Standardizing Neonatal Body Composition Assessment Using Air Displacement Plethysmography: Insights from the Bavarian Experience
Abstract
1. Introduction
- To systematically review the literature on the proportion of eligible preterm infants who undergo assessment (eligibility-to-assessment rate) and to evaluate the safety of ADP assessments conducted in experimental settings.
- To systematically review the literature for comparable routine clinical protocols and standard operating procedures for ADP in body composition assessments.
- To develop and utilize a standardized clinical protocol for routine ADP use in preterm and term infants: The Bavarian Clinical Protocol (BCP).
2. Materials and Methods
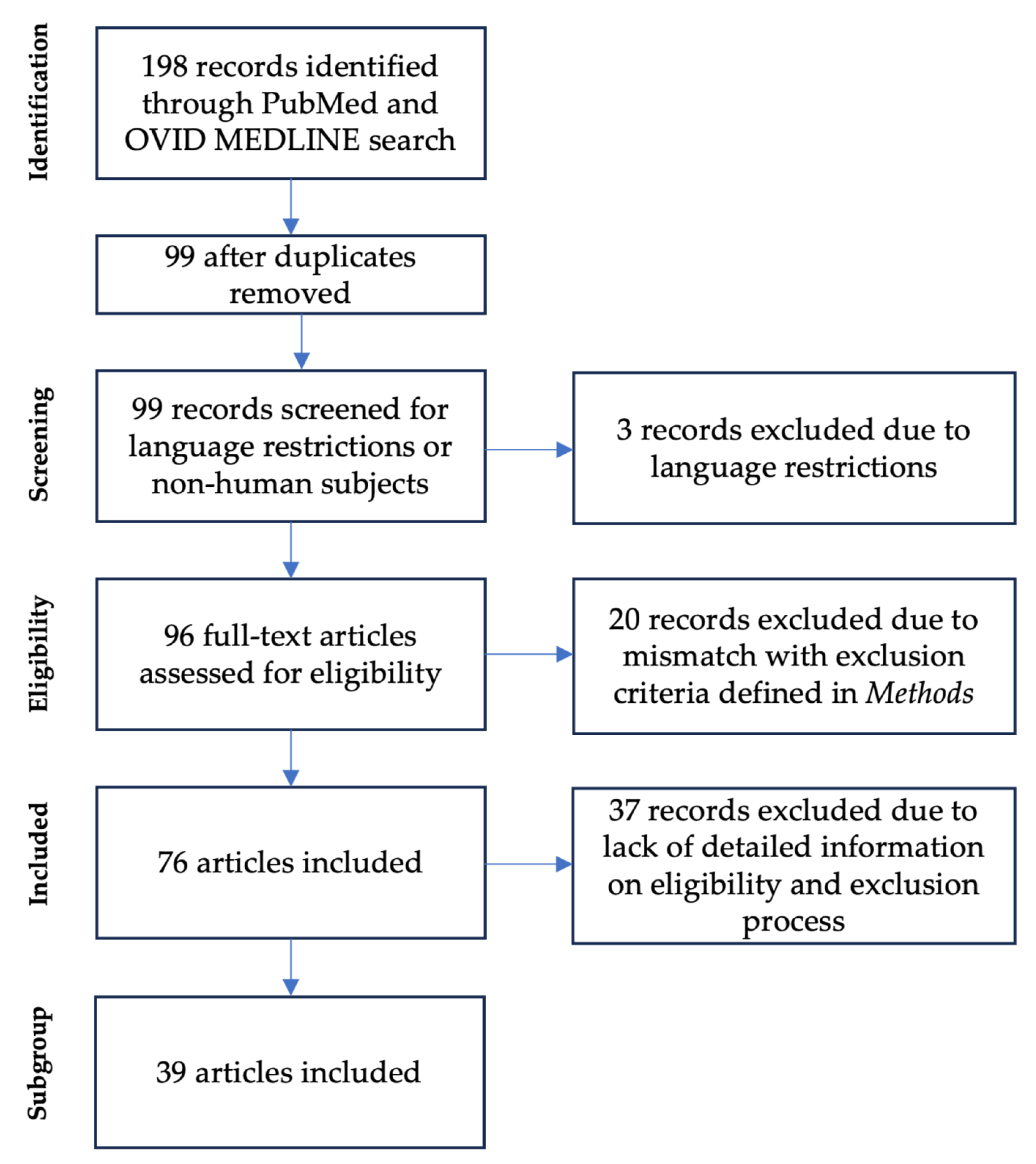
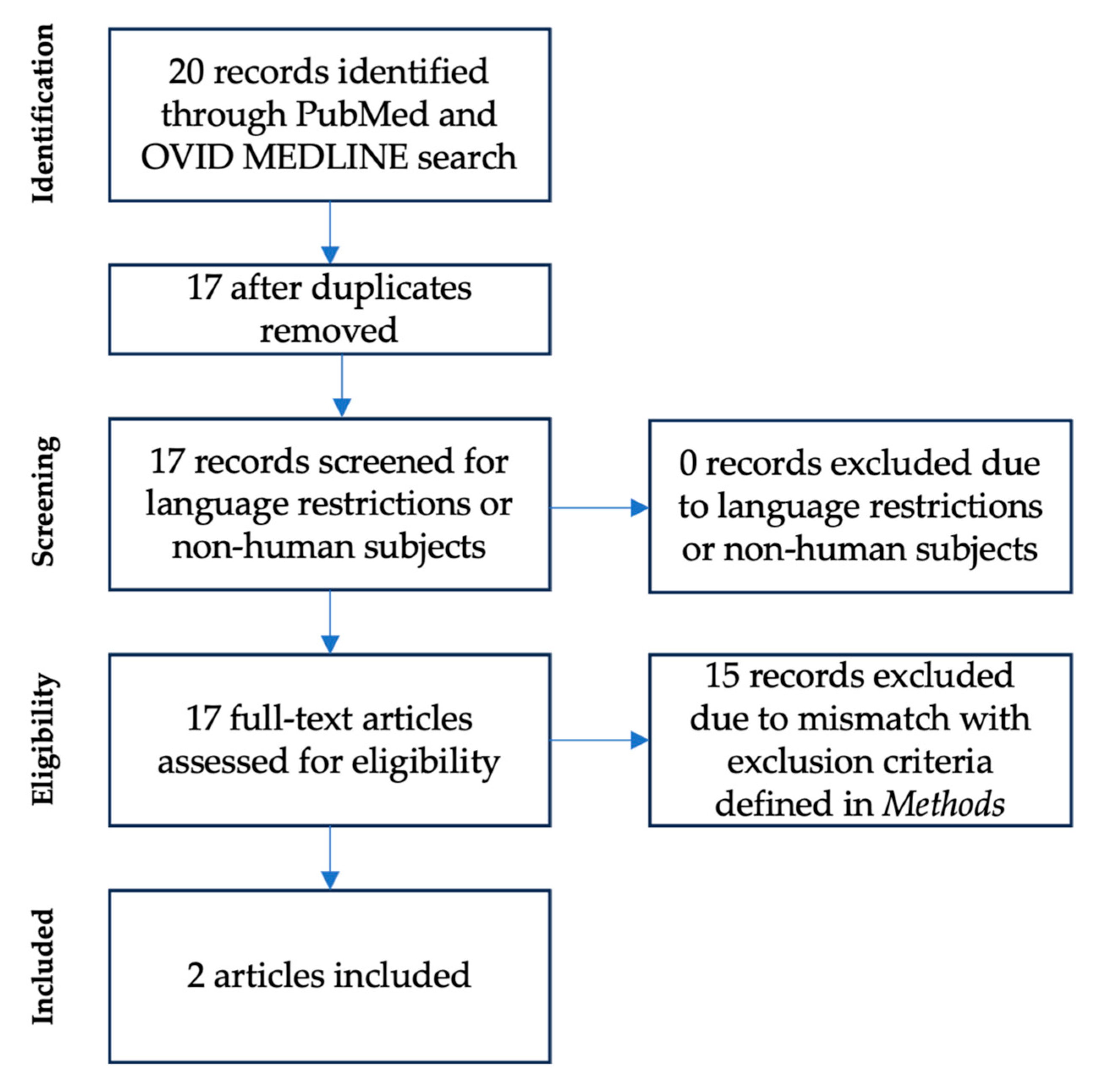
2.1. Literature Review (#1 and #2)
2.2. Retrospective Analysis of the Bavarian Clinical Protocol
3. Results
3.1. Literature Review (#1)—Air Displacement Plethysmography in Preterm Infants
3.2. Literature Review (#2)—Clinical Routine Protocols
3.3. Comparison of Clinical Routines
- Eligibility and exclusion criteria: Clinical stability is a common prerequisite for all three institutions. Definitions varied slightly: for example, Cincinnati Children’s Hospital excluded infants with tubes deemed critical by the surgical team, whereas at MetroHealth Medical Center, the exclusion criteria was a birth weight of >1500 g. COVID-19 infection was an exclusion criteria at both institutions. Nuremberg’s initial protocol emphasized clinical stability with no bradycardia, desaturation, or respiratory instability within 48 h before testing.
- Screening procedures: Screening was performed by dietitians at Cincinnati, integrated into electronic medical records at MetroHealth, and handled collaboratively by nurses and physicians at Nuremberg. Here, primary screening by nurses was recommended because of their frequent patient interactions, with the attending physician confirming eligibility.
- Testing frequency: Nuremberg and Cincinnati performed weekly assessments to track body composition changes, whereas MetroHealth limited testing to a single session during the hospital stay.
- Time and personnel requirements: ADP assessments typically require two staff members; however, involving three operators was shown to improve efficiency, reducing the assessment time from 13 to 8 min. Approximately 12 weekly assessments at Nuremberg required up to 5 h of total staff time.
| Nuremberg Children’s University Hospital [18] | Cincinnati Children’s Medical Center [17] | MetroHealth Medical Center [17] | |
|---|---|---|---|
| Eligibility criteria | All infants admitted to NICU | All NICU admissions regardless of birth weight | VLBW infants (birth weight < 1500 g) |
| Exclusion criteria | 1—no stable breathing on room air 2—episode of significant desaturation 3—bradycardia (<60/min) requiring stimulation within the last 48 h. 4—positive for multidrug-resistant infections on routine microbiological tests (e.g., 3-MRGN, MRSA) | 1—Respiratory support or Oxygen requirement 2—Chest tube to suction 3—Tubes deemed critical by surgical team 4—COVID infection | 1—Birth weight > 1500 g 2—Infants being discharged on O2 support and failed the ‘room air challenge’ of 2 min 3—COVID infection |
| Screening for readiness of eligible infants | The PEAPOD nurse screens all neonates at the units. Eligibility for testing is evaluated using inclusion and exclusion criteria. Clinical stability is confirmed by the attending physician on test day | Neonatal dietician brings up infant readiness for testing during daily rounds | Incorporated into NICU discharge guidelines Reminders of testing eligibility incorporated into electronic medical records |
| Test frequency | Weekly, once infant is weaned to room air | Weekly, once infant is weaned to room air | Once, at term corrected gestation or prior to dischargewhichever comes first |
| Testing time | Once weekly, Tuesdays, at predefined time window between 8.30 AM and 11.00 AM | On Wednesdays, for infants with central lines test done on ‘line change’ day | Whenever infants are ready for testing |
| Testing location | In an examination room near NICU | In the NICU | In the NICU |
| Personnel | PEAPOD certified nurse handles PEAPOD and PEAPOD nurses handle the infants | Monitor technician handles the PEAPOD device and bedside nurse handles the infant | Assigned trained NICU nurses, nurse managers and dieticians |
| Time requirements | 8 min with a workforce of 3 persons (see section Time and staffing requirements for estimation of total work-load) | The measurement itself only takes 5–7 min and includes a body mass measurement | The measurement itself only takes 5–7 min and includes a body mass measurement |
| Clinical utility of Body Composition Data | Data is trended in reference graphs and available for physicians. No nutrional intervention plan is established | Trended data is used to adjust nutritional management on weekly basis. | Adjust discharge feeding regimens Evaluate/Adjust unit’s nutritional practices |
3.4. Bavarian Clinical Protocol
4. Discussion
4.1. Current Utilization of Routine ADP Assessments for Preterm Infants
4.2. Comparison of Protocols for Routine Clinical ADP
- Eligibility criteria: Most centers exclude infants with active infections, central lines, or critical tubes, prioritizing safety and preventing cross-contamination. Formalizing these criteria across institutions may further reduce risks [62].
- Testing frequency: Weekly assessments, as supported by our prior findings, offer sufficient reproducibility for monitoring changes in body composition. Institutions may opt for single or repeated assessments on the basis of priorities—while repeated tests improve monitoring, single assessments reduce workload [14].
- Time and Staffing Requirements: Streamlined workflows with at least two nurses significantly reduce assessment duration, from an average of 13 min to approximately 8 min [18]. This is slightly longer than the 5–7 min reported by Alja’nini et al.; although, it is unclear if their estimate included tasks such as dressing and undressing the infant or transporting them to and from the PEAPOD room. Furthermore, the number of personnel involved in their assessments was not specified, making it difficult to directly compare total work time across these two studies [17]. Efficient scheduling, including preparing one infant while another is being assessed, further enhances time management.
4.3. Bavarian Clinical Protocol (BCP)
- Serve as a practical blueprint for implementing body composition testing in various clinical settings.
- Enhance the reliability and comparability of ADP-based measurements across institutions.
- Improve neonatal safety by standardizing exclusion criteria and operational workflows.
- Facilitate multicenter studies and broader benchmarking efforts in neonatal care.
4.4. Implementing ADP in Routine Neonatal Care: Practical Considerations
- Weekly planning: Initiate planning sessions at the beginning of each week to schedule assessments and ensure staff alignment.
- Morning Setup: Perform early setup of the ADP device on the test day to minimize delays and interruptions.
- Fixed Location: Designate a permanent location for the device to reduce transport time and eliminate frequent recalibration needs.
- Streamlined Workflow: Adopt an assembly line model, with assigned roles for measurement, infant transport, and dressing/undressing.
- Comprehensive Growth Monitoring: Pair ADP assessments with weekly anthropometric measurements to track overall growth and optimize resource allocation.
4.5. Interpretation and Use of Body Composition Data
4.6. Clinical Significance
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costeloe, K.L.; Hennessy, E.M.; Haider, S.; Stacey, F.; Marlow, N.; Draper, E.S. Short term outcomes after extreme preterm birth in England: Comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012, 345, e7976. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.L.; Regan, F.; Jackson, W.E.; Jefferies, C.; Knight, D.B.; Robinson, E.M.; Cutfield, W.S. Premature birth and later insulin resistance. N. Engl. J. Med. 2004, 351, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.K.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Edstedt Bonamy, A.-K. Preterm Birth and Risk of Heart Failure Up to Early Adulthood. J. Am. Coll. Cardiol. 2017, 69, 2634–2642. [Google Scholar] [CrossRef]
- Ramel, S.E.; Gray, H.L.; Christiansen, E.; Boys, C.; Georgieff, M.K.; Demerath, E.W. Greater Early Gains in Fat-Free Mass, but Not Fat Mass, Are Associated with Improved Neurodevelopment at 1 Year Corrected Age for Prematurity in Very Low Birth Weight Preterm Infants. J. Pediatr. 2016, 173, 108–115. [Google Scholar] [CrossRef]
- Pfister, K.M.; Zhang, L.; Miller, N.C.; Ingolfsland, E.C.; Demerath, E.W.; Ramel, S.E. Early body composition changes are associated with neurodevelopmental and metabolic outcomes at 4 years of age in very preterm infants. Pediatr. Res. 2018, 84, 713–718. [Google Scholar] [CrossRef]
- Bua, J.; Risso, F.M.; Bin, M.; Vallon, F.; Travan, L.; Paviotti, G. Association between body composition at term equivalent age and Bayley scores at 2 years in preterm infants. J. Perinatol. 2021, 41, 1852–1858. [Google Scholar] [CrossRef]
- Bell, K.A.; Ramel, S.E.; Robinson, D.T.; Wagner, C.L.; Scottoline, B.; Belfort, M.B. Body composition measurement for the preterm neonate: Using a clinical utility framework to translate research tools into clinical care. J. Perinatol. 2022, 42, 1550–1555. [Google Scholar] [CrossRef]
- Dung, N.Q.; Fusch, G.; Armbrust, S.; Jochum, F.; Fusch, C. Body composition of preterm infants measured during the first months of life: Bioelectrical impedance provides insignificant additional information compared to anthropometry alone. Eur. J. Pediatr. 2007, 166, 215–222. [Google Scholar] [CrossRef]
- Koo, W.W.K.; Walters, J.C.; Hockman, E.M. Body Composition in Neonates: Relationship Between Measured and Derived Anthropometry with Dual-Energy X-Ray Absorptiometry Measurements. Pediatr. Res. 2004, 56, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Nagel, E.; Hickey, M.; Teigen, L.; Kuchnia, A.; Curran, K.; Soumekh, L.; Earthman, C.; Demerath, E.; Ramel, S. Clinical Application of Body Composition Methods in Premature Infants. J. Parenter. Enter. Nutr. 2020, 44, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Urlando, A.; Dempster, P.; Aitkens, S. A New Air Displacement Plethysmograph for the Measurement of Body Composition in Infants. Pediatr. Res. 2003, 53, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Lücke, L.; Fusch, C.; Knab, K.; Schäfer, S.; Zimmermann, J.L.; Felderhoff-Müser, U.; Meis, A.; Lohmüller-Weiß, S.; Szakacs-Fusch, A.; Rochow, N. Reproducibility of Air Displacement Plethysmography in Term and Preterm Infants—A Study to Enhance Body Composition Analysis in Clinical Routine. Nutrients 2024, 16, 1810. [Google Scholar] [CrossRef]
- COSMED PEA POD®. Infant Body Composition System Operator’s Manual; COSMED: Rome, Italy, 2019. [Google Scholar]
- Salas, A.A.; Jerome, M.L.; Chandler-Laney, P.; Ambalavanan, N.; Carlo, W.A. Serial assessment of fat and fat-free mass accretion in very preterm infants: A randomized trial. Pediatr. Res. 2020, 88, 733–738. [Google Scholar] [CrossRef]
- Alja’nini, Z.; McNelis, K.M.; Viswanathan, S.; Goddard, G.R.; Merlino-Barr, S.; Collin, M.; Groh-Wargo, S. Infant body composition assessment in the neonatal intensive care unit (NICU) using air displacement plethysmography: Strategies for implementation into clinical workflow. Clin. Nutr. ESPEN 2021, 43, 212–222. [Google Scholar] [CrossRef]
- Lücke, L.A.; Rochow, N.; Knab, K.; Schäfer, S.; Zimmermann, J.L.; Meis, A.; Lohmüller-Weiß, S.; Szakacs-Fusch, A.; Felderhoff-Müser, U.; Fusch, C. Body Composition Analysis of the Clinical Routine Using Air Displacement Plethysmography: Age-Group-Specific Feasibility Analysis among Preterm Infants. Nutrients 2024, 16, 2694. [Google Scholar] [CrossRef]
- Fusch, S.; Fusch, G.; Yousuf, E.I.; Rochow, M.; So, H.Y.; Fusch, C.; Rochow, N. Individualized Target Fortification of Breast Milk: Optimizing Macronutrient Content Using Different Fortifiers and Approaches. Front. Nutr. 2021, 8, 652641. [Google Scholar] [CrossRef]
- Rochow, N.; Fusch, G.; Ali, A.; Bhatia, A.; So, H.Y.; Iskander, R.; Chessell, L.; el Helou, S.; Fusch, C. Individualized target fortification of breast milk with protein, carbohydrates, and fat for preterm infants: A double-blind randomized controlled trial. Clin. Nutr. 2021, 40, 54–63. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Yumani, D.F.J.; de Jongh, D.; Lafeber, H.N.; van Weissenbruch, M.M. A comparative study using dual-energy X-ray absorptiometry, air displacement plethysmography, and skinfolds to assess fat mass in preterms at term equivalent age. Eur. J. Pediatr. 2021, 180, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Gianni, M.L.; Roggero, P.; Taroni, F.; Liotto, N.; Piemontese, P.; Mosca, F. Adiposity in small for gestational age preterm infants assessed at term equivalent age. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F368–F372. [Google Scholar] [CrossRef]
- Binder, C.; Buchmayer, J.; Thajer, A.; Giordano, V.; Schmidbauer, V.; Harreiter, K.; Klebermass-Schrehof, K.; Berger, A.; Goeral, K. Association between Fat-Free Mass and Brain Size in Extremely Preterm Infants. Nutrients 2021, 13, 4205. [Google Scholar] [CrossRef]
- McGee, M.; Unger, S.; Hamilton, J.; Birken, C.S.; Pausova, Z.; Kiss, A.; Bando, N.; O’Connor, D.L. Associations between Diet Quality and Body Composition in Young Children Born with Very Low Body Weight. J. Nutr. 2020, 150, 2961–2968. [Google Scholar] [CrossRef]
- Bell, K.A.; Matthews, L.G.; Cherkerzian, S.; Prohl, A.K.; Warfield, S.K.; Inder, T.E.; Onishi, S.; Belfort, M.B. Associations of body composition with regional brain volumes and white matter microstructure in very preterm infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2022, 107, 533–538. [Google Scholar] [CrossRef]
- Bell, K.A.; Matthews, L.G.; Cherkerzian, S.; Palmer, C.; Drouin, K.; Pepin, H.L.; Ellard, D.; Inder, T.E.; Ramel, S.E.; Belfort, M.B. Associations of Growth and Body Composition with Brain Size in Preterm Infants. J. Pediatr. 2019, 214, 20–26.e2. [Google Scholar] [CrossRef]
- Macedo, I.; Pereira-da-Silva, L.; Cardoso, M. Associations of Measured Protein and Energy Intakes with Growth and Adiposity in Human Milk-Fed Preterm Infants at Term Postmenstrual Age: A Cohort Study. Am. J. Perinatol. 2018, 35, 882–891. [Google Scholar] [CrossRef]
- Lach, L.E.; Chetta, K.E.; Ruddy-Humphries, A.L.; Ebeling, M.D.; Gregoski, M.J.; Katikaneni, L.D. Body Composition and “Catch-Up” Fat Growth in Healthy Small for Gestational Age Preterm Infants and Neurodevelopmental Outcomes. Nutrients 2022, 14, 3051. [Google Scholar] [CrossRef]
- Ramel, S.E.; Gray, H.L.; Davern, B.A.; Demerath, E.W. Body composition at birth in preterm infants between 30 and 36 weeks gestation. Pediatr. Obes. 2015, 10, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Olhager, E.; Tornqvist, C. Body composition in late preterm infants in the first 10 days of life and at full term. Acta Paediatr. 2014, 103, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, C.; Avellina, V.; Luger, B.; Bockmann, K.; Minarski, M.; Maas, C.; Bernhard, W.; Poets, C.F.; Franz, A.R. Body Composition of Preterm Infants following Rapid Transition to Enteral Feeding. Neonatology 2022, 119, 246–254. [Google Scholar] [CrossRef] [PubMed]
- McNelis, K.; Liu, C.; Ehrlich, S.; Fields, C.; Fields, T.; Poindexter, B. Body Composition of Very Low-Birth-Weight Infants Fed Fortified Human Milk: A Pilot Study. JPEN J. Parenter. Enteral Nutr. 2021, 45, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, J.M.; Zhang, L.; Gray, H.L.; Weir, K.; Demerath, E.W.; Ramel, S.E. Body Composition Trajectories From Infancy to Preschool in Children Born Premature Versus Full-term. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e147–e153. [Google Scholar] [CrossRef]
- Gianni, M.L.; Roggero, P.; Piemontese, P.; Morlacchi, L.; Bracco, B.; Taroni, F.; Garavaglia, E.; Mosca, F. Boys who are born preterm show a relative lack of fat-free mass at 5 years of age compared to their peers. Acta Paediatr. 2015, 104, e119–e123. [Google Scholar] [CrossRef]
- Nagel, E.; Hickey, M.; Teigen, L.; Kuchnia, A.; Holm, T.; Earthman, C.; Demerath, E.; Ramel, S. Can Ultrasound Measures of Muscle and Adipose Tissue Thickness Predict Body Composition of Premature Infants in the Neonatal Intensive Care Unit? JPEN J. Parenter. Enteral Nutr. 2021, 45, 323–330. [Google Scholar] [CrossRef]
- Olhager, E.; Danielsson, I.; Sauklyte, U.; Tornqvist, C. Different feeding regimens were not associated with variation in body composition in preterm infants. J. Matern.-Fetal Neonatal Med. 2022, 35, 6403–6410. [Google Scholar] [CrossRef]
- Scheurer, J.M.; Gray, H.L.; Demerath, E.W.; Rao, R.; Ramel, S.E. Diminished growth and lower adiposity in hyperglycemic very low birth weight neonates at 4 months corrected age. J. Perinatol. 2016, 36, 145–150. [Google Scholar] [CrossRef]
- Gianni, M.L.; Consonni, D.; Liotto, N.; Roggero, P.; Morlacchi, L.; Piemontese, P.; Menis, C.; Mosca, F. Does Human Milk Modulate Body Composition in Late Preterm Infants at Term-Corrected Age? Nutrients 2016, 8, 664. [Google Scholar] [CrossRef]
- Beunders, V.A.A.; Roelants, J.A.; Hulst, J.M.; Rizopoulos, D.; Hokken-Koelega, A.C.S.; Neelis, E.G.; de Fluiter, K.S.; Jaddoe, V.W.V.; Reiss, I.K.M.; Joosten, K.F.M.; et al. Early weight gain trajectories and body composition in infancy in infants born very preterm. Pediatr. Obes. 2021, 16, e12752. [Google Scholar] [CrossRef] [PubMed]
- Calek, E.; Binder, J.; Palmrich, P.; Eibensteiner, F.; Thajer, A.; Kainz, T.; Harreiter, K.; Berger, A.; Binder, C. Effects of Intrauterine Growth Restriction (IUGR) on Growth and Body Composition Compared to Constitutionally Small Infants. Nutrients 2023, 15, 4158. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.A.T.; Meio, M.D.B.B.; Moreira, M.E.L.; de Abranches, A.D.; Milanesi, B.G.; Gomes Junior, S.C.S. Energy expenditure and body composition in infants with bronchopulmonary dysplasia at term age. Eur. J. Pediatr. 2022, 181, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.; Menis, C.; Piemontese, P.; Tabasso, C.; Mallardi, D.; Orsi, A.; Amato, O.; Liotto, N.; Roggero, P.; Mosca, F. Energy Expenditure, Protein Oxidation and Body Composition in a Cohort of Very Low Birth Weight Infants. Nutrients 2021, 13, 3962. [Google Scholar] [CrossRef]
- Atchley, C.B.; Cloud, A.; Thompson, D.; Blunt, M.H.; Satnes, K.J.; Szyld, E.; Ernst, K.D. Enhanced Protein Diet for Preterm Infants: A Prospective, Randomized, Double-blind, Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 218–223. [Google Scholar] [CrossRef]
- Bruckner, M.; Khan, Z.; Binder, C.; Morris, N.; Windisch, B.; Holasek, S.; Urlesberger, B. Extremely Preterm Infants Have a Higher Fat Mass Percentage in Comparison to Very Preterm Infants at Term-Equivalent Age. Front. Pediatr. 2020, 8, 61. [Google Scholar] [CrossRef]
- McLeod, G.; Simmer, K.; Sherriff, J.; Nathan, E.; Geddes, D.; Hartmann, P. Feasibility study: Assessing the influence of macronutrient intakes on preterm body composition, using air displacement plethysmography. J. Paediatr. Child Health 2015, 51, 862–869. [Google Scholar] [CrossRef]
- Da Silva Martins, A.; Barbosa Baker Meio, M.D.; Gomes, S.C.S.; Lima, P.A.T.; Milanesi, B.G.; Moreira, M.E.L. Growth and body composition in preterm newborns with bronchopulmonary dysplasia: A cohort study. J. Perinat. Med. 2018, 46, 913–918. [Google Scholar] [CrossRef]
- Van de Lagemaat, M.; Ruys, C.A.; Muts, J.; Finken, M.J.; Rotteveel, J.; van Goudoever, J.B.; Lafeber, H.N.; van den Akker, C.H.; Schrijver-Levie, N.S.; Boonstra, V.; et al. Growth and body composition of infants born moderate-to-late preterm fed a protein- and mineral-enriched postdischarge formula compared with a standard term formula until 6 months corrected age, a randomized controlled trial. Am. J. Clin. Nutr. 2024, 120, 111–120. [Google Scholar] [CrossRef]
- Villela, L.D.; Meio, M.D.B.B.; de Matos Fonseca, V.; de Abranches, A.D.; Junior, S.-C.G.; da Costa, A.C.C.; Murta, M.M.; Nehab, S.R.G.; Soares, F.V.M.; Moreira, M.E.L. Growth and body composition of preterm infants less than or equal to 32 weeks: Cohort study. Early Hum. Dev. 2018, 117, 90–95. [Google Scholar] [CrossRef]
- Ong, M.L.; Cherkerzian, S.; Bell, K.A.; Berger, P.K.; Furst, A.; Sejane, K.; Bode, L.; Belfort, M.B. Human Milk Oligosaccharides, Growth, and Body Composition in Very Preterm Infants. Nutrients 2024, 16, 1200. [Google Scholar] [CrossRef] [PubMed]
- Demerath, E.W.; Johnson, W.; Davern, B.A.; Anderson, C.G.; Shenberger, J.S.; Misra, S.; Ramel, S.E. New body composition reference charts for preterm infants. Am. J. Clin. Nutr. 2017, 105, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.A.; Travers, C.P.; Jerome, M.L.; Chandler-Laney, P.; Carlo, W.A. Percent Body Fat Content Measured by Plethysmography in Infants Randomized to High- or Usual-Volume Feeding after Very Preterm Birth. J. Pediatr. 2021, 230, 251–254.e3. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.M.; Greecher, C.P.; Shaffer, M.L.; Shenberger, J.S. Potential influence of total parenteral nutrition on body composition at discharge in preterm infants. J. Matern.-Fetal Neonatal Med. 2013, 26, 1548–1553. [Google Scholar] [CrossRef]
- Morris, E.E.; Miller, N.C.; Haapala, J.L.; Georgieff, M.K.; Ramel, S.E. Preterm infant body composition, working memory, and temperament. Infant Behav. Dev. 2023, 70, 101808. [Google Scholar] [CrossRef]
- Morlacchi, L.; Roggero, P.; Gianni, M.L.; Bracco, B.; Porri, D.; Battiato, E.; Menis, C.; Liotto, N.; Mallardi, D.; Mosca, F. Protein use and weight-gain quality in very-low-birth-weight preterm infants fed human milk or formula. Am. J. Clin. Nutr. 2018, 107, 195–200. [Google Scholar] [CrossRef]
- Roggero, P.; Giannì, M.L.; Liotto, N.; Taroni, F.; Orsi, A.; Amato, O.; Morlacchi, L.; Piemontese, P.; Agosti, M.; Mosca, F. Rapid recovery of fat mass in small for gestational age preterm infants after term. PLoS ONE 2011, 6, e14489. [Google Scholar] [CrossRef]
- Parat, S.; Raza, P.; Kamleh, M.; Super, D.; Groh-Wargo, S. Targeted Breast Milk Fortification for Very Low Birth Weight (VLBW) Infants: Nutritional Intake, Growth Outcome and Body Composition. Nutrients 2020, 12, 1156. [Google Scholar] [CrossRef]
- Roggero, P.; Gianni, M.L.; Amato, O.; Piemontese, P.; Morniroli, D.; Wong, W.W.; Mosca, F. Evaluation of air-displacement plethysmography for body composition assessment in preterm infants. Pediatr. Res. 2012, 72, 316–320. [Google Scholar] [CrossRef]
- Pereira-da-Silva, L.; Barradas, S.; Moreira, A.C.; Alves, M.; Papoila, A.L.; Virella, D.; Cordeiro-Ferreira, G. Evolution of Resting Energy Expenditure, Respiratory Quotient, and Adiposity in Infants Recovering from Corrective Surgery of Major Congenital Gastrointestinal Tract Anomalies: A Cohort Study. Nutrients 2020, 12, 3093. [Google Scholar] [CrossRef]
- Infection Control. Isolation Precautions–Guidelines Library. Available online: https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html (accessed on 26 March 2023).
- Hamatschek, C.; Yousuf, E.I.; Möllers, L.S.; So, H.Y.; Morrison, K.M.; Fusch, C.; Rochow, N. Fat and Fat-Free Mass of Preterm and Term Infants from Birth to Six Months: A Review of Current Evidence. Nutrients 2020, 12, 288. [Google Scholar] [CrossRef]
- Norris, T.; Ramel, S.E.; Catalano, P.; Caoimh, C.N.; Roggero, P.; Murray, D.; Fields, D.A.; Demerath, E.W.; Johnson, W. New charts for the assessment of body composition, according to air-displacement plethysmography, at birth and across the first 6 month of life. Am. J. Clin. Nutr. 2019, 109, 1353–1360. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luecke, L.A.; Fusch, C.; Weiss, G.A.; Knab, K.; Schäfer, S.; Zimmermann, J.L.; Meis, A.; Lohmüller-Weiß, S.; Simon, K.; Welsch, J.; et al. Standardizing Neonatal Body Composition Assessment Using Air Displacement Plethysmography: Insights from the Bavarian Experience. Children 2025, 12, 733. https://doi.org/10.3390/children12060733
Luecke LA, Fusch C, Weiss GA, Knab K, Schäfer S, Zimmermann JL, Meis A, Lohmüller-Weiß S, Simon K, Welsch J, et al. Standardizing Neonatal Body Composition Assessment Using Air Displacement Plethysmography: Insights from the Bavarian Experience. Children. 2025; 12(6):733. https://doi.org/10.3390/children12060733
Chicago/Turabian StyleLuecke, Lennart A., Christoph Fusch, Gisela Adrienne Weiss, Katja Knab, Stefan Schäfer, Jasper L. Zimmermann, Anastasia Meis, Stephanie Lohmüller-Weiß, Kerstin Simon, Julia Welsch, and et al. 2025. "Standardizing Neonatal Body Composition Assessment Using Air Displacement Plethysmography: Insights from the Bavarian Experience" Children 12, no. 6: 733. https://doi.org/10.3390/children12060733
APA StyleLuecke, L. A., Fusch, C., Weiss, G. A., Knab, K., Schäfer, S., Zimmermann, J. L., Meis, A., Lohmüller-Weiß, S., Simon, K., Welsch, J., Felderhoff-Müser, U., & Rochow, N. (2025). Standardizing Neonatal Body Composition Assessment Using Air Displacement Plethysmography: Insights from the Bavarian Experience. Children, 12(6), 733. https://doi.org/10.3390/children12060733







