Reconceptualizing Pediatric Strabismus as a Condition Rooted in Sensory Processing Disorder: A Novel Case-Based Hypothesis
Abstract
1. Introduction
2. Case Report
2.1. Patient Description
2.2. Clinical Evaluations
2.3. Differential Diagnosis
3. Discussion
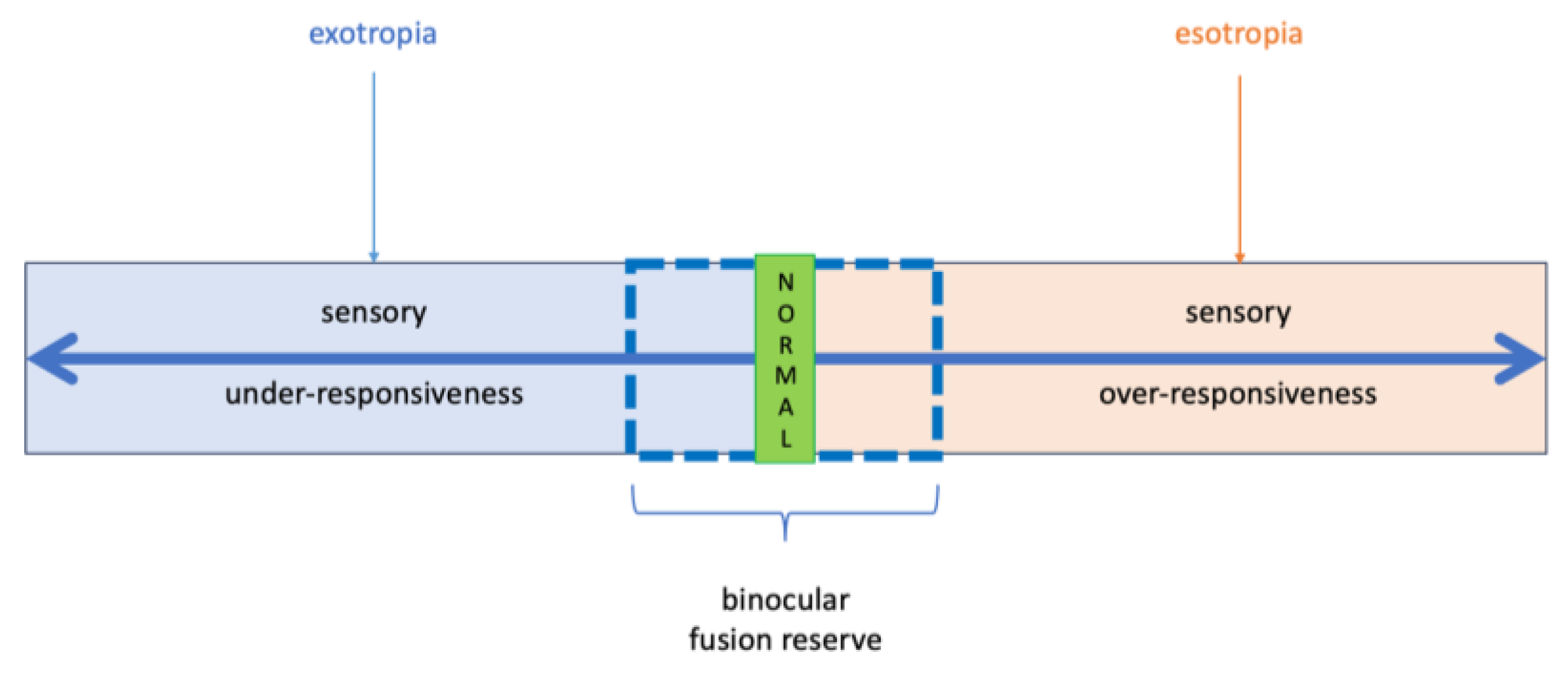
3.1. Strabismus Reconsidered: A Sensory Processing-Based Framework for Fusion Dysregulation
3.2. Limitations
3.3. Future Direction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanukollu, V.M.; Sood, G. Strabismus. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560782/ (accessed on 8 June 2025).
- Hashemi, H.; Pakzad, R.; Heydarian, S.; Yekta, A.; Aghamirsalim, M.; Shokrollahzadeh, F.; Khoshhal, F.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and regional prevalence of strabismus: A comprehensive systematic review and meta-analysis. Strabismus 2019, 27, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Adinanto, F.C.; French, A.N.; Rose, K.A. The prevalence of strabismus: A systematic literature review. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2457. [Google Scholar]
- Zhang, X.J.; Lau, Y.H.; Wang, Y.M.; Kam, K.W.; Ip, P.; Yip, W.W.; Ko, S.T.; Young, A.L.; Tham, C.C.; Pang, C.P.; et al. Prevalence of strabismus and its risk factors among school aged children: The Hong Kong Children Eye Study. Sci. Rep. 2021, 11, 13820. [Google Scholar] [CrossRef]
- Qanat, A.S.; Alsuheili, A.; Alzahrani, A.M.; Faydhi, A.A.; Albadri, A.; Alhibshi, N. Assessment of Different Types of Strabismus Among Pediatric Patients in a Tertiary Hospital in Jeddah. Cureus 2020, 12, e11978. [Google Scholar] [CrossRef]
- Peng, J.; Yao, F.; Li, Q.; Ge, Q.; Shi, W.; Su, T.; Tang, L.; Pan, Y.; Liang, R.; Zhang, L.; et al. Alternations of interhemispheric functional connectivity in children with strabismus and amblyopia: A resting-state fMRI study. Sci. Rep. 2021, 11, 15059. [Google Scholar] [CrossRef]
- Passarello, N.; Tarantino, V.; Chirico, A.; Menghini, D.; Costanzo, F.; Sorrentino, P.; Fucà, E.; Gigliotta, O.; Alivernini, F.; Oliveri, M.; et al. Sensory Processing Disorders in Children and Adolescents: Taking Stock of Assessment and Novel Therapeutic Tools. Brain. Sci. 2022, 12, 1478. [Google Scholar] [CrossRef]
- Ayres, A.J. Sensory Integration and Learning Disorders; Western Psychological Services: Los Angeles, CA, USA, 1972. [Google Scholar]
- Miller, L.J. Sensational Kids Hope and Help for Children with Sensory Processing Disorder; Perigee: New York, NY, USA, 2006. [Google Scholar]
- American Psychiatric Associtation. Diagnostic and Statistical Manual of Mental Disorders Text Revision (DSM-5-TR™), 5th ed.; American Psychiatric Associtation: Washington, DC, USA, 2024. [Google Scholar]
- International Classification of Diseases (ICD-11) 11th Revision. Available online: https://icd.who.int/en/ (accessed on 10 June 2025).
- Section on Complementary and Integrative Medicine; Council on Children with Disabilities; American Academy of Pediatrics; Zimmer, M.; Desch, L. Sensory integration therapies for children with developmental and behavioral disorders. Pediatrics 2012, 129, 1186–1189. [Google Scholar] [PubMed]
- Mezer, E.; Wygnanski-Jaffe, T. Do children and adolescents with attention deficit hyperactivity disorder have ocular abnormalities? Eur. J. Ophthalmol. 2012, 22, 931–935. [Google Scholar] [CrossRef]
- Wilmer, J.B.; Buchanan, G.M. Synergies between neuroscience and visual perception. Curr. Dir. Psychol. Sci. 2009, 18, 389–393. [Google Scholar]
- Owen, J.P.; Marco, E.J.; Desai, S.; Fourie, E.; Harris, J.; Hill, S.S.; Arnett, A.B.; Mukherjee, P. Abnormal white matter microstructure in children with sensory processing disorders. Neuroimage. Clin. 2013, 2, 844–853. [Google Scholar] [CrossRef]
- McArthur, A. L-H. The Debate Over Sensory Processing Disorder. Am. J. Psychiatry. Resid. J. 2022, 17, 14–15. [Google Scholar] [CrossRef]
- Choi, W.J.; Jang, Y.; Kim, S.J.; Jung, J.H. Investigation of transient eye closure evoked with bright light in the patients with intermittent exotropia. BMC Ophthalmol. 2021, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.C.; Cipolli, C. Binocularity and photophobia in intermittent exotropia. Percept. Mot. Skills 1992, 74, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept Evolution in Sensory Integration: A Proposed Nosology for Diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef]
- Walker, K.; Redman-Bentley, D.; Remick-Waltman, K.; Armstrong, D.C. Differences in Oculomotor Function between Children with Sensory Processing Disorder and Typical Development. Optom. Vis. Sci. 2019, 96, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Quick, M.W.; Tigges, M.; Gammon, J.A.; Boothe, R.G. Early abnormal visual experience induces strabismus in infant monkeys. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1012–1017. [Google Scholar]
- Kinard, K.; Miller, N.R.; Digre, K.B.; Katz, B.J.; Crum, A.V.; Warner, J.E. Blepharospasm in children and adolescents. Childs. Nerv. Syst. 2016, 32, 355–358. [Google Scholar] [CrossRef]
- Jethani, J. Marin-Amat syndrome: A rare facial synkinesis. Indian J. Ophthalmol. 2007, 55, 402–403. [Google Scholar] [CrossRef]
- Jung, H.Y.; Chung, S.J.; Hwang, J.M. Tic disorders in children with frequent eye blinking. J. AAPOS 2004, 8, 171–174. [Google Scholar] [CrossRef]
- Johnson, K.A.; Worbe, Y.; Foote, K.D.; Butson, C.R.; Gunduz, A.; Okun, M.S. Tourette syndrome: Clinical features, pathophysiology, and treatment. Lancet. Neurol. 2023, 22, 147–158. [Google Scholar] [CrossRef]
- Brodsky, M.C. The Halpern syndrome of monocular visual vertigo: A rare cause of monocular eye closure. Strabismus. 2011, 19, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, O.; Burgu, B.; Demirel, F.; Soygur, T.; Ozcakar, Z.B.; Yalcinkaya, F.; Tekgul, S. Ochoa syndrome: A spectrum of urofacial syndrome. Eur. J. Pediatr. 2010, 169, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Granet, D.B.; Gomi, C.F.; Ventura, R.; Miller-Scholte, A. The relationship between convergence insufficiency and ADHD. Strabismus 2005, 13, 163–168. [Google Scholar] [CrossRef]
- Li, X.; Fan, F.; Chen, X.; Li, J.; Ning, L.; Lin, K.; Chen, Z.; Qin, Z.; Yeung, A.S.; Li, X.; et al. Computer Vision for Brain Disorders Based Primarily on Ocular Responses. Front. Neurol. 2021, 12, 584270. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. Calming effects of deep touch pressure in patients with autistic disorder, college students, and animals. J. Child. Adolesc. Psychopharmacol. 1992, 2, 63–72. [Google Scholar] [CrossRef]
- Schaaf, R.C.; Nightlinger, K.M. Occupational therapy using a sensory integrative approach: A case study of effectiveness. Am. J. Occup. Ther. 2007, 61, 239–246. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Santoni, J.L.M.; Merino, P.; de Liaño, P.G. Ophthalmologic Manifestations in Autism Spectrum Disorder. Turk. J. Ophthalmol. 2022, 52, 246–251. [Google Scholar] [CrossRef]
- Etičko Povjerenstvo KBSD-Upute za Slanje Prijava. Available online: https://www.kbsd.hr/media/2021/10/Eticko-povjerenstvo-upute-i-naputci.pdf (accessed on 27 June 2025).


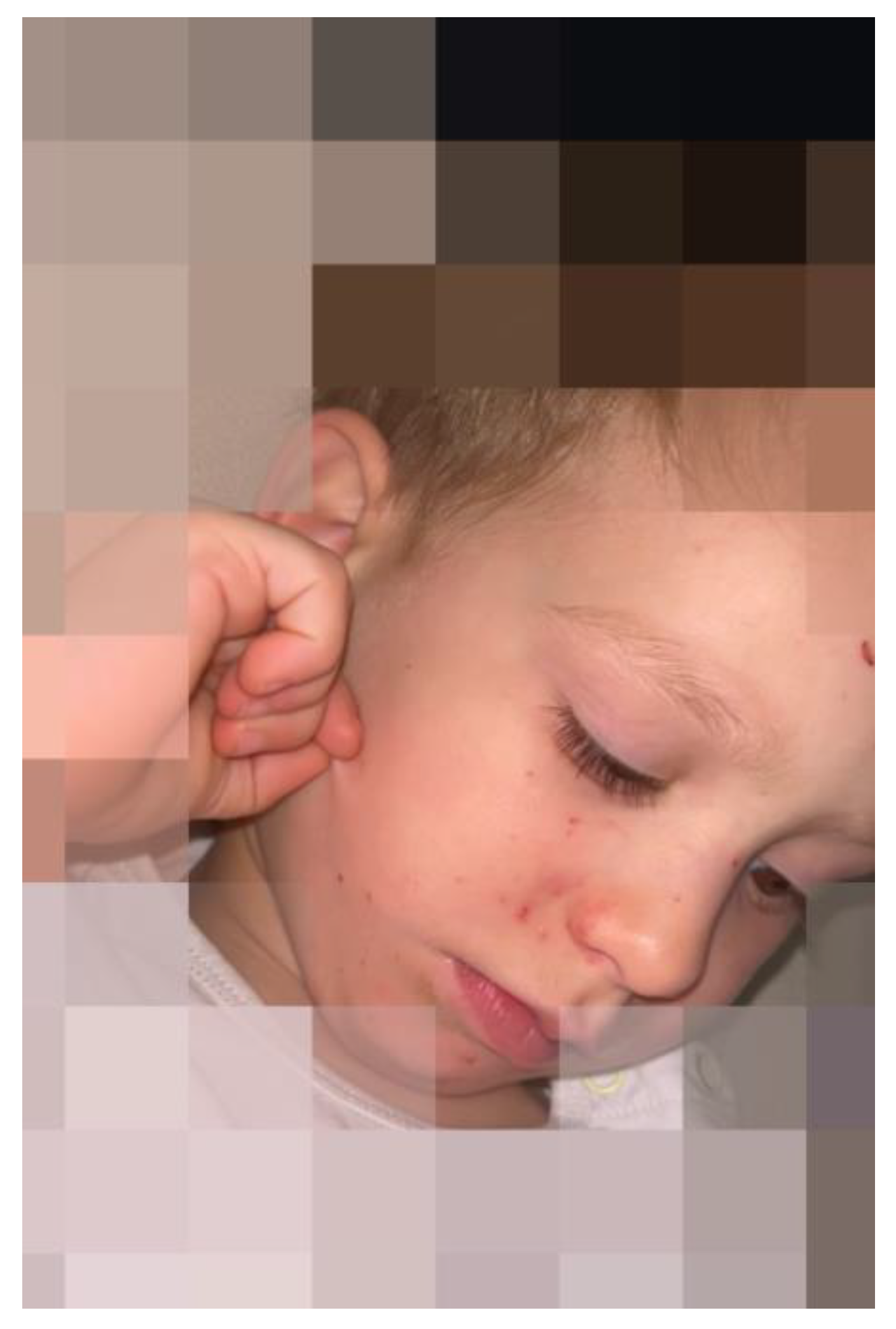
| Age | Sensory Triggers | Observed Responses | Developmental Context |
|---|---|---|---|
| 2 months | Breastfeeding Camera flash | Tight right-eye closure; Facial grimacing | Reflexive avoidance of multisensory input due to limited motor control; Grimacing as a primitive motor strategy to reinforce eye closure in the absence of voluntary self-regulation |
| 3–4 months | Camera flash | Eye poking | Emergence of hand coordination; Early attempt at self-directed monocular eye closure |
| 8–10 months | Bright sunlight Camera flash Strong breeze Air puff Spoon feeding | Frequent monocular/binocular eye blinking | Growing visual awareness; Beginning intentional avoidance responses; Voluntary control required for monocular winking is not yet present |
| 1–2 years | Moderate light or wind, especially during teething pain | Prolonged monocular closure (sometimes alternating); Eye/face rubbing | Improved fine motor skills allow for tactile self-regulation; Lower sensory threshold noted during teething |
| Falling snowflakes | Eye closure Head turning away Covering face with hands Mental distress | Increased sensory-motor integration; Distress triggered by combined visual, tactile and vibratory stimuli | |
| 2–3 years | Vibrations from digger | Eye closure | Increased sensory-motor integration; Distress triggered by combined visual, tactile and vibratory stimuli, attempts to physically reject stimulus |
| Bright light, wind, digital screens | Requests sunglasses; covers eye with hand/fist; Limits screen time; Rubs eyes/forehead; Pulls hair; face pinching shields face in wind | Developed cognitive awareness of triggers; Initiates preventive and self-regulatory behaviors (e.g., requesting sunglasses, discontinuing screen use); Combines visual avoidance with proprioceptive strategies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjeloš, M.; Ćurić, A.; Bušić, M.; Rončević, K.; Elabjer, A. Reconceptualizing Pediatric Strabismus as a Condition Rooted in Sensory Processing Disorder: A Novel Case-Based Hypothesis. Children 2025, 12, 904. https://doi.org/10.3390/children12070904
Bjeloš M, Ćurić A, Bušić M, Rončević K, Elabjer A. Reconceptualizing Pediatric Strabismus as a Condition Rooted in Sensory Processing Disorder: A Novel Case-Based Hypothesis. Children. 2025; 12(7):904. https://doi.org/10.3390/children12070904
Chicago/Turabian StyleBjeloš, Mirjana, Ana Ćurić, Mladen Bušić, Katja Rončević, and Adrian Elabjer. 2025. "Reconceptualizing Pediatric Strabismus as a Condition Rooted in Sensory Processing Disorder: A Novel Case-Based Hypothesis" Children 12, no. 7: 904. https://doi.org/10.3390/children12070904
APA StyleBjeloš, M., Ćurić, A., Bušić, M., Rončević, K., & Elabjer, A. (2025). Reconceptualizing Pediatric Strabismus as a Condition Rooted in Sensory Processing Disorder: A Novel Case-Based Hypothesis. Children, 12(7), 904. https://doi.org/10.3390/children12070904






