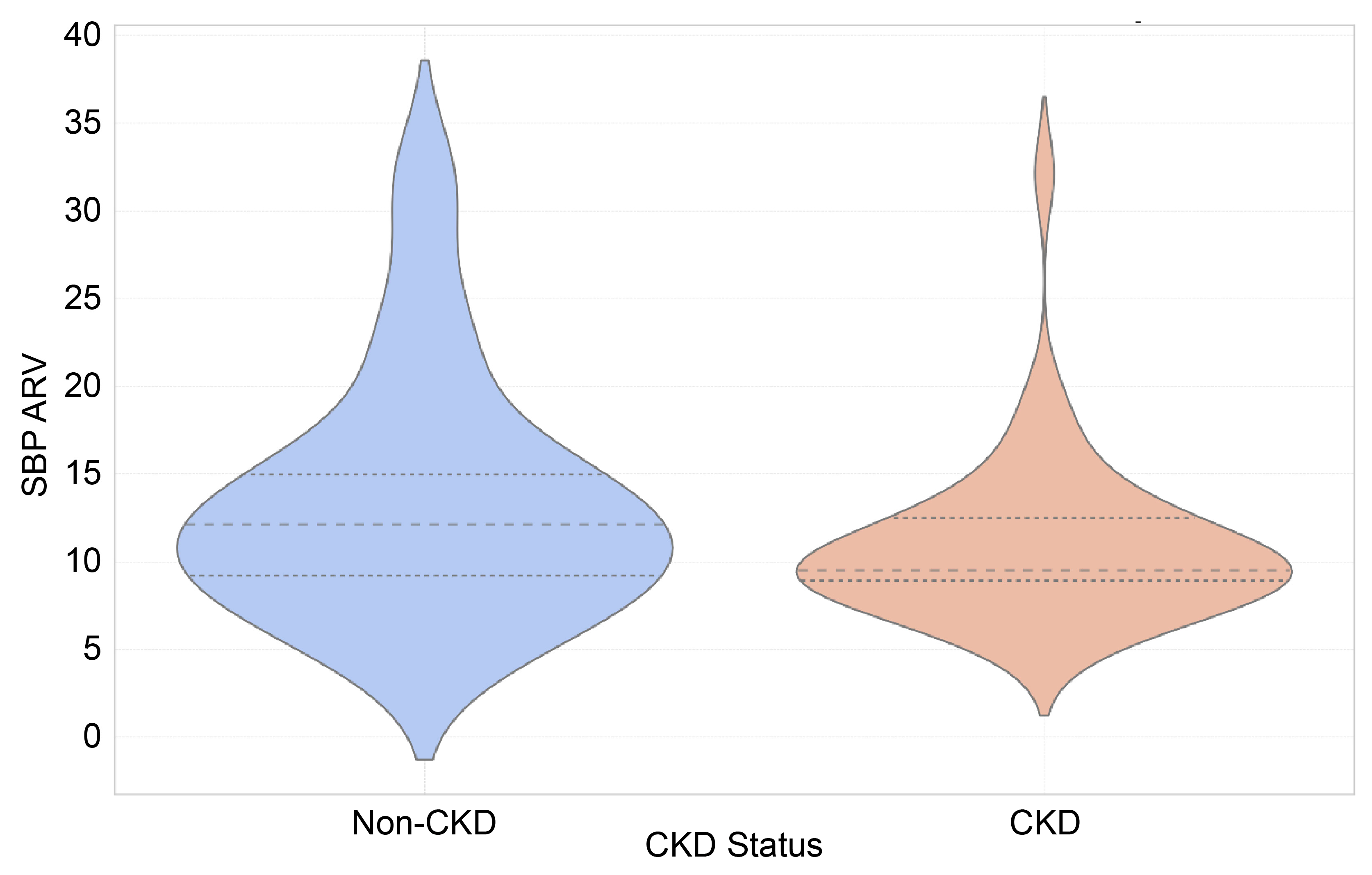
| SBP ARV (median [IQR]) | 10.2 [8.9–13.5] | 9.5 [8.9–12.5] | 12.1 [8.7–15.3] | 0.08 |
| DBP ARV (median [IQR]) | 9.0 [7.3–12.4] | 8.71 [7.1–11.1] | 9.3 [7.4–15.3] | 0.14 |
| SBP SD (median [IQR]) | 11.98 [10.19–15.8] | 11.34 [10.17–14.85] | 13.42 [10.68–19.12] | 0.9 |
| DBP SD (median [IQR]) | 10.95 [9.23–15.62] | 10.75 [9.06–14.09] | 11.45 [9.93–24.65] | 0.19 |
| ABPM Systolic percentile (>95th, n [%]) | 30 (42.9) | 16 (39) | 14 (48.3) | 0.47 |
| ABPM Diastolic percentile (>95th, n [%]) | 22 (31.4) | 15 (36.6) | 7 (24.1) | 0.31 |
| ABPM percentile (>95th, n [%]) | 34 (48.6) | 20 (48.8) | 14 (48.3) | >0.99 |
| Masked Hypertension (n [%]) | 4 (5.7) | 3 (7.3) | 1 (3.4) | 0.64 |
| White Coat Hypertension (n [%]) | 19 (27.1) | 10 (24.4) | 9 (31) | 0.59 |
| Non-Dipping BP (Yes, n [%]) | 30 (42.9) | 15 (36.6) | 15 (51.7) | 0.23 |
| Antihypertensive medications | ||||
| Antihypertensive medications (Yes, n [%]) | 37 (52.9) | 24 (58.9) | 13(44.8) | 0.33 |
| ACEIs or ARBs (n [%]) | 23 (32.9) | 13 (31.7) | 10 (34.5) | >0.99 |
| CCB (n [%]) | 21 (35.6) | 15 (42.9) | 6 (25) | 0.18 |
| BB (n [%]) | 6 (8.6) | 5 (12.2) | 1 (3.4) | 0.40 |
| Vasodilators (n [%]) | 5 (7.1) | 4 (9.8) | 1 (3.4) | 0.40 |
| Central acting (n [%]) | 1 (1.7) | 1 (2.9) | 0 | >0.99 |
| SBP Load | ||||
| <25% (n [%]) | 22 (31.4) | 14 (34.1) | 8 (27.6) | 0.72 |
| 25–50% (n [%]) | 14 (20) | 7 (17.1) | 7 (24.1) | |
| >50% (n [%]) | 34 (48.6) | 20 (48.8) | 14(48.3) | |
| DBP Load | ||||
| <25% (n [%]) | 34 (48.6) | 18 (43.9) | 16 (55.2) | 0.59 |
| 25–50% (n [%]) | 15 (21.4) | 9 (22) | 6 (20.7) | |
| >50% (n [%]) | 21 (30) | 14 (34.1) | 7 (24.1) | |
| Variable | OR | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|
| Age | −0.09 | −0.49 | 0.31 | 0.667 |
| Weight | 0.05 | −0.01 | 0.10 | 0.132 |
| Height | 0.02 | −0.05 | 0.09 | 0.592 |
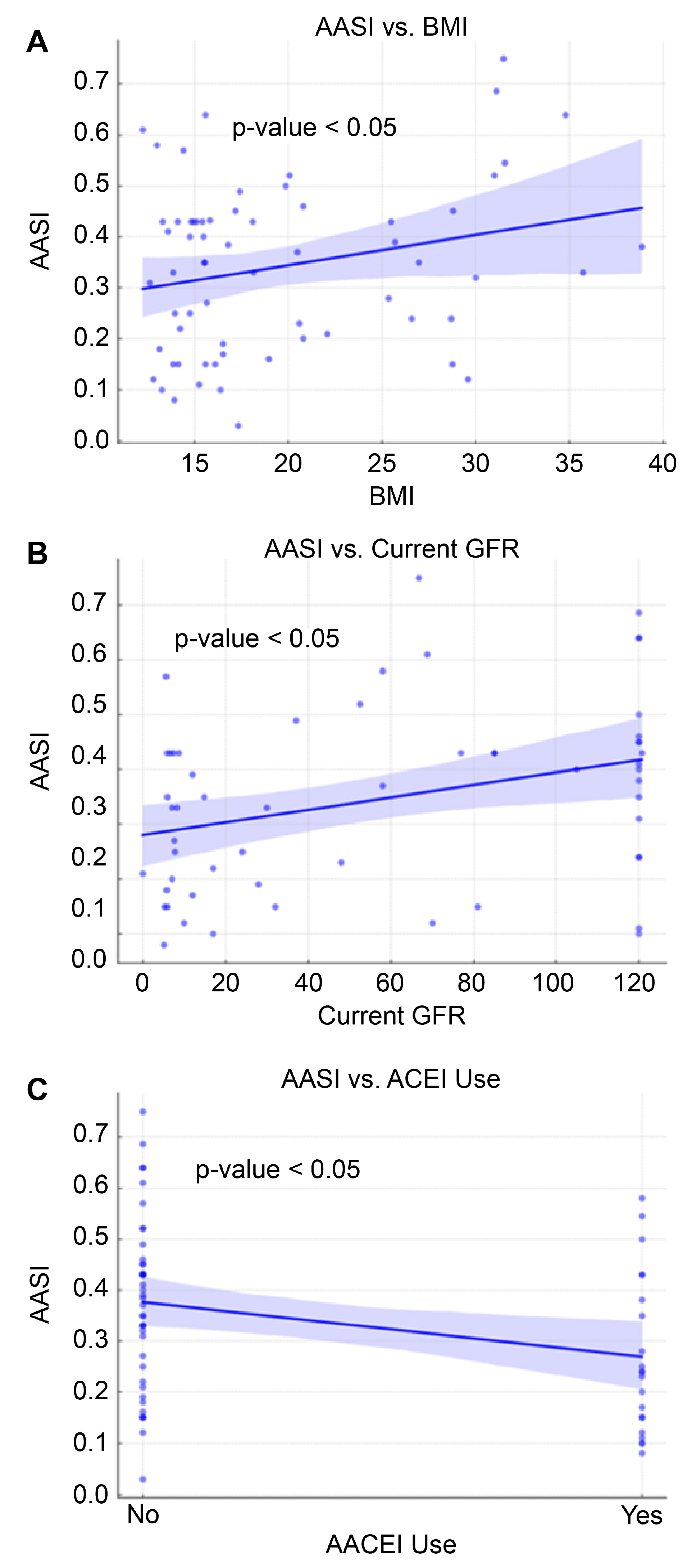
| BMI | 0.19 | 0.00 | 0.39 | 0.049 |
| Initial GFR | −0.01 | −0.03 | 0.02 | 0.810 |
| Current GFR | 0.01 | −0.01 | 0.04 | 0.190 |
| Overall SBP | 0.15 | 0.05 | 0.24 | 0.002 |
| Overall DBP | 0.18 | 0.08 | 0.27 | 0.001 |
| SBP HTN | 3.11 | 0.42 | 5.80 | 0.024 |
| DBP HTN | 3.43 | 0.57 | 6.29 | 0.019 |
| ABPM HTN | 2.71 | 0.02 | 5.39 | 0.049 |
| Dipping | −1.40 | −4.17 | 1.38 | 0.319 |
| WCH | −0.60 | −3.70 | 2.51 | 0.702 |
| Masked | −0.03 | −5.99 | 5.93 | 0.992 |
| ASSI High | 1.94 | −2.62 | 6.51 | 0.398 |
| AASI | 2.45 | −5.72 | 10.62 | 0.552 |
| SBP Load | 1.03 | −0.53 | 2.58 | 0.19 |
| DBP Load | 1.55 | −0.01 | 3.10 | 0.051 |
| PPI | −4.09 | −12.62 | 4.44 | 0.34 |
| PP | 5.07 | −0.16 | 0.16 | 0.99 |
| Office SBP | 0.03 | −0.06 | 0.11 | 0.514 |
| Office DBP | 0.03 | −0.07 | 0.13 | 0.545 |
| Non-CKD | 2.62 | −0.12 | 5.35 | 0.06 |
| CCB | −0.47 | −1.24 | 0.3 | 0.23 |
| ACEI | 0.28 | 0.71 | −1.19 | 1.75 |
| BB | 0.55 | −0.43 | 1.53 | 0.27 |
| Vasodilators | 0.87 | 0.00 | 1.74 | 0.05 |
| Central acting | 0.08 | −1.58 | 1.75 | 0.92 |
| Variable | OR | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|
| Age | −0.26 | −0.75 | 0.23 | 0.29 |
| Weight | 0.04 | −0.04 | 0.11 | 0.31 |
| Height | 0.01 | −0.08 | 0.09 | 0.858 |
| BMI | 0.18 | −0.06 | 0.41 | 0.14 |
| Initial GFR | −0.02 | −0.03 | 0.03 | 0.87 |
| Current GFR | 0.02 | −0.01 | 0.04 | 0.25 |
| SBP HTN | 3.47 | 0.16 | 6.77 | 0.04 |
| DBP HTN | 3.86 | 0.35 | 7.37 | 0.03 |
| ABPM HTN | 2.61 | −0.70 | 5.93 | 0.12 |
| Dipping | −1.44 | −4.83 | 1.95 | 0.40 |
| WCH | −1.76 | −5.53 | 2.00 | 0.35 |
| Masked | 0.19 | −7.08 | 7.45 | 0.96 |
| ASSI High | 2.67 | −2.57 | 7.91 | 0.31 |
| AASI | 2.12 | −7.35 | 11.58 | 0.66 |
| SBP Load | 1.12 | −0.79 | 3.02 | 0.25 |
| DBP Load | 2.08 | 0.20 | 3.96 | 0.03 |
| PPI | −6.16 | −16.5 | 4.12 | 0.24 |
| PP | −0.02 | −0.21 | 0.17 | 0.80 |
| Office SBP | 0.01 | −0.09 | 0.11 | 0.84 |
| Office DBP | 0.02 | −0.11 | 0.14 | 0.77 |
| Non-CKD | 2.69 | −0.67 | 6.05 | 0.11 |
| CCB | −0.33 | −1.28 | 0.61 | 0.48 |
| ACEI | 0.39 | −1.4 | 2.19 | 0.66 |
| BB | 0.98 | −0.20 | 2.16 | 0.10 |
| Vasodilators | 0.98 | −0.09 | 2.04 | 0.07 |
| Central acting | −0.57 | −2.60 | 1.45 | 0.58 |
| Variable | OR | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|
| Age | 0.001 | −0.01 | 0.013 | 0.825 |
| Weight | 0.002 | 0.000 | 0.003 | 0.06 |
| Height | 0.001 | −0.001 | 0.003 | 0.48 |
| BMI | 0.006 | 0.001 | 0.012 | 0.048 |
| Initial GFR | 0.001 | 0.000 | 0.002 | 0.16 |
| Current GFR | 0.001 | 0.000 | 0.002 | 0.006 |
| SYS HTN | 0.06 | −0.02 | 0.14 | 0.15 |
| DBP HTN | −0.002 | −0.09 | 0.09 | 0.97 |
| ABPM HTN | 0.063 | −0.02 | 0.15 | 0.13 |
| Dipping | −0.03 | −0.11 | 0.06 | 0.54 |
| WCH | −0.09 | −0.18 | −0.004 | 0.04 |
| Masked | −0.04 | −0.22 | 0.13 | 0.62 |
| SBP Load | 0.01 | −0.04 | 0.05 | 0.81 |
| DBP Load | −0.01 | −0.05 | 0.04 | 0.82 |
| PPI | 0.31 | 0.07 | 0.54 | 0.013 |
| PP | 0.004 | −0.001 | 0.008 | 0.12 |
| Office SBP | −0.001 | −0.004 | 0.001 | 0.36 |
| Office DBP | −0.002 | −0.005 | 0.001 | 0.28 |
| Non-CKD | −0.02 | −0.101 | 0.07 | 0.72 |
| CCB | −0.01 | −0.033 | 0.012 | 0.34 |
| ACEI | −0.05 | −0.09 | −0.01 | 0.02 |
| BB | −0.02 | −0.05 | 0.01 | 0.18 |
| Vasodilators | −0.01 | −0.04 | 0.02 | 0.35 |
| Central acting | 0.01 | −0.04 | 0.06 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, S.K.; Sandokji, I.A.; Al-Ansari, A.K.; Alsubhi, H.A.; Bahassan, A.; Nawawi, E.; Alqahtani, F.H.; Flimban, M.N.; Shalaby, M.A.; Kari, J.A. Ambulatory Blood Pressure Monitoring in Children: A Cross-Sectional Study of Blood Pressure Indices. Children 2025, 12, 939. https://doi.org/10.3390/children12070939
Abdullah SK, Sandokji IA, Al-Ansari AK, Alsubhi HA, Bahassan A, Nawawi E, Alqahtani FH, Flimban MN, Shalaby MA, Kari JA. Ambulatory Blood Pressure Monitoring in Children: A Cross-Sectional Study of Blood Pressure Indices. Children. 2025; 12(7):939. https://doi.org/10.3390/children12070939
Chicago/Turabian StyleAbdullah, Sulaiman K., Ibrahim A. Sandokji, Aisha K. Al-Ansari, Hadeel A. Alsubhi, Abdulaziz Bahassan, Esraa Nawawi, Fawziah H. Alqahtani, Marwan N. Flimban, Mohamed A. Shalaby, and Jameela A. Kari. 2025. "Ambulatory Blood Pressure Monitoring in Children: A Cross-Sectional Study of Blood Pressure Indices" Children 12, no. 7: 939. https://doi.org/10.3390/children12070939
APA StyleAbdullah, S. K., Sandokji, I. A., Al-Ansari, A. K., Alsubhi, H. A., Bahassan, A., Nawawi, E., Alqahtani, F. H., Flimban, M. N., Shalaby, M. A., & Kari, J. A. (2025). Ambulatory Blood Pressure Monitoring in Children: A Cross-Sectional Study of Blood Pressure Indices. Children, 12(7), 939. https://doi.org/10.3390/children12070939







