Main Processed Hypoallergenic Foods: A Potential Tool to Improve Informed Dietary Choices in Children with IgE-Mediated Food Allergies
Abstract
1. Introduction
2. Research Strategies and Literature Analysis
3. Food Processing and Modification of Allergenicity
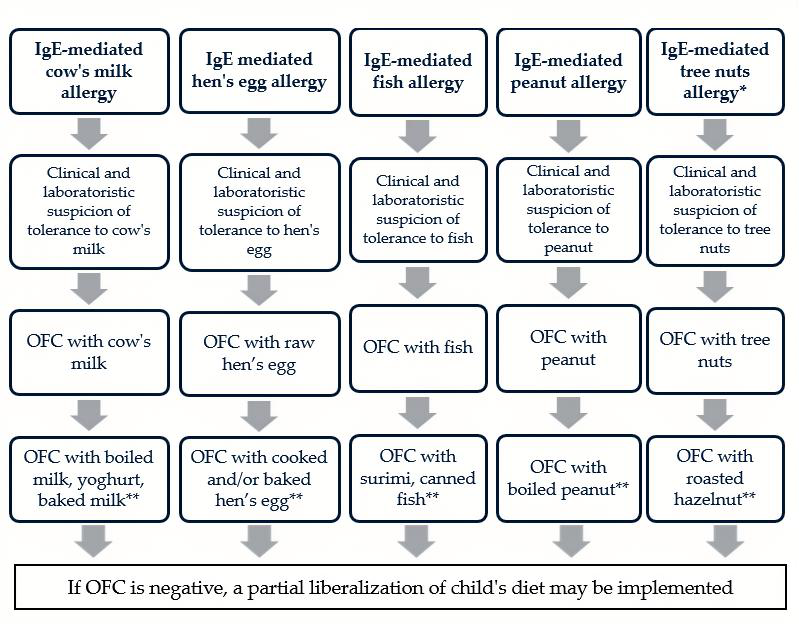
4. Cow’s Milk
5. Hen’s Eggs
6. Soy
7. Fish
8. Shellfish
9. Wheat
10. Peanut
11. Tree Nuts
12. Sesame
13. The Children’s Perspective
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortolani, C.; Ispano, M.; Pastorello, E.; Bigi, A.; Ansaloni, R. The oral allergy syndrome. Ann. Allergy Asthma Immunol. 1997, 78, 417–424. [Google Scholar]
- Muraro, A.; de Silva, D.; Halken, S.; Worm, M.; Khaleva, E.; Arasi, S.; Dunn-Galvin, A.; Nwaru, B.I.; De Jong, N.W.; Rodríguez Del Río, P.; et al. Managing food allergy: GA2LEN guideline 2022. World Allergy Organ. J. 2022, 15, 100687. [Google Scholar]
- Wood, R.A.; Togias, A.; Sicherer, S.H.; Shreffler, W.G.; Kim, E.H.; Jones, S.M.; Leung, D.Y.M.; Vickery, B.P.; Bird, J.A.; Spergel, J.M.; et al. Omalizumab for the Treatment of Multiple Food Allergies. N. Engl. J. Med. 2024, 390, 889–899. [Google Scholar]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar]
- Oriel, R.C.; Wang, J. Tolerance thresholds in food allergy: Molecular and clinical perspectives. J. Allergy Clin. Immunol. 2018, 141, 1595–1606. [Google Scholar]
- Cafarotti, A.; Fiocchi, A. Emerging strategies for hypoallergenic food processing. Pediatr. Allergy Immunol. 2023, 34, e13921. [Google Scholar]
- Mori, F.; Pecoraro, L.; Giovannini, M. Allergen identification in pediatric food allergy. Curr. Opin. Allergy Clin. Immunol. 2024, 24, 1–8. [Google Scholar]
- Novembre, E.; Gelsomino, M.; Liotti, L.; Barni, S.; Mori, F.; Giovannini, M.; Mastrorilli, C.; Pecoraro, L.; Saretta, F.; Castagnoli, R.; et al. Fatal food anaphylaxis in adults and children. Ital. J. Pediatr. 2024, 50, 40. [Google Scholar]
- Tomei, S.; Martelli, A.; Galli, E. The allergist’s role in food technology. Clin. Mol. Allergy 2023, 21, 12. [Google Scholar]
- Albuquerque, T.G.; Oliveira, M.B.P.P.; Costa, H.S. Food processing and nutritional quality. Trends Food Sci. Technol. 2022, 120, 1–12. [Google Scholar]
- Stadler, R.H.; Lineback, D.R. Process-Induced Food Toxicants: Occurrence, Formation, Mitigation, and Health Risks; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Knorr, D.; Froehling, A.; Jaeger, H.; Reineke, K.; Schlueter, O.; Schoessler, K. Emerging technologies in food processing. Annu. Rev. Food Sci. Technol. 2011, 2, 203–235. [Google Scholar]
- Koidl, L.; Untersmayr, E. Modulation of food allergenicity by processing. Mol. Nutr. Food Res. 2023, 67, e2200231. [Google Scholar]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency. Compr. Rev. Food Sci. Food Saf. 2018, 17, 309–330. [Google Scholar]
- De Angelis, E.; Pilolli, R.; Monaci, L. Combined autoclaving and enzymatic hydrolysis to reduce allergenicity of food allergens. Food Chem. 2018, 250, 47–55. [Google Scholar]
- Stuckler, D.; Nestle, M. Big food, food systems, and global health. PLoS Med. 2012, 9, e1001242. [Google Scholar]
- Huby, R.D.; Dearman, R.J.; Kimber, I. Why are some proteins allergens? Toxicol. Sci. 2000, 55, 235–246. [Google Scholar]
- Jiménez-Saiz, R.; Benedé, S.; Molina, E.; López-Expósito, I. Effect of processing technologies on the allergenicity of food products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1902–1917. [Google Scholar]
- Mills, E.N.; Sancho, A.I.; Rigby, N.M.; Jenkins, J.A.; Mackie, A.R. Impact of food processing on the structural and allergenic properties of food allergens. Mol. Nutr. Food Res. 2009, 53, 963–969. [Google Scholar]
- Thomas, K.; Herouet-Guicheney, C.; Ladics, G.; Bannon, G.; Cockburn, A.; Crevel, R.; Fitzpatrick, J.; Mills, C.; Privalle, L.; Vieths, S. Evaluating the effect of food processing on the potential human allergenicity of novel proteins: International workshop report. Food Chem. Toxicol. 2007, 45, 1116–1122. [Google Scholar]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S3–S23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eiwegger, T.; Hung, L. IgE-mediated food allergy in children: Cellular and molecular mechanisms. Pediatr. Allergy Immunol. 2019, 30, 263–272. [Google Scholar]
- Bannon, G.A. What makes a food protein an allergen? Curr. Allergy Asthma Rep. 2004, 4, 43–46. [Google Scholar]
- Spolidoro, G.C.; Marseglia, G.L. Prevalence of the “Big 9” food allergens in pediatric populations. Allergy 2023, 78, 789–801. [Google Scholar]
- US Food and Drug Administration. Food Allergies: What You Need to Know. Available online: https://www.fda.gov/food/buy-store-serve-safe-food/food-allergies-what-you-need-know (accessed on 13 November 2024).
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [PubMed]
- Gelsomino, M.; Liotti, L.; Barni, S.; Mori, F.; Giovannini, M.; Mastrorilli, C.; Pecoraro, L.; Saretta, F.; Castagnoli, R.; Arasi, S.; et al. Elimination Diets in Lactating Mothers of Infants with Food Allergy. Nutrients 2024, 16, 2317. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.; Toschi Vespasiani, G.; Ricci, G.; Miniaci, A.; di Palmo, E.; Pession, A. Cow’s Milk Protein Allergy as a Model of Food Allergies. Nutrients 2021, 13, 1525. [Google Scholar] [CrossRef]
- Baker, J.R., Jr.; Gangwar, R.S.; Platts-Mills, T.A. The Processed Milk Hypothesis: A major factor in the development of Eosinophilic Esophagitis (EoE)? J. Allergy Clin. Immunol. 2024, 154, 1123–1126. [Google Scholar]
- Norgaard, A.; Skov, P.S.; Bindslev-Jensen, C. Egg and milk allergy in adults: Comparison between fresh foods and commercial allergen extracts in skin prick test and histamine release from basophils. Clin. Exp. Allergy 1992, 22, 940–947. [Google Scholar]
- Fu, L.; Cherayil, B.J.; Shi, H.; Wang, Y.; Zhu, Y. Food processing to eliminate food allergens and development of hypoallergenic foods. In Food Allergy—From Molecular Mechanisms to Control Strategies; Springer Nature: Singapore, 2019; pp. 123–147. [Google Scholar]
- Martos, G.; Lopez-Exposito, I.; Bencharitiwong, R.; Berin, M.C.; Nowak-Węgrzyn, A. Mechanisms underlying differential food allergy response to heated egg. J. Allergy Clin. Immunol. 2011, 127, 990–997.e1-2. [Google Scholar]
- Alessandri, C.; Sforza, S.; Palazzo, P.; Lambertini, F.; Paolella, S.; Zennaro, D.; Rafaiani, C.; Ferrara, R.; Bernardi, M.L.; Santoro, M.; et al. Tolerability of a fully maturated cheese in cow’s milk allergic children: Biochemical, immunochemical, and clinical aspects. PLoS ONE 2012, 7, e40945. [Google Scholar]
- Miceli Sopo, S.; Greco, M.; Monaco, S.; Bianchi, A.; Cuomo, B.; Liotti, L.; Iacono, I.D. Matrix effect on baked milk tolerance in children with IgE cow milk allergy. Allergol. Immunopathol. 2016, 44, 517–523. [Google Scholar]
- Zenker, H.E.; Wichers, H.J.; Tomassen, M.M.M.; Boeren, S.; De Jong, N.W.; Hettinga, K.A. Peptide release after simulated infant in vitro digestion of dry heated cow’s milk protein and transport of potentially immunoreactive peptides across the Caco-2 cell monolayer. Nutrients 2020, 12, 2483. [Google Scholar] [CrossRef]
- Bavaro, S.L.; De Angelis, E.; Barni, S.; Pilolli, R.; Mori, F.; Novembre, E.M.; Monaci, L. Modulation of Milk Allergenicity by Baking Milk in Foods: A Proteomic Investigation. Nutrients 2019, 11, 1536. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Lawson, K.; Masilamani, M.; Kattan, J.; Bahnson, H.T.; Sampson, H.A. Increased Tolerance to Less Extensively Heat-Denatured (Baked) Milk Products in Milk-Allergic Children. J. Allergy Clin. Immunol. Pract. 2018, 6, 486–495.e5. [Google Scholar]
- Teodorowicz, M.; Zenker, H.E.; Ewaz, A.; Tsallis, T.; Mauser, A.; Gensberger-Reigl, S.; de Jong, N.W.; Hettinga, K.A.; Wichers, H.J.; van Neerven, R.J.J.; et al. Enhanced Uptake of Processed Bovine β-Lactoglobulin by Antigen Presenting Cells: Identification of Receptors and Implications for Allergenicity. Mol. Nutr. Food Res. 2021, 65, e2000834. [Google Scholar]
- Platts-Mills, T.A.E.; Boyd, K.K.; Medernach, J.G. Can we alter the course of allergic disease? Ann. Allergy Asthma Immunol. 2022, 129, 271–273. [Google Scholar]
- Abbring, S.; Kusche, D.; Roos, T.C.; Diks, M.A.P.; Hols, G.; Garssen, J.; Baars, T.; van Esch, B.C.A.M. Milk processing increases the allergenicity of cow’s milk-Preclinical evidence supported by a human proof-of-concept provocation pilot. Clin. Exp. Allergy 2019, 49, 1013–1025. [Google Scholar]
- Liu, X.; Feng, B.S.; Kong, X.; Xu, H.; Li, X.; Yang, P.C.; Liu, Z. Food-cooking processes modulate allergenic properties of hen’s egg white proteins. Int. Arch. Allergy Immunol. 2013, 160, 134–142. [Google Scholar]
- Julia, S.; Sanchez, L.; Perez, M.D.; Lavilla, M.; Conesa, C.; Calvo, M. Effect of heat treatment on hen’s egg ovomucoid: An immunochemical and calorimetric study. Food Res. Int. 2007, 40, 603–612. [Google Scholar]
- Hefle, S.L. Impact of processing on food allergens. Adv. Exp. Med. Biol. 1999, 459, 107–119. [Google Scholar]
- Yamada, A.; Hasegawa, T.; Fujieda, M.; Morita, H.; Matsumoto, K. Protease-digested egg-white products induce oral tolerance in mice but elicit little IgE production upon epicutaneous exposure. Allergol. Int. 2022, 71, 528–535. [Google Scholar]
- Ma, X.; Liang, R.; Xing, Q.; Lozano-Ojalvo, D. Can food processing produce hypoallergenic egg? J. Food Sci. 2020, 85, 2635–2644. [Google Scholar] [PubMed]
- Jiang, S.; Huang, Y.; Tang, X.; Wang, T.; Li, Q.; Wang, H.; Meng, X. Traditional cooking methods decreased the allergenicity of egg proteins. J. Food Sci. 2024, 89, 3847–3857. [Google Scholar] [PubMed]
- Vapor, A.; Mendonça, A.; Tomaz, C.T. Processes for reducing egg allergenicity: Advances and different approaches. Food Chem. 2022, 367, 130568. [Google Scholar]
- Leonard, S.A.; Sampson, H.A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Godbold, J.; Nowak-Węgrzyn, A. Dietary baked egg accelerates resolution of egg allergy in children. J. Allergy Clin. Immunol. 2012, 130, 473–480.e1. [Google Scholar]
- De Vlieger, L.; Nuyttens, L.; Matton, C.; Diels, M.; Verelst, S.; Leus, J.; Coppens, K.; Sauer, K.; Dilissen, E.; Coorevits, L.; et al. Guided Gradual Egg-Tolerance Induction in Hen’s Egg Allergic Children Tolerating Baked Egg: A Prospective Randomized Trial. Front. Allergy 2022, 3, 886094. [Google Scholar]
- Michelfelder, A.J. Soy: A complete source of protein. Am. Fam. Physician 2009, 79, 43–47. [Google Scholar]
- Ebisawa, M.; Brostedt, P.; Sjölander, S.; Sato, S.; Borres, M.P.; Ito, K. Gly m 2S albumin is a major allergen with a high diagnostic value in soybean-allergic children. J. Allergy Clin. Immunol. 2013, 132, 976–978.e1-5. [Google Scholar]
- Kleine-Tebbe, J.; Vogel, L.; Crowell, D.N.; Haustein, U.F.; Vieths, S. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1- related PR-10 protein in soybean, SAM22. J. Allergy Clin. Immunol. 2002, 110, 797–804. [Google Scholar]
- Pecoraro, L.; Giovannini, M.; Mori, F.; Barni, S.; Castagnoli, R.; Arasi, S.; Mastrorilli, C.; Saretta, F.; Liotti, L.; Caminiti, L.; et al. Imported allergens in Italy: An emerging issue. Ital. J. Pediatr. 2024, 50, 36. [Google Scholar]
- Magishi, N.; Yuikawa, N.; Kobayashi, M.; Taniuchi, S. Degradation and removal of soybean allergen in Japanese soy sauce. Mol. Med. Rep. 2017, 16, 2264–2268. [Google Scholar]
- Kobayashi, M. Immunological functions of soy sauce: Hypoallergenicity and antiallergic activity of soy sauce. J. Biosci. Bioeng. 2005, 100, 144–151. [Google Scholar]
- Dramburg, S.; Hilger, C.; Santos, A.F.; de Las Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34 (Suppl. 28), e13854. [Google Scholar]
- Buyuktiryaki, B.; Masini, M.; Mori, F.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Giovannini, M.; du Toit, G.; Lopata, A.L.; et al. IgE-Mediated Fish Allergy in Children. Medicina 2021, 57, 76. [Google Scholar]
- Schrama, D.; Czolk, R.; Raposo de Magalhães, C.; Kuehn, A.; Rodrigues, P.M. Fish Allergenicity Modulation Using Tailored Enriched Diets-Where Are We? Front. Physiol. 2022, 13, 897168. [Google Scholar]
- Carrera, M.; González-Fernández, Á.; Magadán, S.; Mateos, J.; Pedrós, L.; Medina, I.; Gallardo, J.M. Molecular characterization of B-cell epitopes for the major fish allergen, parvalbumin, by shotgun proteomics, protein-based bioinformatics and IgE-reactive approaches. J. Proteom. 2019, 200, 123–133. [Google Scholar]
- Mastrorilli, C.; Arasi, S.; Barni, S.; Caimmi, D.; Chiera, F.; Comberiati, P.; Dinardo, G.; Giannetti, A.; Gismondi, M.; Gracci, S.; et al. IgE-Mediated and Non-IgE-Mediated Fish Allergy in Pediatric Age: A Holistic Approach-A Consensus by Diagnostic Commission of the Italian Society of Pediatric Allergy and Immunology. Medicina 2023, 59, 1651. [Google Scholar]
- Sletten, G.; Van Do, T.; Lindvik, H.; Egaas, E.; Florvaag, E. Effects of industrial processing on the immunogenicity of commonly ingested fish species. Int. Arch. Allergy Immunol. 2010, 151, 223–236. [Google Scholar]
- Pérez-Tavarez, R.; Moreno, H.M.; Borderias, J.; Loli-Ausejo, D.; Pedrosa, M.; Hurtado, J.L.; Rodriguez-Pérez, R.; Gasset, M. Fish muscle processing into seafood products reduces β-parvalbumin allergenicity. Food Chem. 2021, 364, 130308. [Google Scholar] [PubMed]
- Pecoraro, L.; Tenero, L.; Pietrobelli, A.; Dalle Carbonare, L.; Czernin, S.; Widhalm, K.; Alvarez-Perea, A.; Piacentini, G. Canned tuna tolerance in children with IgE-mediated fish allergy: An allergological and nutritional view. Minerva Pediatr. 2020, 72, 408–415. [Google Scholar]
- Pecoraro, L.; Dalle Carbonare, L.; Castagnoli, R.; Marseglia, G.L.; Piacentini, G.; Pietrobelli, A. IgE-mediated fish allergy in children: Is omega-3 supplementation useful? Int. J. Food Sci. Nutr. 2022, 73, 154–157. [Google Scholar]
- Zuidmeer-Jongejan, L.; Huber, H.; Swoboda, I.; Rigby, N.; Versteeg, S.A.; Jensen, B.M.; Quaak, S.; Akkerdaas, J.H.; Blom, L.; Asturias, J.; et al. Development of a hypoallergenic recombinant parvalbumin for first-in-man subcutaneous immunotherapy of fish allergy. Int. Arch. Allergy Immunol. 2015, 166, 41–51. [Google Scholar]
- Giovannini, M.; Beken, B.; Buyuktiryaki, B.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Pontone, M.; Bartha, I.; Mori, F.; et al. IgE-Mediated Shellfish Allergy in Children. Nutrients 2023, 15, 2714. [Google Scholar]
- Gelis, S.; Rueda, M.; Valero, A.; Fernández, E.A.; Moran, M.; Fernández-Caldas, E. Shellfish Allergy: Unmet Needs in Diagnosis and Treatment. J. Investig. Allergol. Clin. Immunol. 2020, 30, 409–420. [Google Scholar]
- Cheng, J.H.; Wang, H.; Sun, D.W. An overview of tropomyosin as an important seafood allergen: Structure, cross-reactivity, epitopes, allergenicity, and processing modifications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 127–147. [Google Scholar]
- Liu, K.; Lin, S.; Gao, X.; Wang, S.; Liu, Y.; Liu, Q.; Sun, N. Reduced Allergenicity of Shrimp (Penaeus vannamei) by Altering the Protein Fold, Digestion Susceptibility, and Allergen Epitopes. J. Agric. Food Chem. 2023, 71, 9120–9134. [Google Scholar]
- Dong, X.; Wang, J.; Raghavan, V. Impact of microwave processing on the secondary structure, in-vitro protein digestibility and allergenicity of shrimp (Litopenaeus vannamei) proteins. Food Chem. 2021, 337, 127811. [Google Scholar]
- Ozawa, H.; Yamamura, A.; Kimijima, T.; Ishizaki, S.; Ochiai, Y. Elimination of the major allergen tropomyosin from shrimp muscle by boiling treatment. Fish. Sci. 2020, 86, 197–202. [Google Scholar]
- Naqpal, S.; Rajappa, L.; Metcalfe, D.D.; Rao, P.V. Isolation and characterization of heat-stable allergens from shrimp (Penaeus indicus). J. Allergy Clin. Immunol. 1989, 83, 26–36. [Google Scholar] [PubMed]
- Zhao, J.; Timira, V.; Ahmed, I.; Chen, Y.; Wang, H.; Zhang, Z.; Lin, H.; Li, Z. Crustacean shellfish allergens: Influence of food processing and their detection strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 3794–3822. [Google Scholar]
- Zhang, J.; Liu, W.; Zhang, R.; Zhao, X.; Fang, L.; Qin, X.; Gu, R.; Lu, J.; Li, G. Hypoallergenic mutants of the major oyster allergen Cra g 1 alleviate oyster tropomyosin allergenic potency. Int. J. Biol. Macromol. 2020, 164, 1973–1983. [Google Scholar]
- Li, M.S.; Xia, F.; Liu, Q.M.; Chen, Y.Y.; Yun, X.; Liu, M.; Chen, G.X.; Wang, L.; Cao, M.J.; Liu, G.M. Hypoallergenic derivatives of Scylla paramamosain heat-stable allergens alleviated food allergy symptoms in Balb/c mice. Food Funct. 2022, 13, 11518–11531. [Google Scholar]
- Sabença, C.; Ribeiro, M.; Sousa, T.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Wheat/Gluten-Related Disorders and Gluten-Free Diet Misconceptions: A Review. Foods 2021, 10, 1765. [Google Scholar]
- Fingerle, M.; Salaorni, S.; Pietrobelli, A.; Piacentini, G.; Banzato, C.; Pecoraro, L. Wheat-Related Disorders in Children: A 360-Degree View. Children 2024, 11, 707. [Google Scholar] [CrossRef]
- Morita, E.; Matsuo, H.; Kohno, K.; Yokooji, T.; Yano, H.; Endo, T. A Narrative Mini Review on Current Status of Hypoallergenic Wheat Development for IgE-Mediated Wheat Allergy, Wheat-Dependent Exercise-Induced Anaphylaxis. Foods 2023, 12, 954. [Google Scholar]
- Buchanan, B.B.; Adamidi, C.; Lozano, R.M.; Yee, B.C.; Momma, M.; Kobrehel, K.; Ermel, R.; Frick, O.L. Thioredoxin-linked mitigation of allergic responses to wheat. Proc. Natl. Acad. Sci. USA 1997, 94, 5372–5377. [Google Scholar]
- Yamada, Y.; Yokooji, T.; Kunimoto, K.; Inoguchi, K.; Ogino, R.; Taogoshi, T.; Morita, E.; Matsuo, H. Hypoallergenic Wheat Line (1BS-18H) Lacking ω5-Gliadin Induces Oral Tolerance to Wheat Gluten Proteins in a Rat Model of Wheat Allergy. Foods 2022, 11, 2181. [Google Scholar]
- Liu, M.; Dai, S.; Yin, L.; Huang, Z.; Jia, X. Wheat gluten deamidation: Structure, allergenicity and its application in hypoallergenic noodles. J. Sci. Food Agric. 2024, 104, 2477–2483. [Google Scholar]
- Xia, T.; Kim, K.; Kweon, M. Quality of Low-Allergy Wheat (‘O-Free’) Flour and Optimization of Its Bread-Baking Performance. Foods 2022, 11, 3399. [Google Scholar]
- Matsumoto, T.; Miyazaki, T. Systemic urticaria in an infant after ingestion of processing food that contained a trace quantity of wheat. Ann. Allergy Asthma Immunol. 2004, 93, 98–100. [Google Scholar]
- Babaie, D.; Ebisawa, M.; Soheili, H.; Ghasemi, R.; Zandieh, F.; Sahragard, M.; Seifi, H.; Fallahi, M.; Khoshmirsafa, M.; Darougar, S.; et al. Oral Wheat Immunotherapy: Long-Term Follow-Up in Children with Wheat Anaphylaxis. Int. Arch. Allergy Immunol. 2022, 183, 306–314. [Google Scholar]
- Palladino, C.; Breiteneder, H. Peanut allergens. Mol. Immunol. 2018, 100, 58–70. [Google Scholar]
- Prodic, I.; Stanic-Vucinic, D.; Apostolovic, D.; Mihailovic, J.; Radibratovic, M.; Radosavljevic, J.; Burazer, L.; Milcic, M.; Smiljanic, K.; van Hage, M.; et al. Influence of peanut matrix on stability of allergens in gastric-simulated digesta: 2S albumins are main contributors to the IgE reactivity of short digestion-resistant peptides. Clin. Exp. Allergy 2018, 48, 731–740. [Google Scholar]
- de Jongh, H.H.J.; de Jong, G.A.H.; Apostolovic, D.; Taylor, S.L.; Baumert, J.L.; Koppelman, S.J. Effect of heat treatment on the conformational stability of intact and cleaved forms ofthe peanut allergen Ara h 6 in relation to its IgE-binding potency. Food Chem. 2020, 326, 127027. [Google Scholar]
- Luo, C.; Hu, C.; Gao, J.; Li, X.; Wu, Z.; Yang, A.; Chen, H. A potential practical approach to reduce Ara h 6 allergenicity by gamma irradiation. Food Chem. 2013, 136, 1141–1147. [Google Scholar]
- Wang, M.; Gelfand, E.W. Targeting Pim1 kinase in the treatment of peanut allergy. Expert. Opin. Ther. Targets 2014, 18, 177–183. [Google Scholar] [CrossRef]
- Hourihane, J.O.; Bedwani, S.J.; Dean, T.P.; Warner, J.O. Randomised, double blind, crossover challenge study of allergenicity of peanut oils in subjects allergic to peanuts. BMJ 1997, 14, 1084–1088. [Google Scholar]
- Keating, M.U.; Jones, R.T.; Worley, N.J.; Shively, C.A.; Yunginger, J.W. Immunoassay of peanut allergens in food-processing materials and finished foods. J. Allergy Clin. Immunol. 1990, 86, 41–44. [Google Scholar]
- Turner, P.J.; Mehr, S.; Sayers, R.; Wong, M.; Shamji, M.H.; Campbell, D.E.; Mills, E.N. Loss of allergenic proteins during boiling explains tolerance to boiled peanut in peanut allergy. J. Allergy Clin. Immunol. 2014, 134, 751–753. [Google Scholar]
- Mondoulet, L.; Paty, E.; Drumare, M.F.; Ah-Leung, S.; Scheinmann, P.; Willemot, R.M.; Wal, J.M.; Bernard, H. Influence of thermal processing on the allergenicity of peanut proteins. J. Agric. Food Chem. 2005, 53, 4547–4553. [Google Scholar]
- Immormino, R.M.; Smeekens, J.M.; Mathai, P.I.; Kesselring, J.R.; Turner, A.V.; Kulis, M.D.; Moran, T.P. Peanut butter feeding induces oral tolerance in genetically diverse collaborative cross mice. Front. Allergy 2023, 4, 1219268. [Google Scholar]
- PALISADE. Group of Clinical Investigators. AR101 Oral Immunotherapy for Peanut Allergy. N. Engl. J. Med. 2018, 379, 1991–2001. [Google Scholar]
- Jones, S.M.; Kim, E.H.; Nadeau, K.C.; Nowak-Wegrzyn, A.; Wood, R.A.; Sampson, H.A.; Scurlock, A.M.; Chinthrajah, S.; Wang, J.; Pesek, R.D.; et al. Efficacy and safety of oral immunotherapy in children aged 1-3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): A randomised placebo-controlled study. Lancet 2022, 399, 359–371. [Google Scholar]
- Foti Randazzese, S.; Panasiti, I.; Caminiti, L.; Catamerò, F.; Landi, M.; De Filippo, M.; Votto, M.; Olcese, R.; Favuzza, F.; Giovannini, M.; et al. Current state and advances in desensitization for peanut allergy in pediatric age. Pediatr. Allergy Immunol. 2024, 35, e14127. [Google Scholar]
- Zeng, J.; Ma, F.; Zhai, L.; Du, C.; Zhao, J.; Li, Z.; Wang, J. Recent advance in sesame allergens: Influence of food processing and their detection methods. Food Chem. 2024, 448, 139058. [Google Scholar]
- Tagliati, S.; Barni, S.; Giovannini, M.; Liccioli, G.; Sarti, L.; Alicandro, T.; Paladini, E.; Perferi, G.; Azzari, C.; Novembre, E.; et al. Nut Allergy: Clinical and Allergological Features in Italian Children. Nutrients 2021, 13, 4076. [Google Scholar] [CrossRef]
- Geroldinger-Simic, M.; Zelniker, T.; Aberer, W.; Ebner, C.; Egger, C.; Greiderer, A.; Prem, N.; Lidholm, J.; Ballmer-Weber, B.K.; Vieths, S.; et al. Birch pollen-related food allergy: Clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J. Allergy Clin. Immunol. 2011, 127, 616–622.e1. [Google Scholar]
- Giovannini, M.; Skypala, I.J.; Caubet, J.C.; Du Toit, G.; Nowak-Wegrzyn, A. Diagnosis and Management of Pollen Food Allergy Syndrome to Nuts. J. Allergy Clin. Immunol. Pract. 2024, 12, 599–604. [Google Scholar]
- Hansen, K.S.; Ballmer-Weber, B.K.; Lüttkopf, D.; Skov, P.S.; Wüthrich, B.; Bindslev-Jensen, C.; Vieths, S.; Poulsen, L.K. Roasted hazelnuts--allergenic activity evaluated by double-blind, placebo-controlled food challenge. Allergy 2003, 58, 132–138. [Google Scholar]
- Worm, M.; Hompes, S.; Fiedler, E.M.; Illner, A.K.; Zuberbier, T.; Vieths, S. Impact of native, heat-processed and encapsulated hazelnuts on the allergic response in hazelnut-allergic patients. Clin. Exp. Allergy 2009, 39, 159–166. [Google Scholar]
- Cabanillas, B.; Pedrosa, M.M.; Rodríguez, J.; Muzquiz, M.; Maleki, S.J.; Cuadrado, C.; Burbano, C.; Crespo, J.F. Influence of enzymatic hydrolysis on the allergenicity of roasted peanut protein extract. Int. Arch. Allergy Immunol. 2012, 157, 41–50. [Google Scholar]
- Huang, J.; Puglisi, L.H.; Cook, K.A.; Kelso, J.M.; Wangberg, H. Safety and Feasibility of Peanut, Tree Nut, and Sesame Oral Immunotherapy in Infants and Toddlers in a Real-World Setting. J. Allergy Clin. Immunol. Pract. 2024, 13, 185–191.e3. [Google Scholar]
- Penumarti, A.; Szczepanski, N.; Kesselring, J.; Gabel, E.; Sheth, R.; Berglund, J.; Kim, E.H.; Burks, A.W.; Kulis, M.D. Irradiated Tree Nut Flours for Use in Oral Immunotherapy. J. Allergy Clin. Immunol. Pract. 2021, 9, 321–327. [Google Scholar]
- Oriel, R.C.; Elizur, A.; Sicherer, S.H. Comprehensive Diagnosis, Management, and Treatment of Sesame Allergy. J. Allergy Clin. Immunol. Pract. 2024, 12, 590–597. [Google Scholar]
- Ocak, M.; Sahiner, U.M.; Soyer, O.; Sekerel, B.E. The role of diagnostic tests and oral food challenge results to predict sesame allergy. Ann. Allergy Asthma Immunol. 2022, 128, 46–52.e1. [Google Scholar]
- Nachshon, L.; Westerhout, J.; Blom, W.M.; Remington, B.; Levy, M.B.; Goldberg, M.R.; Epstein-Rigbi, N.; Katz, Y.; Elizur, A. Sesame eliciting and safe doses in a large sesame allergic population. Allergy 2023, 78, 3212–3220. [Google Scholar]
- Zielinska, J.; Zagórska, W.; Krupa-Łaska, A.; Łyżwa, K.; Lewandowski, Z.; Kulus, M.; Grzela, K. Efficacy and safety of low-dose sesame oral immunotherapy in paediatric patients: A protocol for a single-centre, randomised controlled trial. BMJ Open 2024, 14, e085811. [Google Scholar]
- Chua, G.T.; Soller, L.; Kapur, S.; McHenry, M.; Rex, G.A.; Cook, V.E.; Cameron, S.B.; Chan, E.S.; Yeung, J.; Erdle, S.C. Real-world safety and effectiveness analysis of low-dose preschool sesame oral immunotherapy. J. Allergy Clin. Immunol. Glob. 2023, 3, 100171. [Google Scholar]
- Kaman, K.; Factor, J.M. A practical focus on sesame allergy and a brief review of other seed allergies. J. Food Allergy 2022, 4, 151–157. [Google Scholar]
- Pang, L.; Chen, C.; Liu, M.; Huang, Z.; Zhang, W.; Shi, J.; Yang, X.; Jiang, Y. A comprehensive review of effects of ultrasound pretreatment on processing technologies for food allergens: Allergenicity, nutritional value, and technofunctional properties and safety assessment. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70100. [Google Scholar]
- Kosti, R.I.; Triga, M.; Tsabouri, S.; Priftis, K.N. Food allergen selective thermal processing regimens may change oral tolerance in infancy. Allergol. Immunopathol. 2013, 41, 407–417. [Google Scholar]
- Eigenmann, P.A.; Ebisawa, M.; Greenhawt, M.; Hourihane, J.O.; Perry, T.T.; Remington, B.C.; Wood, R.A. Addressing risk management difficulties in children with food allergies. Pediatr. Allergy Immunol. 2021, 32, 658–666. [Google Scholar]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar]
- Koosakulchai, V.; Park, S.; Ban, M.; Fusayasu, N.; Yanagida, N.; Sato, S.; Ebisawa, M. Safe consumption of processing foods after negative medium-dose cow’s milk oral food challenge. Allergol. Int. 2024, 73, 335–337. [Google Scholar]
- Monaco, S.; Russo, G.; Romano, A.; Liotti, L.; Verga, M.C.; Miceli Sopo, S. Yogurt is tolerated by the majority of children with IgE-mediated cow’s milk allergy. Allergol. Immunopathol. 2019, 47, 322–327. [Google Scholar]
- Miceli Sopo, S.; Greco, M.; Cuomo, B.; Bianchi, A.; Liotti, L.; Monaco, S.; Dello Iacono, I. Matrix effect on baked egg tolerance in children with IgE-mediated hen’s egg allergy. Pediatr. Allergy Immunol. 2016, 27, 465–470. [Google Scholar]
- Saifi, M.; Swamy, N.; Crain, M.; Brown, L.S.; Bird, J.A. Tolerance of a high-protein baked-egg product in egg-allergic children. Ann. Allergy Asthma Immunol. 2016, 116, 415–419. [Google Scholar]
- Pecoraro, L.; Infante, S.; Fuentes-Aparicio, V.; Cabrera-Freitag, P.; Antonucci, N.; Alvarez-Perea, A. IgE-mediated fish allergy in pediatric age: Does canned tuna have a chance for tolerance? Pediatr. Allergy Immunol. 2021, 32, 1114–1117. [Google Scholar]
- Fiocchi, A.; Bouygue, G.R.; Sarratud, T.; Terracciano, L.; Martelli, A.; Restani, P. Clinical tolerance of processing foods. Ann. Allergy Asthma Immunol. 2004, 93 (Suppl. 3), S38–S46. [Google Scholar] [PubMed]
- Upton, J.E.M.; Correa, N.; Eiwegger, T. Oral immunotherapy for food allergy: What’s age got to do with it? Allergy 2023, 78, 626–628. [Google Scholar]
- Loke, P.; Vickery, B.P.; Jones, S.M.; Peters, R.L.; Roberts, G.; Koplin, J.J. Food Allergen Immunotherapy in Preschool Children: Do We Have the Evidence? J. Allergy Clin. Immunol. Pract. 2023, 11, 1028–1035. [Google Scholar] [PubMed]
- Hubbard, S. Nutrition and food allergies: The dietitian’s role. Ann. Allergy Asthma Immunol. 2003, 90 (Suppl. 3), 115–116. [Google Scholar] [PubMed]
- Pecoraro, L.; Mastrorilli, C.; Arasi, S.; Barni, S.; Caimmi, D.; Chiera, F.; Dinardo, G.; Gracci, S.; Miraglia Del Giudice, M.; Bernardini, R.; et al. Nutritional and Psychosocial Impact of Food Allergy in Pediatric Age. Life 2024, 14, 695. [Google Scholar] [CrossRef]
| Big 9—Foods | Potentially Less Allergenic Processing Method | Demonstrated Tolerance to Processed Forms | Potential Use in Oral Immunotherapy |
|---|---|---|---|
| Cow’s milk | Sterilization, denaturation, non-enzymatic glycation, boiling, fermentation, and baking | Boiled milk, yoghurt, and baked milk | Baked milk |
| Hen’s egg | Boiling, baking, frying, and steaming | Cooked hen’s egg and baked hen’s egg | Baked hen’s eggs |
| Soy | Fermentation | Soy sauce | This statement is not demonstrated |
| Fish | Heating, boiling, steaming, and freezing | Surimi, canned fish | This statement is not demonstrated |
| Shellfish | Heat in combination with reverse-pressure sterilization, microwaving | This statement is not demonstrated | This statement is not demonstrated |
| Wheat | Acid-hydrolysis, fermentation, irradiation, deamidation of wheat gliadin, and thioredoxin process | "ω-5 gliadin-free" wheat flour, “O-free” wheat flour | This statement is not demonstrated |
| Peanut | Boiling, boiling in combination with autoclave, irradiation, fermentation, and protease hydrolysis | Raffinate peanut oil and boiled peanut | This statement is not demonstrated |
| Tree nuts | Boiling and irradiation | Roasted hazelnut (only in patients with cosensitization to birch) | This statement is not demonstrated |
| Sesame | High-pressure processing and fermentation | This statement is not demonstrated | Tahini |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecoraro, L.; Barni, S.; Mori, F.; Giovannini, M.; Castagnoli, R.; Arasi, S.; Mastrorilli, C.; Saretta, F.; Liotti, L.; Caminiti, L.; et al. Main Processed Hypoallergenic Foods: A Potential Tool to Improve Informed Dietary Choices in Children with IgE-Mediated Food Allergies. Children 2025, 12, 915. https://doi.org/10.3390/children12070915
Pecoraro L, Barni S, Mori F, Giovannini M, Castagnoli R, Arasi S, Mastrorilli C, Saretta F, Liotti L, Caminiti L, et al. Main Processed Hypoallergenic Foods: A Potential Tool to Improve Informed Dietary Choices in Children with IgE-Mediated Food Allergies. Children. 2025; 12(7):915. https://doi.org/10.3390/children12070915
Chicago/Turabian StylePecoraro, Luca, Simona Barni, Francesca Mori, Mattia Giovannini, Riccardo Castagnoli, Stefania Arasi, Carla Mastrorilli, Francesca Saretta, Lucia Liotti, Lucia Caminiti, and et al. 2025. "Main Processed Hypoallergenic Foods: A Potential Tool to Improve Informed Dietary Choices in Children with IgE-Mediated Food Allergies" Children 12, no. 7: 915. https://doi.org/10.3390/children12070915
APA StylePecoraro, L., Barni, S., Mori, F., Giovannini, M., Castagnoli, R., Arasi, S., Mastrorilli, C., Saretta, F., Liotti, L., Caminiti, L., Klain, A., Gelsomino, M., Miraglia Del Giudice, M., Marseglia, G. L., & Novembre, E. (2025). Main Processed Hypoallergenic Foods: A Potential Tool to Improve Informed Dietary Choices in Children with IgE-Mediated Food Allergies. Children, 12(7), 915. https://doi.org/10.3390/children12070915







