Clinical Significance of the 5T;12TG Genotype in Pediatric CFSPID: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Generalities
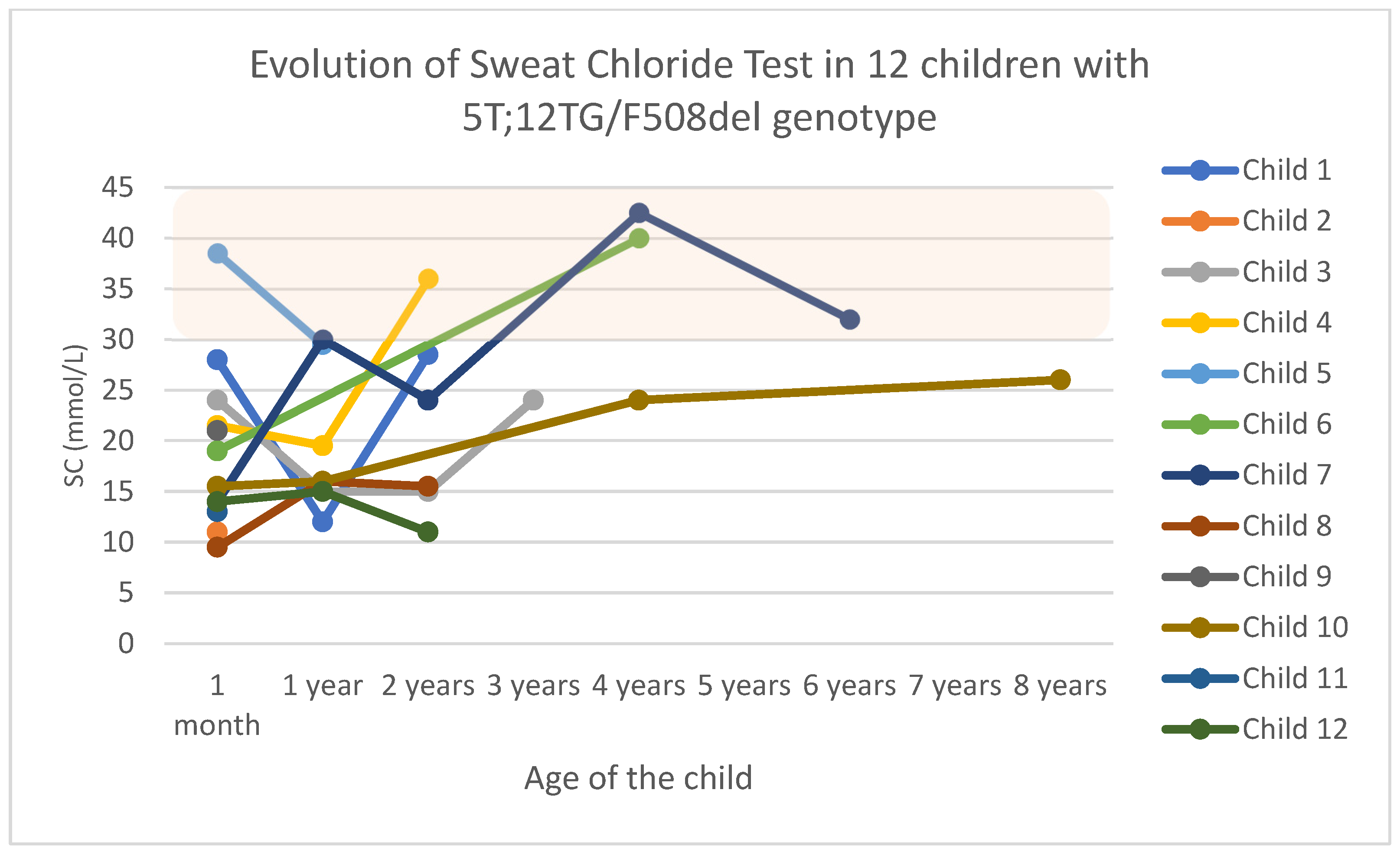
3.2. Group 1: Genotype 5T;12TG/F508del
3.3. Group 2: Genotype 5T;12TG/Cystic Fibrosis-Causing Variant (Other than F508del)
3.4. Group 3: Genotype 5T;12TG/Variant of Varying Clinical Consequences
3.5. Group 4: 5T;12TG/Variant of Unknown Clinical Significance at Present
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| CFSPID | CF screen-positive inconclusive diagnosis |
| SC | Sweat chloride |
| CFTR-RD | CFTR-related disorder |
| NBS | Newborn bloodspot screening |
| IRT | Immunoreactive trypsinogen |
| CFc | CF-causing variants |
| VVCC | Variants of varying clinical consequences |
| VUS | Variants of uncertain significance |
| nCFc | Non-CF-causing variant |
| FEV1 | Forced expiratory volume in 1 s |
| PI | Pancreatic insufficiency |
| IQR | Interquartile range |
| ETI | Elexacaftor–Tezacaftor–Ivacaftor modulator treatment |
References
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.-R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The Lancet Respiratory Medicine Commission on the Future of Care of Cystic Fibrosis. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [PubMed]
- Boucher, R.C. Airway Surface Dehydration in Cystic Fibrosis: Pathogenesis and Therapy. Annu. Rev. Med. 2007, 58, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Massie, J.; Sontag, M.; Southern, K.W. Newborn screening for cystic fibrosis. Lancet Respir. Med. 2016, 4, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Welcome to CFTR2|CFTR2. n.d. Available online: https://cftr2.org/ (accessed on 5 February 2024).
- Munck, A.; Mayell, S.; Winters, V.; Shawcross, A.; Derichs, N.; Parad, R.; Barben, J.; Southern, K. Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID): A new designation and management recommendations for infants with an inconclusive diagnosis following newborn screening. J. Cyst. Fibros. 2015, 14, 706–713. [Google Scholar] [CrossRef]
- Bombieri, C.; Claustres, M.; De Boeck, K.; Derichs, N.; Dodge, J.; Girodon, E.; Sermet, I.; Schwarz, M.; Tzetis, M.; Wilschanski, M.; et al. Recommendations for the classification of diseases as CFTR-related disorders. J. Cyst. Fibros. 2011, 10, S86–S102. [Google Scholar] [CrossRef]
- Green, D.M.; Lahiri, T.; Raraigh, K.S.; Ruiz, F.; Spano, J.; Antos, N.; Bonitz, L.; Christon, L.; Gregoire-Bottex, M.; Hale, J.E.; et al. Cystic Fibrosis Foundation Evidence-Based Guideline for the Management of CRMS/CFSPID. Pediatrics 2024, 153, e2023064657. [Google Scholar] [CrossRef]
- Barben, J.; Castellani, C.; Munck, A.; Davies, J.C.; Groot, K.M.d.W.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.J.; McColley, S.; et al. Updated guidance on the management of children with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome/cystic fibrosis screen positive, inconclusive diagnosis (CRMS/CFSPID). J. Cyst. Fibros. 2021, 20, 810–819. [Google Scholar] [CrossRef]
- Padoan, R.; Corbetta, C.; Bassotti, A.; Seia, M. Identification of the 5T-12TG allele of the cystic fibrosis transmembrane conductance regulator gene in hypertrypsinaemic newborns. Acta Paediatr. 2006, 95, 871–873. [Google Scholar] [CrossRef]
- Groman, J.D.; Hefferon, T.W.; Casals, T.; Bassas, L.; Estivill, X.; Georges, M.D.; Guittard, C.; Koudova, M.; Fallin, M.D.; Nemeth, K.; et al. Variation in a Repeat Sequence Determines Whether a Common Variant of the Cystic Fibrosis Transmembrane Conductance Regulator Gene Is Pathogenic or Benign. Am. J. Hum. Genet. 2004, 74, 176–179. [Google Scholar] [CrossRef]
- Tosco, A.; Castaldo, A.; Colombo, C.; Claut, L.; Carnovale, V.; Iacotucci, P.; Lucarelli, M.; Cimino, G.; Fabrizzi, B.; Caporelli, N.; et al. Clinical outcomes of a large cohort of individuals with the F508del/5T;TG12 CFTR genotype. J. Cyst. Fibros. 2022, 21, 850–855. [Google Scholar] [CrossRef]
- Chillón, M.; Casals, T.; Mercier, B.; Bassas, L.; Lissens, W.; Silber, S.; Romey, M.-C.; Ruiz-Romero, J.; Verlingue, C.; Claustres, M.; et al. Mutations in the Cystic Fibrosis Gene in Patients with Congenital Absence of the Vas Deferens. N. Engl. J. Med. 1995, 332, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Vertex Announces U.S. FDA Approval for TRIKAFTA (Elexacaftor/Tezacaftor/Ivacaftor and Ivacaftor) to Include Additional Non-F508del TRIKAFTA-Responsive Variants|Vertex Pharmaceuticals Newsroom. n.d. Available online: https://news.vrtx.com/news-releases/news-release-details/vertex-announces-us-fda-approval-trikafta (accessed on 23 March 2025).
- Fibrosis Quística—Panel Ibérico—Diagnóstica Longwood. n.d. Available online: https://www.dlongwood.com/catalogo/fibrosis-quistica-panel-iberico/ (accessed on 26 April 2025).
- Flume, P.A.; Mogayzel, P.J.; Robinson, K.A.; Goss, C.H.; Rosenblatt, R.L.; Kuhn, R.J.; Marshall, B.C.; the Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic Fibrosis Pulmonary Guidelines: Treatment of Pulmonary Exacerbations. Am. J. Respir. Crit. Care Med. 2009, 180, 802–808. [Google Scholar] [CrossRef] [PubMed]
- ATS GUIDELINES Bundle—Routine Lung Function Tests. n.d. Available online: https://eguideline.guidelinecentral.com/i/1463892-routine-lung-function-tests/0? (accessed on 29 January 2025).
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Welcome to CFTR-France. n.d. Available online: https://cftr.iurc.montp.inserm.fr/cftr (accessed on 5 February 2024).
- Munck, A.; Bourmaud, A.; Bellon, G.; Picq, P.; Farrell, P.M.; Group on behalf of the DS. Phenotype of children with inconclusive cystic fibrosis diagnosis after newborn screening. Pediatr. Pulmonol. 2020, 55, 918–928. [Google Scholar] [CrossRef]
- Groves, T. Long-Term Outcomes of Children with Intermediate Sweat Chloride Values in Infancy. J. Pediatr. 2015, 166, 1469–1474.e3. [Google Scholar] [CrossRef]
- Tosco, A.; Carnovale, V.; Claut, L.; Fabrizzi, B.; Majo, F.; Castellani, C.; Sepe, A.; Castaldo, G.; Terlizzi, V. Clinical outcome of individuals carrying 5T;TG12 in trans with CFTR variants with varying clinical consequences. Pediatr. Pulmonol. 2023, 58, 1253–1255. [Google Scholar] [CrossRef]
- Chillón, M.; Dörk, T.; Casals, T.; Giménez, J.; Fonknechten, N.; Will, K.; Ramos, D.; Nunes, V.; Estivill, X. A novel donor splice site in intron 11 of the CFTR gene, created by mutation 1811+1.6kbA-->G, produces a new exon: High frequency in Spanish cystic fibrosis chromosomes and association with severe phenotype. Am. J. Hum. Genet. 1995, 56, 623–629. [Google Scholar]
- Noone, P.G.; Pue, C.A.; Zhou, Z.; Friedman, K.J.; Wakeling, E.L.; Ganeshananthan, M.; Simon, R.H.; Silverman, L.M.; Knowles, M.R. Lung Disease Associated with the IVS8 5T Allele of the CFTR Gene. Am. J. Respir. Crit. Care Med. 2000, 162, 1919–1924. [Google Scholar] [CrossRef]
- Praticò, A.D.; Praticò, E.R.; Rotolo, N.; Salafia, S.; Franzonello, C.; Leonardi, S. Isolated liver disease in a patient with a CFTR genotype F508del/12TG-5T and 470MV: A new face of an old disease. Ann. Hepatol. 2015, 14, 933–936. [Google Scholar] [CrossRef]
- Dray, X.; Fajac, I.; Bienvenu, T.; Chryssostalis, A.; Sogni, P.; Hubert, D. Association of Pancreas Divisum and Recurrent Acute Pancreatitis With the IVS8-5T-12TG Allele of the CFTR Gene and CFTR Dysfunction. Pancreas 2007, 35, 90–93. [Google Scholar] [CrossRef]
- Kier, C.; Kay, D.M.; Langfelder-Schwind, E.; Goetz, D.M.; Berdella, M.; DeCelie-Germana, J.K.; Soultan, Z.N.; Caggana, M.; Fortner, C.N.; Giusti, R.; et al. Variability in evaluation and follow-up of newborns with CRMS/CFSPID in New York State. Pediatr. Pulmonol. 2024, 59, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, M.; Cazzato, S.; Mutignani, M.; Cevenini, M.; Guidetti, E.; Ben Zvi, I.; Aldini, R.; Saraceni, G.; Cavoli, C.; Garagnani, P.; et al. A Patient with Pancreas Divisum, Recurrent Acute Pancreatitis, and Homozygosity for the Cystic Fibrosis Transmembrane Regulator–Associated Protein 5T Allele. Clin. Gastroenterol. Hepatol. 2013, 11, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, M.; Fox, E.; Treyster, Z. Recurrent Sinopulmonary Infections and Poor Growth in a Child Homozygous for the CFTR 5T-TG12 Variant n.d. Available online: https://www.authorea.com/users/486950/articles/571695-recurrentsinopulmonary-infections-and-poor-growth-in-a-child-homozygous-for-the-cftr-5t-tg12variant (accessed on 3 June 2022).
- Terlizzi, V.; Fevola, C.; Presti, S.; Claut, L.; Ambroni, M.; Calderazzo, M.A.; Esposito, I.; Fabrizzi, B.; Leonetti, G.; Lombardo, M.; et al. Critical Issues in the Management of CRMS/CFSPID Children: A National Real-World Survey. Pediatr. Pulmonol. 2025, 60, e27483. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, F.; Lebecque, P.; De Boeck, K.; Leal, T. Biological variability of the sweat chloride in diagnostic sweat tests: A retrospective analysis. J. Cyst. Fibros. 2017, 16, 30–35. [Google Scholar] [CrossRef]
- Terlizzi, V.; Dolce, D. Variability of the sweat test in children with Cystic Fibrosis previously CRMS/CFSPID: A retrospective monocenter experience. J. Cyst. Fibros. 2023, 22, 496–498. [Google Scholar] [CrossRef]
- Terlizzi, V. Elexacaftor/Tezacaftor/Ivacaftor therapy in cystic fibrosis children previously CFSPID: Is it over-medicalization? J. Cyst. Fibros. 2023, 23, 366–367. [Google Scholar] [CrossRef]

| Genotypes (n) | 5T;12TG/F508del (n = 12) | 5T;12TG/CFc non-F508del (n = 6) | 5T;12TG/VVCC (n = 2) | 5T;12TG/VUS (n = 1) |
|---|---|---|---|---|
| Allele non-5T;12TG (n) | F508del (n = 12) | 1811+1634A->G (n = 2) L206W (n = 1) G542X (n = 1) 3120+1G->A (n = 1) V232D (n = 1) | 5T;12TG (n = 1) R117H;7T (n = 1) | 1181+1.6kbA>G (n = 1) |
| Female (%) | 5 (41.7%) | 3 (50%) | 2 (100%) | 1 (100%) |
| Preterm birth (%) | 1 (8.3%) | 2 (33.3%) | 0 (0%) | 0 (0%) |
| IRT median [IQR] (ng/mL) | 71.13 [54.8–78.7] | 80.55 [61.8–110.5] | 89.5 [59–120] | 55.6 |
| CFNBS diagnosis by the initial panel (%) | 12 (100%) | 5 (83.3%) | 2 (100%) | 1 (100%) |
| Age at first visit, median [IQR] (days) | 27 [21–41] | 44.5 [35.5–65.2] | 38 (n = 1) | 21 |
| Age at the end of the study, median [IQR] (years) | 4.25 [3.4–5.3] | 4.59 [4.3–7.1] | 9.9 [7–12.9] | 4.76 |
| Conversion to CF (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Conversion to CFTR-RD | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| First visit SC, median [IQR] (mmol/L) | 17.2 [13.2–23.4] | 22 [16–28.3] | 11.5 [11–12] | 14 |
| Number of children with intermediate SC at the first visit (%) | 1 (8.33%) | 1 (16.7%) | 0 (0%) | 0 (0%) |
| Number of children with intermediate SC at some point during follow-up (%) | 4 (33.3%) | 4 (66.6%) | 0 (0%) | 1 (100%) |
| Number of children with a weight z-score in the range +/− 2SD (%) | 10 (83.3%) | 4 (66.7%) | 2 (100%) | 0 (0%) |
| Number of children with a height z-score in the range +/− 2SD (%) | 12 (100%) | 6 (100%) | 2 (100%) | 0 (0%) |
| Last FEV1 z-score, median (n) | −0.4 (n = 1) | 0.12 (n = 1) | 0.19 (n = 1) | - |
| Respiratory symptoms (%) | Wheezing (16.7%) Bilateral pneumonia (8.3%) | Bronchiolitis (33.3%) Asthma (16.7%) | 0 (0%) | PEx (50%) |
| Hospitalizations (%) | 2 (16.7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Bronchial colonization isolations (%) | H. influenzae (50%) M. catarrhalis (50%) MSSA (33.3%) S. pneumoniae (25%) A. baumanii (8.3%) | MSSA (33.3%) H. influenzae (16.7%) M. catarrhalis (16.7%) S. pneumoniae (16.7%) S. maltophilia (16.7%) Escherichia Coli (16.7%) | MSSA (50%) S. pneumoniae (50%) S. maltophilia (50%) | |
| Digestive symptoms (%) | Constipation (8.3%) | GE reflux (16.7%) Constipation (16.7%) | 0 (0%) | 0 (0%) |
| Pancreatic insufficiency (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Physiotherapy Inhaled corticosteroid | 0 (0%) 2 (16.7%) | 0 (0%) 1 (16.7%) | 0 (0%) 0 (0%) | 0 (0%) 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Tirado, A.; Blitz-Castro, E.; Vicente-Santamaría, S.; Luna-Paredes, C.; Salcedo-Lobato, E.; Tabares-González, A.; Gascón-Galindo, C.; Boutry, S.; Lamas-Ferreiro, A. Clinical Significance of the 5T;12TG Genotype in Pediatric CFSPID: A Retrospective Study. Children 2025, 12, 778. https://doi.org/10.3390/children12060778
Morales-Tirado A, Blitz-Castro E, Vicente-Santamaría S, Luna-Paredes C, Salcedo-Lobato E, Tabares-González A, Gascón-Galindo C, Boutry S, Lamas-Ferreiro A. Clinical Significance of the 5T;12TG Genotype in Pediatric CFSPID: A Retrospective Study. Children. 2025; 12(6):778. https://doi.org/10.3390/children12060778
Chicago/Turabian StyleMorales-Tirado, Ana, Enrique Blitz-Castro, Saioa Vicente-Santamaría, Carmen Luna-Paredes, Enrique Salcedo-Lobato, Ana Tabares-González, Celia Gascón-Galindo, Simon Boutry, and Adelaida Lamas-Ferreiro. 2025. "Clinical Significance of the 5T;12TG Genotype in Pediatric CFSPID: A Retrospective Study" Children 12, no. 6: 778. https://doi.org/10.3390/children12060778
APA StyleMorales-Tirado, A., Blitz-Castro, E., Vicente-Santamaría, S., Luna-Paredes, C., Salcedo-Lobato, E., Tabares-González, A., Gascón-Galindo, C., Boutry, S., & Lamas-Ferreiro, A. (2025). Clinical Significance of the 5T;12TG Genotype in Pediatric CFSPID: A Retrospective Study. Children, 12(6), 778. https://doi.org/10.3390/children12060778





