Histological Grade, Tumor Breadth, and Hypertension Predict Early Recurrence in Pediatric Sarcoma: A LASSO-Regularized Micro-Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Dataset
2.2. Data Cleaning and Encoding
- Missing-value handling:
- ○
- Numeric features: median imputation.
- ○
- Categorical features: mode imputation.
- Feature encoding:
- ○
- Numeric variables were z-standardized (μ = 0, σ = 1).
- ○
- Categorical variables underwent one-hot encoding with drop_first = True; unseen levels were handled via handle_unknown = ‘ignore’.
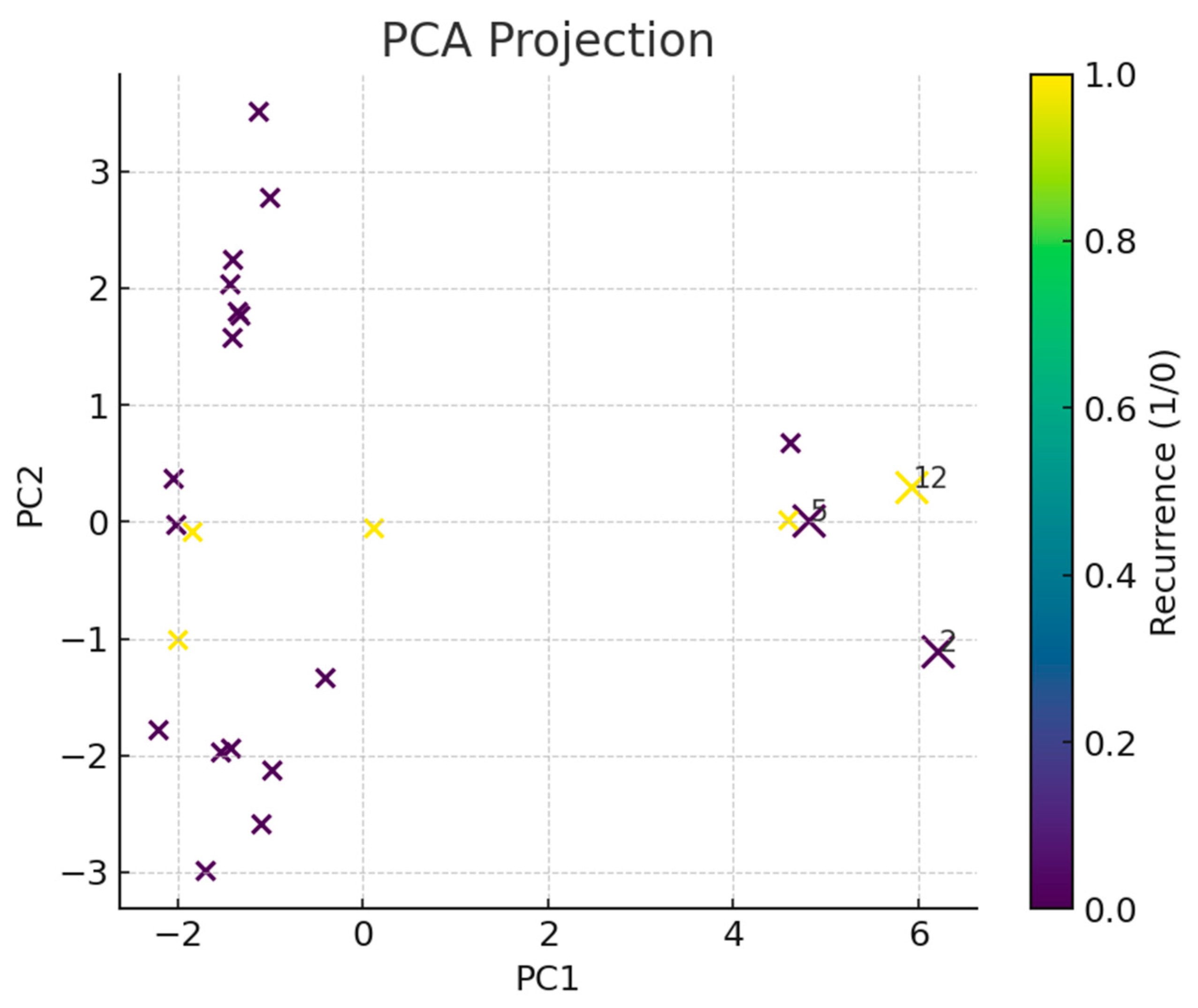
2.3. Dimensionality Reduction and Outlier Detection
2.4. Predictive Modeling
2.5. Variable Importance
2.6. Software and Reproducibility
3. Results
Predictive Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Test | Groups | Χ2 (df) | p-Value | Interpretation |
|---|---|---|---|---|
| Hosmer–Lemeshow | four equal-sized risk quartiles | 1.9 (2) | 0.39 | No evidence of lack-of-fit |
| Predictor | LASSO OR | Firth OR | Concordance |
|---|---|---|---|
| Histological Grade | 2.18 | 2.25 | X |
| Tumor Width | 2.04 | 2.10 | X |
| Arterial Hypertension | 1.70 | 1.72 | X |
| Extremity | 1.90 | 1.88 | X |
References
- Zahm, S.H.; Fraumeni, J.F., Jr. The epidemiology of soft tissue sarcoma. Semin. Oncol. 1997, 24, 504–514. [Google Scholar]
- Linabery, A.M.; Ross, J.A. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer 2008, 113, 2575–2596. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The epidemiology of sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Loeb, D.M.; Thornton, K.; Shokek, O. Pediatric soft tissue sarcomas. Surg. Clin. N. Am. 2008, 88, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Zagars, G.K.; Ballo, M.T.; Pisters, P.W.T.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S.; Evans, H.L. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 2003, 97, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Fernebro, J.; Bladström, A.; Rydholm, A.; Gustafson, P.; Olsson, H.; Engellau, J.; Nilbert, M. Increased risk of malignancies in a population-based study of 818 soft-tissue sarcoma patients. Br. J. Cancer 2006, 95, 986–990. [Google Scholar] [CrossRef]
- Soole, F.; Maupain, C.; Defachelles, A.-S.; Taque, S.; Minard-Colin, V.; Bergeron, C.; De Rycke, Y.; Orbach, D. Synovial sarcoma relapses in children and adolescents: Prognostic factors, treatment, and outcome. Pediatr. Blood Cancer 2014, 61, 1387–1393. [Google Scholar] [CrossRef]
- Garcia-Ortega, D.Y. Comprehensive treatment strategy for improving surgical resection rate of retroperitoneal sarcomas: A histology-specific approach narrative review. Front. Oncol. 2024, 14, 1432900. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Mendez-Guerra, C.; Goh, M.H.; Pretell-Mazzini, J. Principles of Surgical Treatment of Soft Tissue Sarcomas. Cancers 2025, 17, 401. [Google Scholar] [CrossRef]
- Bickels, J.; Malawer, M.M. Adult Soft-Tissue Sarcomas of the Extremities. J. Bone Jt. Surg. Am. 2022, 104, 379–389. [Google Scholar] [CrossRef]
- Harati, K.; Goertz, O.; Pieper, A.; Daigeler, A.; Joneidi-Jafari, H.; Niggemann, H.; Stricker, I.; Lehnhardt, M. Soft Tissue Sarcomas of the Extremities: Surgical Margins Can Be Close as Long as the Resected Tumor Has No Ink on It. Oncologist 2017, 22, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Sangkhathat, S. Current management of pediatric soft tissue sarcomas. World J. Clin. Pediatr. 2015, 4, 94–105. [Google Scholar] [CrossRef]
- Dracham, C.B.; Shankar, A.; Madan, R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 2018, 36, 85–94. [Google Scholar] [CrossRef]
- Smolle, M.A.; Andreou, D.; Tunn, P.-U.; Szkandera, J.; Liegl-Atzwanger, B.; Leithner, A. Diagnosis and treatment of soft-tissue sarcomas of the extremities and trunk. EFORT Open Rev. 2017, 2, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, C.; Springfield, D.S.; Marcus, K.J.; Perez-Atayde, A.R.; Gebhardt, M.C. Factors predicting local recurrence, metastasis, and survival in pediatric soft tissue sarcoma in extremities. Clin. Orthop. Relat. Res. 2010, 468, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Weskamp, P.; Ufton, D.; Drysch, M.; Wagner, J.M.; Dadras, M.; Lehnhardt, M.; Behr, B.; Wallner, C. Risk Factors for Occurrence and Relapse of Soft Tissue Sarcoma. Cancers 2022, 14, 1273. [Google Scholar] [CrossRef]
- Kammer, M.; Dunkler, D.; Michiels, S.; Heinze, G. Evaluating methods for Lasso selective inference in biomedical research: A comparative simulation study. BMC Med. Res. Methodol. 2022, 22, 206. [Google Scholar] [CrossRef]
- Byeon, H.; Gc, P.; Hannan, S.A.; Alghayadh, F.Y.; Soomar, A.M.; Soni, M.; Bhatt, M.W. Deep neural network model for enhancing disease prediction using auto encoder based broad learning. SLAS Technol. 2024, 29, 100145. [Google Scholar] [CrossRef]
- Placido, D.; Yuan, B.; Hjaltelin, J.X.; Zheng, C.; Haue, A.D.; Chmura, P.J.; Yuan, C.; Kim, J.; Umeton, R.; Antell, G.; et al. A deep learning algorithm to predict risk of pancreatic cancer from disease trajectories. Nat. Med. 2023, 29, 1113–1122. [Google Scholar] [CrossRef]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep learning for healthcare: Review, opportunities and challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef]
- Pavlou, M.; Ambler, G.; Seaman, S.; De Iorio, M.; Omar, R.Z. Review and evaluation of penalised regression methods for risk prediction in low-dimensional data with few events. Stat. Med. 2016, 35, 1159–1177. [Google Scholar] [CrossRef] [PubMed]
- Cahlon, O.; Brennan, M.F.; Jia, X.; Qin, L.-X.; Singer, S.; Alektiar, K.M. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann. Surg. 2012, 255, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.A. Soft tissue sarcomas of the extremities. Bayl. Univ. Med. Cent. Proc. 2003, 16, 285–290. [Google Scholar] [CrossRef]
- Lou, S.; Balluff, B.; de Graaff, M.A.; Cleven, A.H.; de Bruijn, I.B.; Bovée, J.V.; McDonnell, L.A. High-grade sarcoma diagnosis and prognosis: Biomarker discovery by mass spectrometry imaging. Proteomics 2016, 16, 1802–1813. [Google Scholar] [CrossRef]
- Neuville, A.; Chibon, F.; Coindre, J.-M. Grading of soft tissue sarcomas: From histological to molecular assessment. Pathology 2014, 46, 113–120. [Google Scholar] [CrossRef]
- Young, R.J.; Litière, S.; Lia, M.; Hogendoorn, P.C.W.; Fisher, C.; Mechtersheimer, G.; Daugaard, S.; Sciot, R.; Collin, F.; Messiou, C.; et al. Predictive and prognostic factors associated with soft tissue sarcoma response to chemotherapy: A subgroup analysis of the European Organisation for Research and Treatment of Cancer 62012 study. Acta Oncol. 2017, 56, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Leidinger, B.; Heyse, T.; Schuck, A.; Buerger, H.; Mommsen, P.; Bruening, T.; Fuchs, S.; Gosheger, G. High incidence of metastatic disease in primary high grade and large extremity soft tissue sarcomas treated without chemotherapy. BMC Cancer 2006, 6, 160. [Google Scholar] [CrossRef]
- Grimer, R.J. Size matters for sarcomas! Ann. R. Coll. Surg. Engl. 2006, 88, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.; Judson, I.; Peake, D.; Seddon, B. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010, 2010, 506182. [Google Scholar] [CrossRef]
- Voss, R.K.; Callegaro, D.; Chiang, Y.-J.; Fiore, M.; Miceli, R.; Keung, E.Z.; Feig, B.W.; Torres, K.E.; Scally, C.P.; Hunt, K.K.; et al. Sarculator is a Good Model to Predict Survival in Resected Extremity and Trunk Sarcomas in US Patients. Ann. Surg. Oncol. 2022, 29, 4376–4385. [Google Scholar] [CrossRef]
- Coindre, J.-M.; Terrier, P.; Guillou, L.; Le Doussal, V.; Collin, F.; Ranchère, D.; Sastre, X.; Vilain, M.-O.; Bonichon, F.; N’Guyen Bui, B. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas. Cancer 2001, 91, 1914–1926. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.-J.; Lin, Y.-F.; Chen, J.-S. Newly developed hypertension as an early marker of recurrence of adrenocortical carcinoma with high renin expression. Int. J. Urol. 2008, 15, 540–542. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2022, 13, 1098725. [Google Scholar] [CrossRef] [PubMed]
- Solak, Y.; Afsar, B.; Vaziri, N.D.; Aslan, G.; Yalcin, C.E.; Covic, A.; Kanbay, M. Hypertension as an autoimmune and inflammatory disease. Hypertens. Res. 2016, 39, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Al-Saleem, T.; Brooks, J.J.; Rogatko, A.; Kraybill, W.G.; Eisenberg, B. Vascular endothelial growth factor and soft tissue sarcomas: Tumor expression correlates with grade. Ann. Surg. Oncol. 2001, 8, 260–267. [Google Scholar] [CrossRef]
- Lahat, G.; Lazar, A.; Wang, X.; Wang, W.-L.; Zhu, Q.-S.; Hunt, K.K.; Pollock, R.E.; Lev, D. Increased vascular endothelial growth factor-C expression is insufficient to induce lymphatic metastasis in human soft-tissue sarcomas. Clin. Cancer Res. 2009, 15, 2637–2646. [Google Scholar] [CrossRef]
- Belaidi, E.; Joyeux-Faure, M.; Ribuot, C.; Launois, S.H.; Levy, P.; Godin-Ribuot, D. Major Role for Hypoxia Inducible Factor-1 and the Endothelin System in Promoting Myocardial Infarction and Hypertension in an Animal Model of Obstructive Sleep Apnea. J. Am. Coll. Cardiol. 2009, 53, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Eisinger-Mathason, T.K.; Zhang, M.; Qiu, Q.; Skuli, N.; Nakazawa, M.S.; Karakasheva, T.; Mucaj, V.; Shay, J.E.; Stangenberg, L.; Sadri, N.; et al. Hypoxia-Dependent Modification of Collagen Networks Promotes Sarcoma Metastasis. Cancer Discov. 2013, 3, 1190–1205. [Google Scholar] [CrossRef]
- Díaz Casas, S.E.; Villacrés, J.M.; Lehmann Mosquera, C.; García Mora, M.; Mariño Lozano, I.; Ángel Aristizábal, J.; Suarez Rodríguez, R.; Duarte Torres, C.A.; Sánchez Pedraza, R. Prognostic Factors Associated with Tumor Recurrence and Overall Survival in Soft Tissue Sarcomas of the Extremities in a Colombian Reference Cancer Center. Curr. Oncol. 2024, 31, 1725–1738. [Google Scholar] [CrossRef]
- Renn, A.; Adejolu, M.; Messiou, C.; Bhaludin, B.; Strauss, D.; Thway, K.; Moskovic, E. Overview of malignant soft-tissue sarcomas of the limbs. Clin. Radiol. 2021, 76, e1–e940. [Google Scholar] [CrossRef]
- Abbatucci, J.; Boulier, N.; de Ranieri, J.; Mandard, A.; Tanguy, A.; Vernhes, J.; Lozier, J.; Busson, A. Local control and survival in soft tissue sarcomas of the limbs, trunk walls and head and neck: A study of 113 cases. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.J.; Huemann, Z.; Hu, J.; Rahmim, A. A Guide to Cross-Validation for Artificial Intelligence in Medical Imaging. Radiol. Artif. Intell. 2023, 5, e220232. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Holmes, S. Ten quick tips for effective dimensionality reduction. PLoS Comput. Biol. 2019, 15, e1006907. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R.; Cohen, J.B. White Coat Hypertension & Cardiovascular Outcomes. Curr. Hypertens. Rep. 2024, 26, 399–407. [Google Scholar] [CrossRef]
- Saccò, M.; Meschi, M.; Regolisti, G.; Detrenis, S.; Bianchi, L.; Bertorelli, M.; Pioli, S.; Magnano, A.; Spagnoli, F.; Giuri, P.G.; et al. The relationship between blood pressure and pain. J. Clin. Hypertens. 2013, 15, 600–605. [Google Scholar] [CrossRef]
- Pan, Y.; Cai, W.; Cheng, Q.; Dong, W.; An, T.; Yan, J. Association between anxiety and hypertension: A systematic review and meta-analysis of epidemiological studies. Neuropsychiatr. Dis. Treat. 2015, 11, 1121–1130. [Google Scholar] [CrossRef]
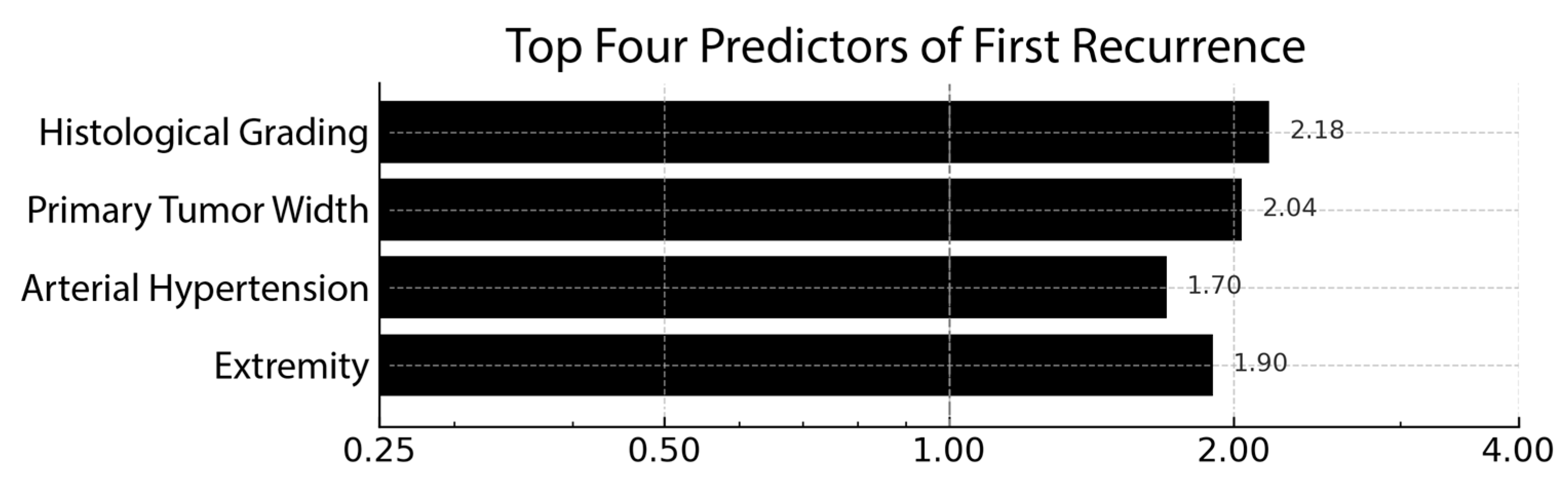
| Rank | Predictor | β (Log-Odds) | OR |
|---|---|---|---|
| 1 | Histological grading (G0–Gx) | +0.78 | 2.18 |
| 2 | Primary tumor width (cm) | +0.71 | 2.04 |
| 3 | Extremity (yes/no) | +0.64 | 1.90 |
| 4 | Arterial hypertension (yes/no) | +0.53 | 1.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedler, A.; Dadras, M.; Drysch, M.; Schmidt, S.V.; Puscz, F.; Reinkemeier, F.; Lehnhardt, M.; Wallner, C. Histological Grade, Tumor Breadth, and Hypertension Predict Early Recurrence in Pediatric Sarcoma: A LASSO-Regularized Micro-Cohort Study. Children 2025, 12, 806. https://doi.org/10.3390/children12060806
Fiedler A, Dadras M, Drysch M, Schmidt SV, Puscz F, Reinkemeier F, Lehnhardt M, Wallner C. Histological Grade, Tumor Breadth, and Hypertension Predict Early Recurrence in Pediatric Sarcoma: A LASSO-Regularized Micro-Cohort Study. Children. 2025; 12(6):806. https://doi.org/10.3390/children12060806
Chicago/Turabian StyleFiedler, Alexander, Mehran Dadras, Marius Drysch, Sonja Verena Schmidt, Flemming Puscz, Felix Reinkemeier, Marcus Lehnhardt, and Christoph Wallner. 2025. "Histological Grade, Tumor Breadth, and Hypertension Predict Early Recurrence in Pediatric Sarcoma: A LASSO-Regularized Micro-Cohort Study" Children 12, no. 6: 806. https://doi.org/10.3390/children12060806
APA StyleFiedler, A., Dadras, M., Drysch, M., Schmidt, S. V., Puscz, F., Reinkemeier, F., Lehnhardt, M., & Wallner, C. (2025). Histological Grade, Tumor Breadth, and Hypertension Predict Early Recurrence in Pediatric Sarcoma: A LASSO-Regularized Micro-Cohort Study. Children, 12(6), 806. https://doi.org/10.3390/children12060806





