Red Cell Distribution Width as a Predictive Biomarker for Early Lung Injury in Pediatric Patients Following Cardiopulmonary Bypass
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Variables
2.3. Main Definitions
- •
- Oxygenation: PaO2/FiO2 < 300 mmHg (regardless of the level of positive end-expiratory pressure, PEEP);
- •
- Chest radiograph: Presence of bilateral infiltrates;
- •
- Pulmonary artery occlusion pressure (PAOP): Less than 18 mmHg, indicating no cardiogenic pulmonary edema (CPE).
2.4. Statistical Analysis
3. Results
3.1. Demographic and Perioperative Characteristics
3.2. Perioperative RDW Dynamics
3.3. RDW as an Independent Predictor of CPB-ALI
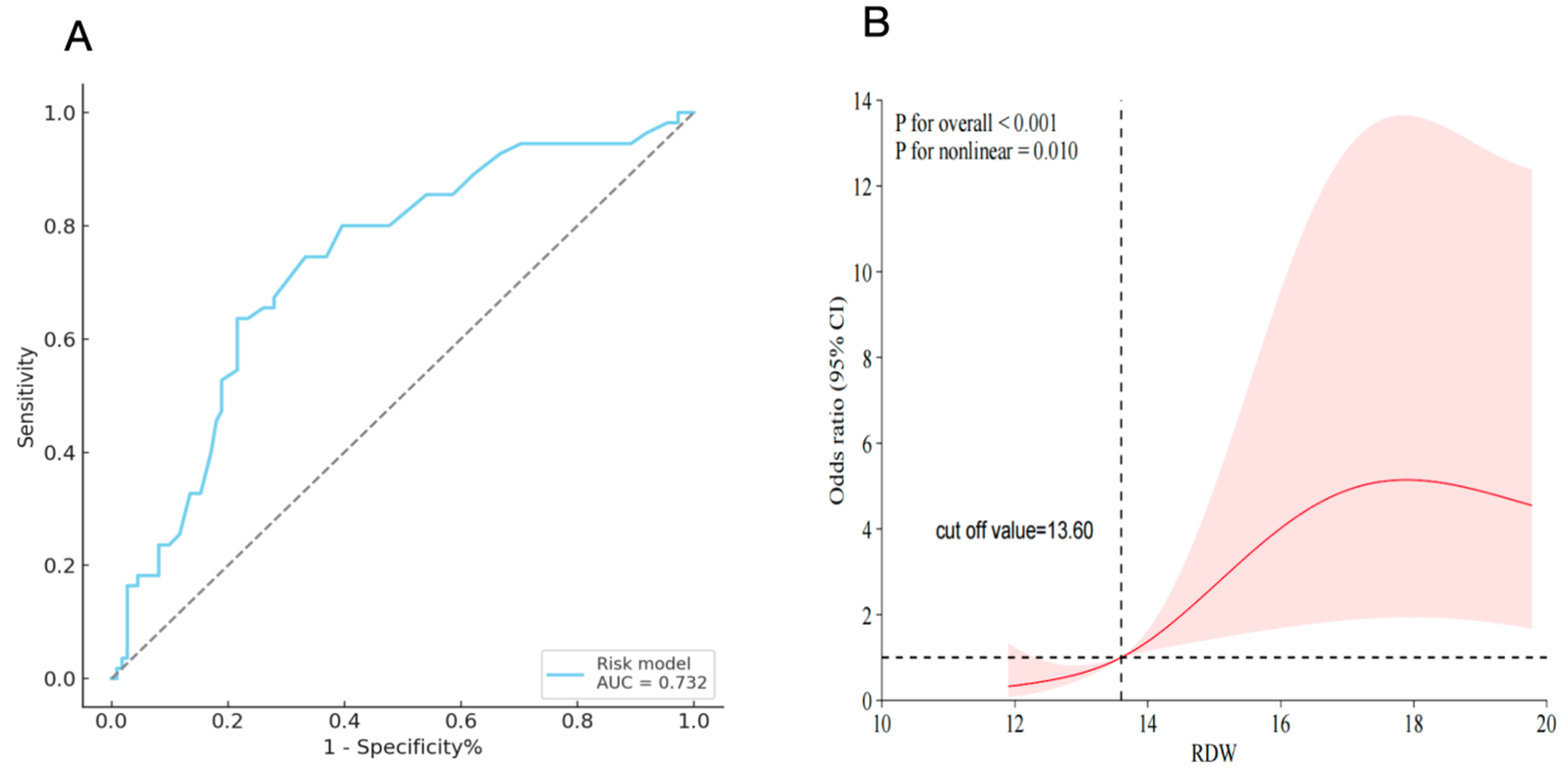
3.4. ROC Curve Analysis
3.5. Nonlinear Association Between RDW and CPB-ALI Risk
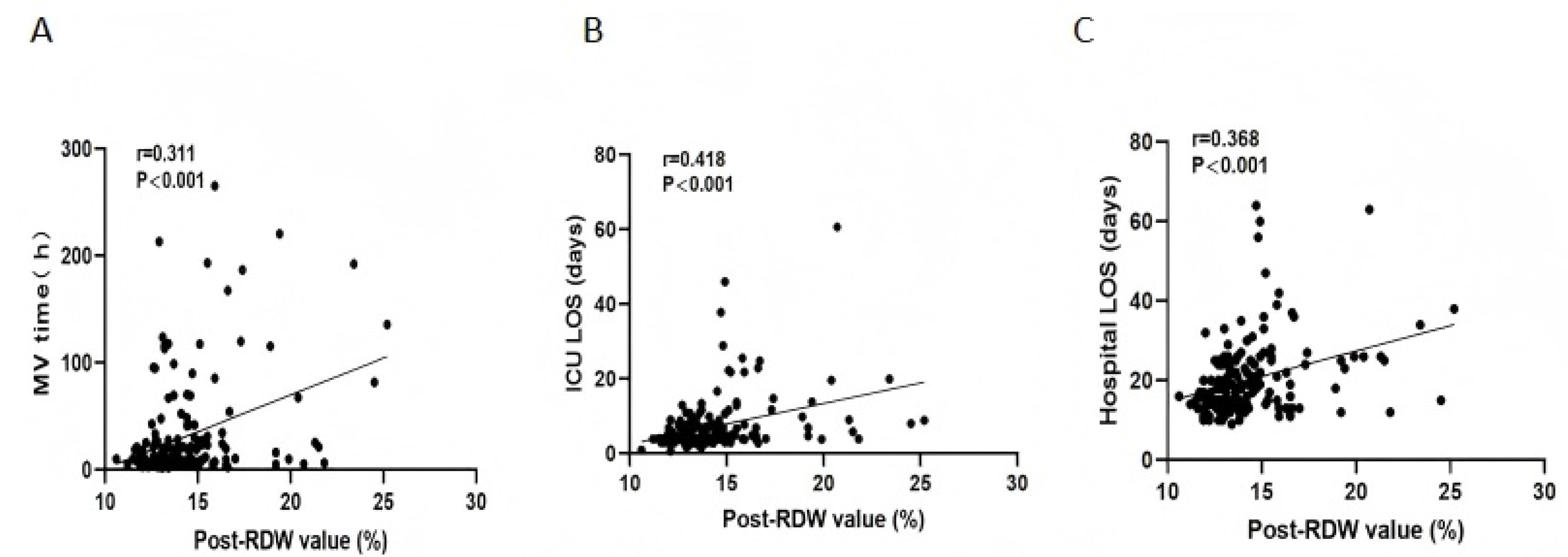
3.6. Association of RDW with Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASD | Atrial septal defect; |
| VSD | Ventricular septal defect; |
| CPB | Cardiopulmonary bypass; |
| ALI | Acute lung injury; |
| CPB-ALI | Cardiopulmonary bypass-associated acute lung injury; |
| CPB-NALI | Cardiopulmonary bypass-associated non-acute lung injury; |
| RDW | Red cell distribution width; |
| ICU | Intensive care unit; |
| LOS | Length of stay; |
| MV | Mechanical ventilation; |
| AUC | Area under the curve; |
| RCS | Restricted cubic spline; |
| ROC | Receiver operating characteristic; |
| OR | Odds ratio; |
| CI | Confidence interval. |
References
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.-K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bernier, P.L.; Stefanescu, A.; Samoukovic, G.; Tchervenkov, C.I. The challenge of congenital heart disease worldwide: Epidemiologic and demographic facts. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card Surg. Annu. 2010, 13, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Linde, D.v.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, E.; Filos, K.S.; Koletsis, E.; Dougenis, D. Lung dysfunction following cardiopulmonary bypass. J. Card. Surg. 2010, 25, 47–55. [Google Scholar] [CrossRef]
- Milot, J.; Perron, J.; Lacasse, Y.; Létourneau, L.; Cartier, P.C.; Maltais, F. Incidence and predictors of ARDS after cardiac surgery. Chest 2001, 119, 884–888. [Google Scholar] [CrossRef]
- Jiang, L.; Cun, Y.; Wang, Q.; Wu, K.; Hu, M.; Wu, Z.; Zhu, T.; Yang, Z.; Patel, N.; Cai, X.; et al. Predicting acute lung injury in infants with congenital heart disease after cardiopulmonary bypass by gut microbiota. Front. Immunol. 2024, 15, 1362040. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Huang, L.; Song, M.; Zhu, G. Association between cardiopulmonary bypass time and mortality among patients with acute respiratory distress syndrome after cardiac surgery. BMC Cardiovasc. Disord. 2023, 23, 622. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, Z.; Liu, X.; Shu, Q.; Tan, L.; Lin, R.; Shi, Z.; Fang, X. Perioperative risk factors for prolonged mechanical ventilation following cardiac surgery in neonates and young infants. Chest 2008, 134, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Chen, C.; Zhao, D.; Liu, X.; Cheng, B.; Wu, S.; Lin, R.; Tan, L.; Fang, X.; Shu, Q. The role of plasma gelsolin in cardiopulmonary bypass induced acute lung injury in infants and young children: A pilot study. BMC Anesthesiol. 2014, 14, 67. [Google Scholar] [CrossRef]
- AlRabeeah, S.M. A Review of Prolonged Mechanical Ventilation in Pediatric Cardiac Surgery Patients: Risk Factors and Implications. J. Multidiscip. Healthc. 2024, 17, 6121–6130. [Google Scholar] [CrossRef]
- Goto, Y.; Hiramatsu, Y.; Ageyama, N.; Sato, S.; Mathis, B.J.; Kitazawa, S.; Matsubara, M.; Sakamoto, H.; Sato, Y. Rolipram plus Sivelestat inhibits bone marrow-derived leukocytic lung recruitment after cardiopulmonary bypass in a primate model. J. Artif. Organs. 2019, 22, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Peng, W.; Zhen, S.; Jiang, X. Postoperative Neutrophil-to-Lymphocyte Ratio Is Associated with Mortality in Adult Patients After Cardiopulmonary Bypass Surgery: A Cohort Study. Med. Sci. Monit. 2021, 27, e932954. [Google Scholar] [CrossRef] [PubMed]
- Pak, O.; Sydykov, A.; Kosanovic, D.; Schermuly, R.T.; Dietrich, A.; Schröder, K.; Brandes, R.P.; Gudermann, T.; Sommer, N.; Weissmann, N. Lung Ischaemia-Reperfusion Injury: The Role of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 967, 195–225. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, X.; Tai, Q.; Xu, G.; Ju, Y.; Gao, W. Endothelial colony-forming cells reduced the lung injury induced by cardiopulmonary bypass in rats. Stem Cell Res. Ther. 2020, 11, 246. [Google Scholar] [CrossRef]
- Li, X.F.; Mao, W.J.; Jiang, R.J.; Yu, H.; Zhang, M.Q.; Yu, H. Effect of Mechanical Ventilation Mode Type on Postoperative Pulmonary Complications After Cardiac Surgery: A Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2024, 38, 437–444. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; Gao, S.; Yan, S.; Diao, X.; Wang, Y.; Xu, X.; Tian, Y.; Ji, B. Quality Management of a Comprehensive Blood Conservation Program During Cardiopulmonary Bypass. Ann. Thorac. Surg. 2022, 114, 142–150. [Google Scholar] [CrossRef]
- den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; Van Schil, P.E.; De Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1283–H1299. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Gao, L.; Liu, S.; Zhao, J.; Liu, G.; Zhang, S. Promising applications of red cell distribution width in diagnosis and prognosis of diseases with or without disordered iron metabolism. Cell Biol. Int. 2023, 47, 1161–1169. [Google Scholar] [CrossRef]
- Guo, T.; Qin, Z.; He, D. Acute Myocardial Infarction (AMI) as the Effect Modifiers to Modify the Association Between Red Blood Cell Distribution Width (RDW) and Mortality in Critically Ill Patients With Stroke. Front. Med. 2022, 9, 754979. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.J.; Kang, H.J.; Son, N.; Bae, S.; Seo, J.; Oh, J.; Rim, S.; Jung, I.H.; Choi, E.; et al. Red cell distribution width as a prognosticator in patients with heart failure. ESC Heart Fail. 2023, 10, 834–845. [Google Scholar] [CrossRef]
- Zhang, C.; Hou, T.H.; Liang, W.R.; Sato, S.; Mathis, B.J.; Kitazawa, S.; Matsubara, M.; Sakamoto, H.; Sato, Y. Prognostic value of red blood cell distribution width combined with chloride in predicting short- and long-term mortality in critically ill patients with congestive heart failure: Findings from the MIMIC-IV database. Heliyon 2024, 10, e23353. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Wang, Y.; Zhao, C.; Tian, Q.; Wang, Q.; He, G.; Lun, L.; Xuan, C. Hematological parameters and early-onset coronary artery disease: A retrospective case-control study based on 3366 participants. Ther. Adv. Chronic. Dis. 2023, 14, 20406223221142670. [Google Scholar] [CrossRef]
- Aslam, H.; Oza, F.; Ahmed, K.; Kopel, J.; Aloysius, M.M.; Ali, A.; Dahiya, D.S.; Aziz, M.; Perisetti, A.; Goyal, H. The Role of Red Cell Distribution Width as a Prognostic Marker in Chronic Liver Disease: A Literature Review. Int. J. Mol. Sci. 2023, 24, 3487. [Google Scholar] [CrossRef]
- Cai, N.; Jiang, M.; Wu, C.; He, F. Red Cell Distribution Width at Admission Predicts the Frequency of Acute Kidney Injury and 28-Day Mortality in Patients With Acute Respiratory Distress Syndrome. Shock 2022, 57, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, Y.; Ying, B.; Cheng, B. Relation between Red Cell Distribution Width and Mortality in Critically Ill Patients with Acute Respiratory Distress Syndrome. Biomed. Res. Int. 2019, 2019, 1942078. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.S.; Chen, Z.Q.; Hu, Y.F.; Chen, J.; Xu, W.; Shu, J.; Pan, J. Red blood cell distribution width is associated with mortality risk in patients with acute respiratory distress syndrome based on the Berlin definition: A propensity score matched cohort study. Heart Lung 2020, 49, 641–645. [Google Scholar] [CrossRef]
- Peng, Y.F.; Zhang, Z.X.; Cao, W.; Meng, C.R.; Xu, S.S.; Zhang, Q. The association between red blood cell distribution width and acute pancreatitis associated lung injury in patients with acute pancreatitis. Open Med. 2015, 10, 176–179. [Google Scholar] [CrossRef]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149 Pt 1, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid. Redox. Signal. 2008, 10, 1923–1940. [Google Scholar] [CrossRef]
- Zakkar, M.; Guida, G.; Suleiman, M.S.; Angelini, G.D. Cardiopulmonary bypass and oxidative stress. Oxid. Med. Cell. Longev. 2015, 2015, 189863. [Google Scholar] [CrossRef]
- Han, L.; Li, L.; Linghu, H.; Zheng, L.; Gou, D. Cardiopulmonary bypass in a rat model may shorten the lifespan of stored red blood cells by activating caspase-3. PLoS ONE 2023, 18, e0290295. [Google Scholar] [CrossRef] [PubMed]
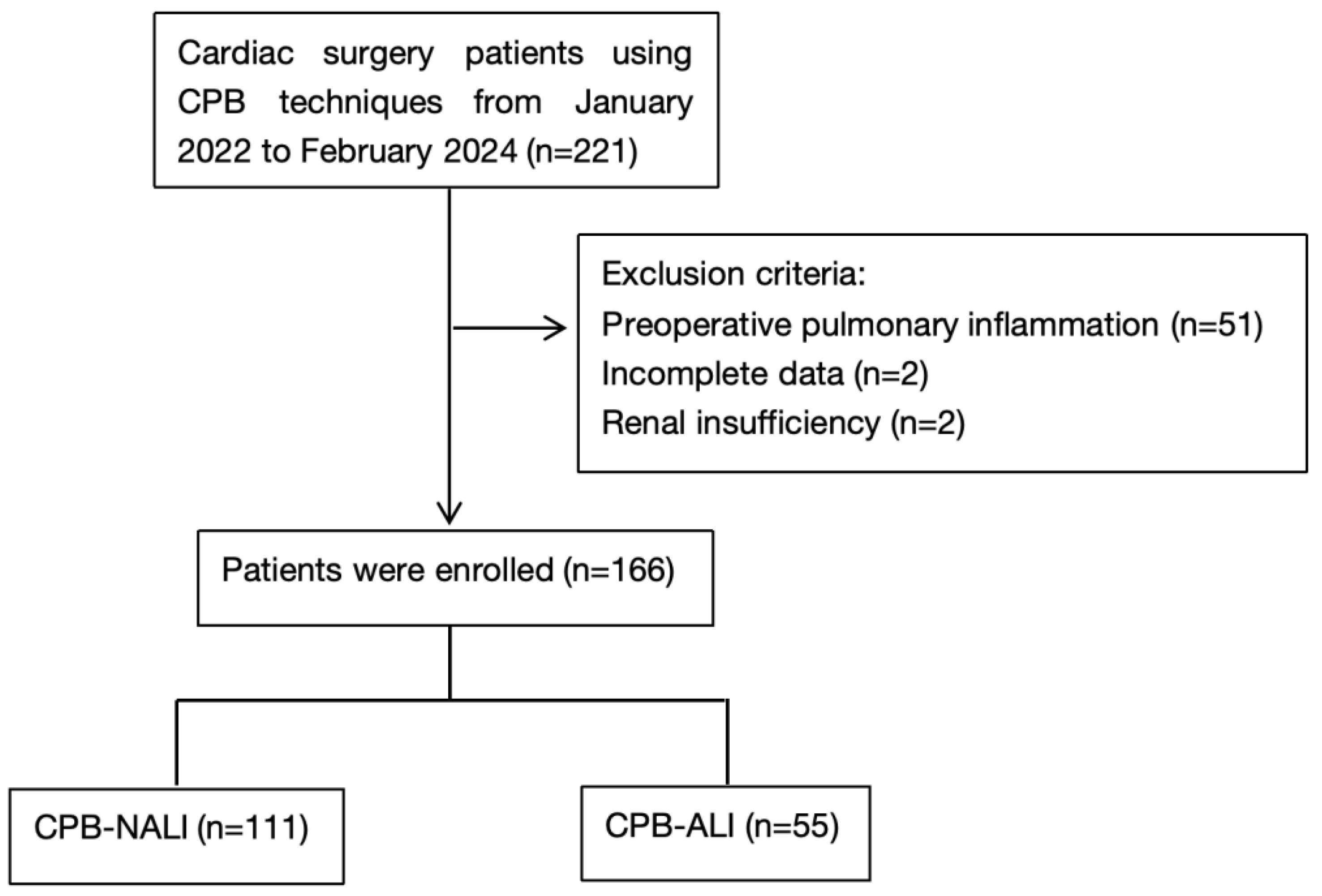
| Characteristics | CPB—NALI (n = 111, 66.9%) | CPB—ALI (n = 55, 33.1%) | p Value |
|---|---|---|---|
| Age (days) | 1132.00 (415.00–2099.00) | 624.00 (376.00–1696.00) | p = 0.091 |
| Weight (kg) | 12.00 (9.00–18.00) | 10.00 (9.00–15.00) | p = 0.054 |
| Gender, male | 60 (54.1%) | 33 (60.0%) | p = 0.47 |
| Hospital LOS (d) | 17.00 (13.00–22.00) | 21.00 (15.00–27.00) | p = 0.001 |
| ICU LOS (d) | 3.98 (3.66–6.58) | 6.90 (4.00–11.72) | p < 0.001 |
| Operative time (h) | 4.47 ± 1.12 | 5.10 ± 1.30 | p = 0.003 |
| CPB time (min) | 96.85 ± 38.13 | 127.76 ± 52.27 | p < 0.001 |
| Aortic clamping time (min) | 54.69 ± 33.50 | 78.67 ± 39.95 | p < 0.001 |
| MV time (h) | 9.21 (5.57–24.28) | 19.11 (7.29–47.95) | p = 0.014 |
| ASD, VSD repair surgery (%) | 48 (43.2%) | 17 (30.9%) | p = 0.133 |
| Vascular surgery (%) | 31 (27.9%) | 11 (20.0%) | p = 0.344 |
| Tetralogy of Fallot repair surgery (%) | 13 (11.7%) | 12 (21.8%) | p = 0.107 |
| Valve repair surgery (%) | 10 (9.0%) | 3 (5.5%) | p = 0.548 |
| Outflow tract surgery (%) | 4 (3.6%) | 6 (10.9%) | p = 0.084 |
| Other surgery (%) | 5 (4.5%) | 6 (10.9%) | p = 0.182 |
| Testing Period | Control | Study | Difference (Control–Study) | p Value (Control vs. Study) |
|---|---|---|---|---|
| Preoperative | 13.91 ± 2.60 | 14.28 ± 2.30 | (−1.18, 0.45) | 0.076 |
| Postoperative | 13.78 ± 2.15 | 15.40 ± 2.72 | (−2.45, −0.78) | <0.001 |
| Difference (pre–post) | (−0.08, 0.35) | (−1.49, −0.75) | – | – |
| p value (pre vs. post) | 0.929 | <0.001 | – | – |
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| CPB time (min) | 1.02 (1.01–1.02) | <0.001 | 1.02 (1.01–1.03) | 0.001 |
| Clamp time (min) | 1.02 (1.01–1.03) | <0.001 | – | 0.643 |
| Operative time (min) | 1.54 (1.17–2.04) | 0.002 | – | 0.896 |
| Post-RDW value (%) | 1.32 (1.13–1.54) | <0.001 | 1.35 (1.10–1.64) | 0.003 |
| Pre–post-RDW change (%) | 4.51 (2.52–8.06) | <0.001 | 4.22 (2.31–7.72) | <0.001 |
| Area Under ROC Curve (95% CI) | At a Cutoff Value of 14.3% | ||||
|---|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | ||
| Postoperative RDW levels | 0.732 (0.658–0.797) | 0.636 (0.605–0.668) | 0.784 (0.745–0.823) | 0.593 (0.564–0.623) | 0.813 (0.773–0.854) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Cheng, J.; Peng, K.; Chen, L.; Kong, Z.; Zhao, Y.; Luo, Z. Red Cell Distribution Width as a Predictive Biomarker for Early Lung Injury in Pediatric Patients Following Cardiopulmonary Bypass. Children 2025, 12, 785. https://doi.org/10.3390/children12060785
Liu H, Cheng J, Peng K, Chen L, Kong Z, Zhao Y, Luo Z. Red Cell Distribution Width as a Predictive Biomarker for Early Lung Injury in Pediatric Patients Following Cardiopulmonary Bypass. Children. 2025; 12(6):785. https://doi.org/10.3390/children12060785
Chicago/Turabian StyleLiu, Hui, Jie Cheng, Kaicheng Peng, Lin Chen, Zhenxuan Kong, Yan Zhao, and Zhengxiu Luo. 2025. "Red Cell Distribution Width as a Predictive Biomarker for Early Lung Injury in Pediatric Patients Following Cardiopulmonary Bypass" Children 12, no. 6: 785. https://doi.org/10.3390/children12060785
APA StyleLiu, H., Cheng, J., Peng, K., Chen, L., Kong, Z., Zhao, Y., & Luo, Z. (2025). Red Cell Distribution Width as a Predictive Biomarker for Early Lung Injury in Pediatric Patients Following Cardiopulmonary Bypass. Children, 12(6), 785. https://doi.org/10.3390/children12060785






