Clinical and Behavioral Correlates of Blood Acylcarnitine Profiles in Children with Autism Spectrum Disorder: A Cross-Sectional Analysis
Abstract
1. Introduction
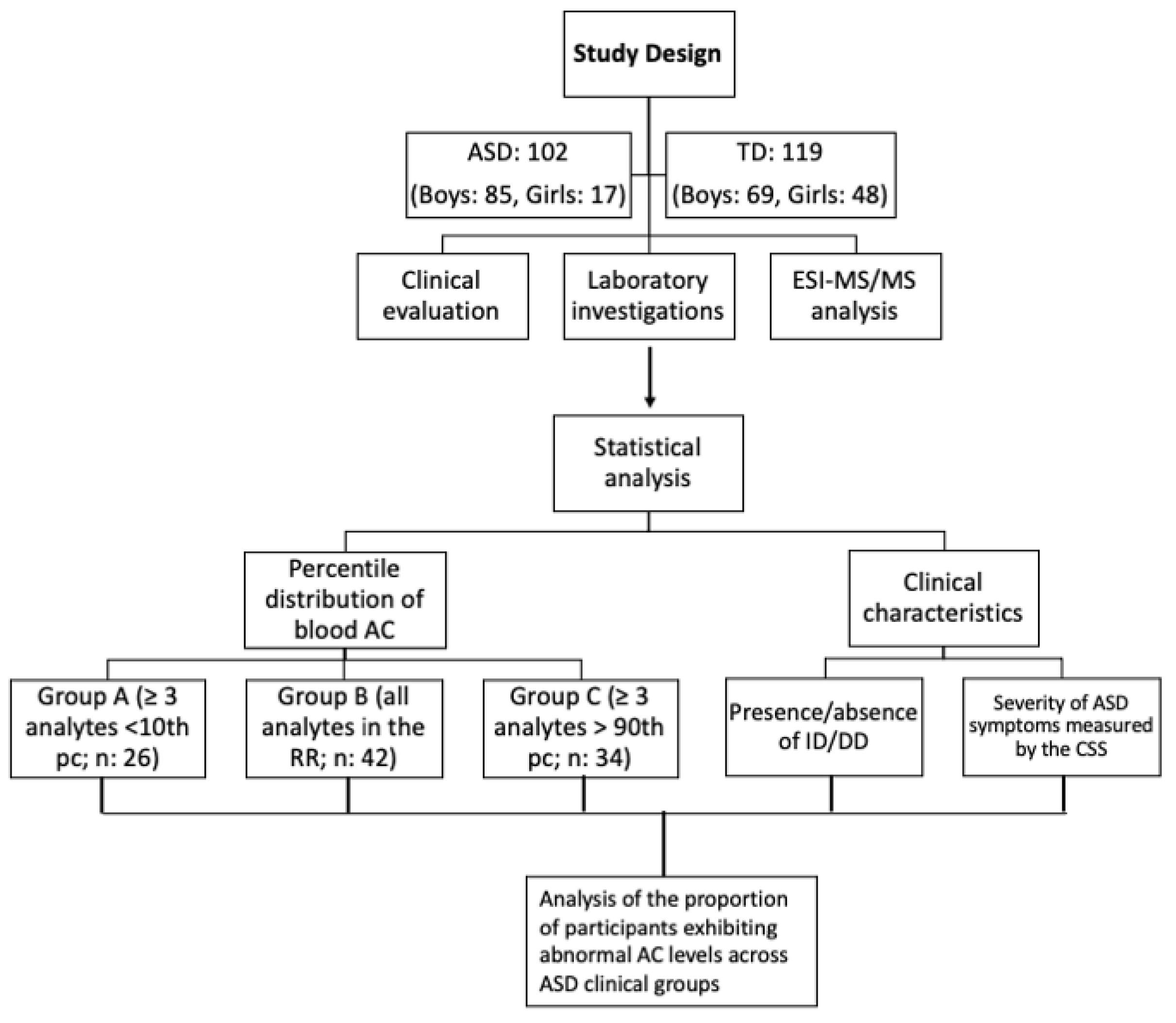
2. Materials and Methods
2.1. Participants
2.2. Sample Collections and ESI-MS/MS Analysis
2.3. Statistical Analysis
3. Results
3.1. Blood AC Levels in ASD and TD Participants
3.2. Clinical Characteristics
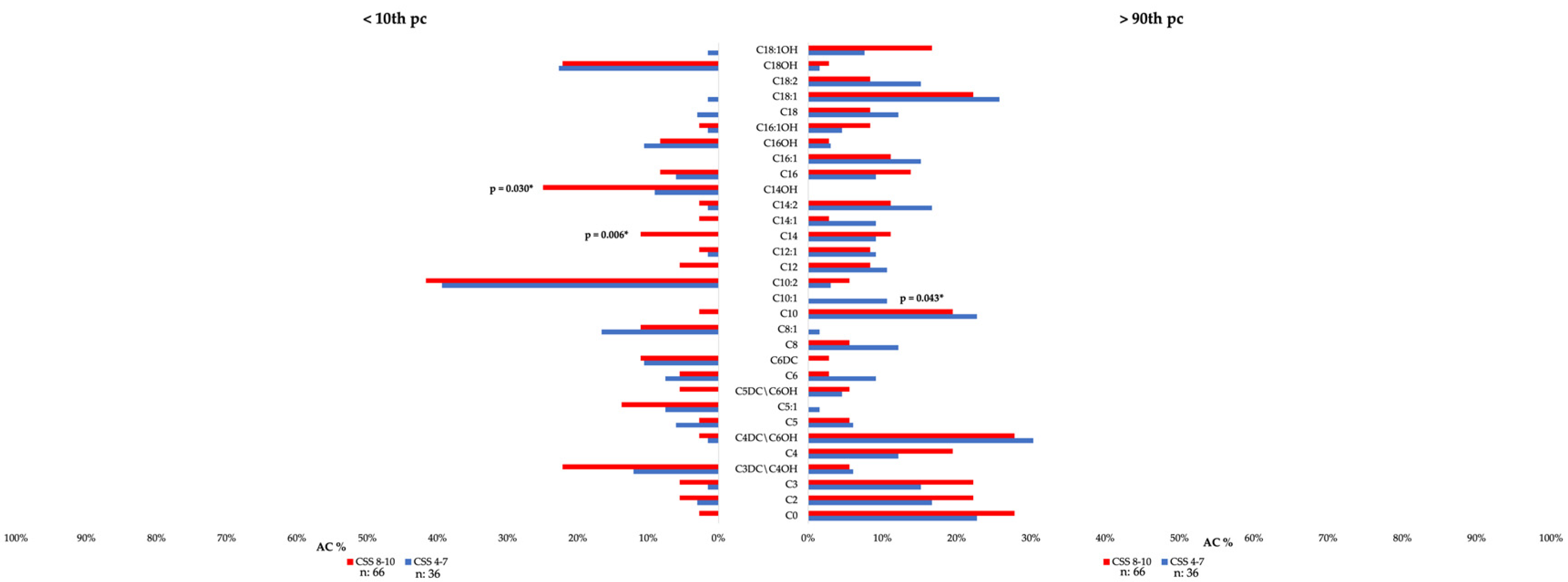
3.3. Clinical Correlates of Individual Blood AC in Patients with ASD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020; Morbidity and Mortality Weekly Report; Surveillance Summaries: Washington, DC, USA, 2023; Volume 72, pp. 1–14. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018; Morbidity and Mortality Weekly Report; Surveillance Summaries: Washington, DC, USA, 2021; Volume 70, pp. 1–16. [Google Scholar] [CrossRef]
- Soke, G.N.; Maenner, M.J.; Christensen, D.; Kurzius-Spencer, M.; Schieve, L.A. Prevalence of Co-occurring Medical and Behavioral Conditions/Symptoms Among 4- and 8-Year-Old Children with Autism Spectrum Disorder in Selected Areas of the United States in 2010. J. Autism Dev. Disord. 2018, 48, 2663–2676. [Google Scholar] [CrossRef]
- Bougeard, C.; Picarel-Blanchot, F.; Schmid, R.; Campbell, R.; Buitelaar, J. Prevalence of Autism Spectrum Disorder and Co-morbidities in Children and Adolescents: A Systematic Literature Review. Front. Psychiatry 2021, 12, 744709. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rincon, N.; McCarty, P.J.; Brister, D.; Scheck, A.C.; Rossignol, D.A. Biomarkers of mitochondrial dysfunction in autism spectrum disorder: A systematic review and meta-analysis. Neurobiol. Dis. 2024, 197, 106520. [Google Scholar] [CrossRef]
- Qu, W.; Yan, G.; Du, Y.; Zhou, X.; Huang, C.; Li, B.; Zhou, J.; Li, Q. Crosstalk Between Mitochondrial DNA and Immune Response: Focus on Autism Spectrum Disorder. Mol. Neurobiol. 2025, 62, 5629–5639. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Napoli, E.; Wong, S.; Hertz-Picciotto, I.; Giulivi, C. Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics 2014, 133, e1405–e1410. [Google Scholar] [CrossRef]
- Frye, R.E.; Naviaux, R.K. Autistic disorder with complex IV overactivity: A new mitochondrial syndrome. J. Pediatr. Neurol. 2011, 9, 427–434. [Google Scholar] [CrossRef]
- Barone, R.; Alaimo, S.; Messina, M.; Pulvirenti, A.; Bastin, J.; MIMIC-Autism Group; Ferro, A.; Frye, R.E.; Rizzo, R. A Subset of Patients with Autism Spectrum Disorders Show a Distinctive Metabolic Profile by Dried Blood Spot Analyses. Front. Psychiatry 2018, 9, 636. [Google Scholar] [CrossRef]
- Frye, R.E. Biomarkers of Abnormal Energy Metabolism in Children with Autism Spectrum Disorder. N. Am. J. Med. Sci. 2012, 5. Available online: https://najms.com/index.php/najms/article/view/305 (accessed on 25 June 2024). [CrossRef]
- Frye, R.E.; Melnyk, S.; Macfabe, D.F. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl. Psychiatry 2013, 3, e220. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Lionnard, L.; Singh, I.; Karim, M.A.; Chajra, H.; Frechet, M.; Kissa, K.; Racine, V.; Ammanamanchi, A.; McCarty, P.J.; et al. Mitochondrial morphology is associated with respiratory chain uncoupling in autism spectrum disorder. Transl. Psychiatry 2021, 11, 527. [Google Scholar] [CrossRef]
- Barone, R.; Bastin, J.; Djouadi, F.; Singh, I.; Karim, M.A.; Ammanamanchi, A.; McCarty, P.J.; Delhey, L.; Shannon, R.; Casabona, A.; et al. Mitochondrial Fatty Acid β-Oxidation and Resveratrol Effect in Fibroblasts from Patients with Autism Spectrum Disorder. J. Pers. Med. 2021, 11, 510. [Google Scholar] [CrossRef]
- Kępka, A.; Ochocińska, A.; Chojnowska, S.; Borzym-Kluczyk, M.; Skorupa, E.; Knaś, M.; Waszkiewicz, N. Potential Role of L-Carnitine in Autism Spectrum Disorder. J. Clin. Med. 2021, 10, 1202. [Google Scholar] [CrossRef]
- Esposito, C.M.; Buoli, M.; Ciappolino, V.; Agostoni, C.; Brambilla, P. The Role of Cholesterol and Fatty Acids in the Etiology and Diagnosis of Autism Spectrum Disorders. Int. J. Mol. Sci. 2021, 22, 3550. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S.L.; Luyster, R.J.; Guthrie, W. Autism Diagnostic Observation Schedule, 2nd ed.; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Green, E.; Stroud, L.; Bloomfield, S.; Cronje, J.; Foxcroft, C.; Hurter, K.; Lane, H.; Marais, R.; Marx, C.; McAlinden, P.; et al. Griffiths Scales of Child Development, 3rd ed.; Part II: Administration and Scoring; Hogrefe: Oxford, UK, 2016. [Google Scholar]
- Stroud, L.; Foxcroft, C.; Green, E.; Bloomfield, S.; Cronje, J.; Hurter, K.; Lane, H.; Marais, R.; Marx, C.; McAlinden, P.; et al. Griffiths Scales of Child Development, 3rd ed.; Part I: Overview, Development and Psychometric Properties; Hogrefe: Oxford, UK, 2016. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; (WISC-IV); The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Gotham, K.; Pickles, A.; Lord, C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 693–705. [Google Scholar] [CrossRef]
- Klei, L.; Sanders, S.J.; Murtha, M.T.; Hus, V.; Lowe, J.K.; Willsey, A.J.; Moreno-De-Luca, D.; Yu, T.W.; Fombonne, E.; Geschwind, D.; et al. Common genetic variants, acting additively, are a major source of risk for autism. Mol. Autism 2012, 3, 9. [Google Scholar] [CrossRef]
- Cirnigliaro, L.; Valle, M.S.; Casabona, A.; Randazzo, M.; La Bruna, F.; Pettinato, F.; Narzisi, A.; Rizzo, R.; Barone, R. The Developmental Autism Early Screening (DAES): A Novel Test for Screening Autism Spectrum Disorder. J. Autism Dev. Disord. 2025, 55, 221–236. [Google Scholar] [CrossRef]
- Lv, Q.Q.; You, C.; Zou, X.B.; Deng, H.Z. Acyl-carnitine, C5DC, and C26 as potential biomarkers for diagnosis of autism spectrum disorder in children. Psychiatry Res. 2018, 267, 277–280. [Google Scholar] [CrossRef]
- Ahrens, A.P.; Hyötyläinen, T.; Petrone, J.R.; Igelström, K.; George, C.D.; Garrett, T.J.; Orešič, M.; Triplett, E.W.; Ludvigsson, J. Infant microbes and metabolites point to childhood neurodevelopmental disorders. Cell 2024, 187, 1853–1873.e15. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Donley, E.L.R.; Ney, D.M.; Amaral, D.G.; Burrier, R.E.; Natowicz, M.R. Metabolomic biomarkers in autism: Identification of complex dysregulations of cellular bioenergetics. Front. Psychiatry 2023, 14, 1249578. [Google Scholar] [CrossRef]
- Nickel, K.; Menke, M.; Endres, D.; Runge, K.; Tucci, S.; Schumann, A.; Domschke, K.; Tebartz van Elst, L.; Maier, S. Altered markers of mitochondrial function in adults with autism spectrum disorder. Autism Res. Off. J. Int. Soc. Autism Res. 2023, 16, 2125–2138. [Google Scholar] [CrossRef]
- Lee, I.H.; Koelliker, E.; Kong, S.W. Quantitative trait locus analysis for endophenotypes reveals genetic substrates of core symptom domains and neurocognitive function in autism spectrum disorder. Transl. Psychiatry 2022, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.D.; Kanabus, M.; Anderson, G.; Hargreaves, I.P.; Rutherford, T.; O’Donnell, M.; Cross, J.H.; Rahman, S.; Eaton, S.; Heales, S.J. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J. Neurochem. 2014, 129, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, N.E.; Orford, M.; Vivekananda, U.; Simpson, Z.; Van de Bor, B.; Smith, H.; Balestrini, S.; Rutherford, T.; Brennan, E.; McKenna, J.; et al. K. Vita: A feasibility study of a blend of medium chain triglycerides to manage drug-resistant epilepsy. Brain Commun. 2021, 3, fcab160. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.S.; Yeo, W.S. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina 2019, 55, 129. [Google Scholar] [CrossRef]
- Barone, R.; Rizzo, R.; Tabbì, G.; Malaguarnera, M.; Frye, R.E.; Bastin, J. Nuclear Peroxisome Proliferator-Activated Receptors (PPARs) as Therapeutic Targets of Resveratrol for Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 1878. [Google Scholar] [CrossRef]
- Steinberg, S.J.; Dodt, G.; Raymond, G.V.; Braverman, N.E.; Moser, A.B.; Moser, H.W. Peroxisome biogenesis disorders. Biochim. Biophys. Acta 2006, 1763, 1733–1748. [Google Scholar] [CrossRef]
- Waterham, H.R.; Ferdinandusse, S.; Wanders, R.J. Human disorders of peroxisome metabolism and biogenesis. Biochim. Biophys. Acta 2016, 1863, 922–933. [Google Scholar] [CrossRef]
- Lismont, C.; Nordgren, M.; Van Veldhoven, P.P.; Fransen, M. Redox interplay between mitochondria and peroxisomes. Front. Cell Dev. Biol. 2015, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Khaliulin, I.; Hamoudi, W.; Amal, H. The multifaceted role of mitochondria in autism spectrum disorder. Mol. Psychiatry 2025, 30, 629–650. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Yau, C.E.; Yaow, C.Y.L.; Chong, R.I.H.; Chong, N.Z.; Teoh, S.E.; Lim, Y.L.; Soh, A.Y.S.; Ng, W.K.; Thumboo, J. What Has Longitudinal ‘Omics’ Studies Taught Us about Irritable Bowel Syndrome? A Systematic Review. Metabolites 2023, 13, 484. [Google Scholar] [CrossRef] [PubMed]
| ASD Group N (%) | A (n: 26) | B (n: 42) | C (n: 34) | p-Value |
|---|---|---|---|---|
| Age at diagnosis | 3.4 ± 1.0 | 3.4 ± 0.9 | 3.3 ± 0.9 | 0.812 |
| Age at sample collection | 7 ± 4.2 | 6.2 ± 3.3 | 5.5 ± 3.0 | 0.474 |
| Sex (Male) | 20 (76.9%) | 35 (83.3%) | 29 (85.3%) | 0.684 |
| Non-verbal ASD | 2 (7.7%) | 3 (7.1%) | 7 (20.6%) | 0.147 |
| Microcephaly | 2 (7.7%) | 5 (11.9%) | 3 (8.8%) | 0.828 |
| Macrocephaly | 2 (7.7%) | 8 (19.05%) | 3 (8.8%) | 0.277 |
| Regression | 10 (38.5%) | 7 (16.7%) | 8 (23.5%) | 0.126 |
| IQ/DQ | 62.3 ± 13.8 | 60.5 ± 18.4 | 60.8 ± 16.7 | 0.278 |
| ID | 15 (57.7%) | 31 (73.8%) | 23 (67.6%) | 0.385 |
| EEG anomalies | 3 (11.5%) | 3 (7.1%) | 4 (11.8%) | 0.751 |
| Epilepsy | 0 (0%) | 1 (2.4%) | 0 (0%) | 0.486 |
| N (%) | ASD Group A (n = 26) | ASD Group C (n = 34) | p-Value | p (Yates’ Correction) |
|---|---|---|---|---|
| C0 | 1 (3.8%) | 17 (50%) | 0.0001 * | 0.0003 * |
| C2 | 4 (15.4%) | 14 (41.2%) | 0.0307 | 0.0606 |
| C3 | 3 (11.5%) | 16 (47.0%) | 0.003 * | 0.008 * |
| C3DC\C4OH | 9 (34.6%) | 5 (14.7%) | 0.071 | 0.134 |
| C4 | 0 (0%) | 13 (38.2%) | 0.0004* | 0.001 * |
| C4DC\C5OH | 2 (7.7%) | 15 (44.1%) | 0.0019* | 0.005 * |
| C5 | 4 (15.4%) | 3 (8.8%) | 0.433 | 0.705 |
| C5:1 | 7 (26.9%) | 1 (2.9%) | 0.007* | 0.02 * |
| C5DC\C6OH | 6 (23.1%) | 3 (8.8%) | 0.125 | 0.243 |
| C6 | 7 (26.9%) | 7 (20.5%) | 0.565 | 0.79 |
| C6DC | 3 (11.5%) | 1 (2.9%) | 0.186 | .423 |
| C8 | 0 (0%) | 9 (26.5%) | 0.004* | 0.013 * |
| C8:1 | 4 (15.4%) | 1 (2.9%) | 0.084 | 0.2087 |
| C10 | 1 (3.8%) | 18 (52.9%) | 0.00005* | 0.0002 * |
| C10:1 | 0 (0%) | 6 (17.6%) | 0.024* | 0.068 |
| C10:2 | 18 (69.2%) | 4 (11.8%) | <0.0001 * | <0.0001 |
| C12 | 2 (7.7%) | 9 (26.5%) | 0.062 | 0.127 |
| C12:1 | 2 (7.7%) | 6 (17.6%) | 0.261 | 0.459 |
| C14 | 2 (7.7%) | 5 (14.7%) | 0.402 | 0.665 |
| C14:1 | 1 (3.8%) | 6 (17.6%) | 0.0989 | 0.213 |
| C14:2 | 2 (7.7%) | 11 (32.35%) | 0.0216 * | 0.0475 * |
| C14OH | 11 (42.3%) | 0 (0%) | <0.0001 * | <0.0001 * |
| C16 | 6 (23.1%) | 10 (29.4%) | 0.583 | 0.799 |
| C16:1 | 0 (0%) | 13 (38.2%) | 0.0004 * | 0.001 * |
| C16OH | 9 (34.6%) | 3 (8.8%) | 0.013 * | 0.03 * |
| C16:1OH | 1 (3.8%) | 3 (8.8%) | 0.444 | 0.808 |
| C18 | 1 (3.8%) | 8 (23.5%) | 0.034* | 0.0799 |
| C18:1 | 0 (0%) | 19 (55.9%) | <0.0001 * | <0.0001 * |
| C18:2 | 0 (0%) | 10 (29.4%) | 0.002 * | 0.007 * |
| C18OH | 14 (53.8%) | 2 (5.9%) | <0.0001 * | <0.0001 * |
| C18:1OH | 1 (3.8%) | 8 (23.5%) | 0.034* | 0.0799 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prato, A.; Randazzo, M.; Messina, M.A.; Puglisi, G.; Aleo, L.R.; Ciantia, F.; Cirnigliaro, L.; Rizzo, R.; Barone, R. Clinical and Behavioral Correlates of Blood Acylcarnitine Profiles in Children with Autism Spectrum Disorder: A Cross-Sectional Analysis. Children 2025, 12, 848. https://doi.org/10.3390/children12070848
Prato A, Randazzo M, Messina MA, Puglisi G, Aleo LR, Ciantia F, Cirnigliaro L, Rizzo R, Barone R. Clinical and Behavioral Correlates of Blood Acylcarnitine Profiles in Children with Autism Spectrum Disorder: A Cross-Sectional Analysis. Children. 2025; 12(7):848. https://doi.org/10.3390/children12070848
Chicago/Turabian StylePrato, Adriana, Martina Randazzo, Maria Anna Messina, Giovanni Puglisi, Laura Rosy Aleo, Fiorella Ciantia, Lara Cirnigliaro, Renata Rizzo, and Rita Barone. 2025. "Clinical and Behavioral Correlates of Blood Acylcarnitine Profiles in Children with Autism Spectrum Disorder: A Cross-Sectional Analysis" Children 12, no. 7: 848. https://doi.org/10.3390/children12070848
APA StylePrato, A., Randazzo, M., Messina, M. A., Puglisi, G., Aleo, L. R., Ciantia, F., Cirnigliaro, L., Rizzo, R., & Barone, R. (2025). Clinical and Behavioral Correlates of Blood Acylcarnitine Profiles in Children with Autism Spectrum Disorder: A Cross-Sectional Analysis. Children, 12(7), 848. https://doi.org/10.3390/children12070848






