Impact of Active and Passive Maxillary Plates on Cleft Width Morphology in Unilateral Cleft Lip and Palate: A Prospective Intervention Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Patients and Study Groups
2.3. Inclusion and Exclusion Criteria
2.4. Sample Size Calculation
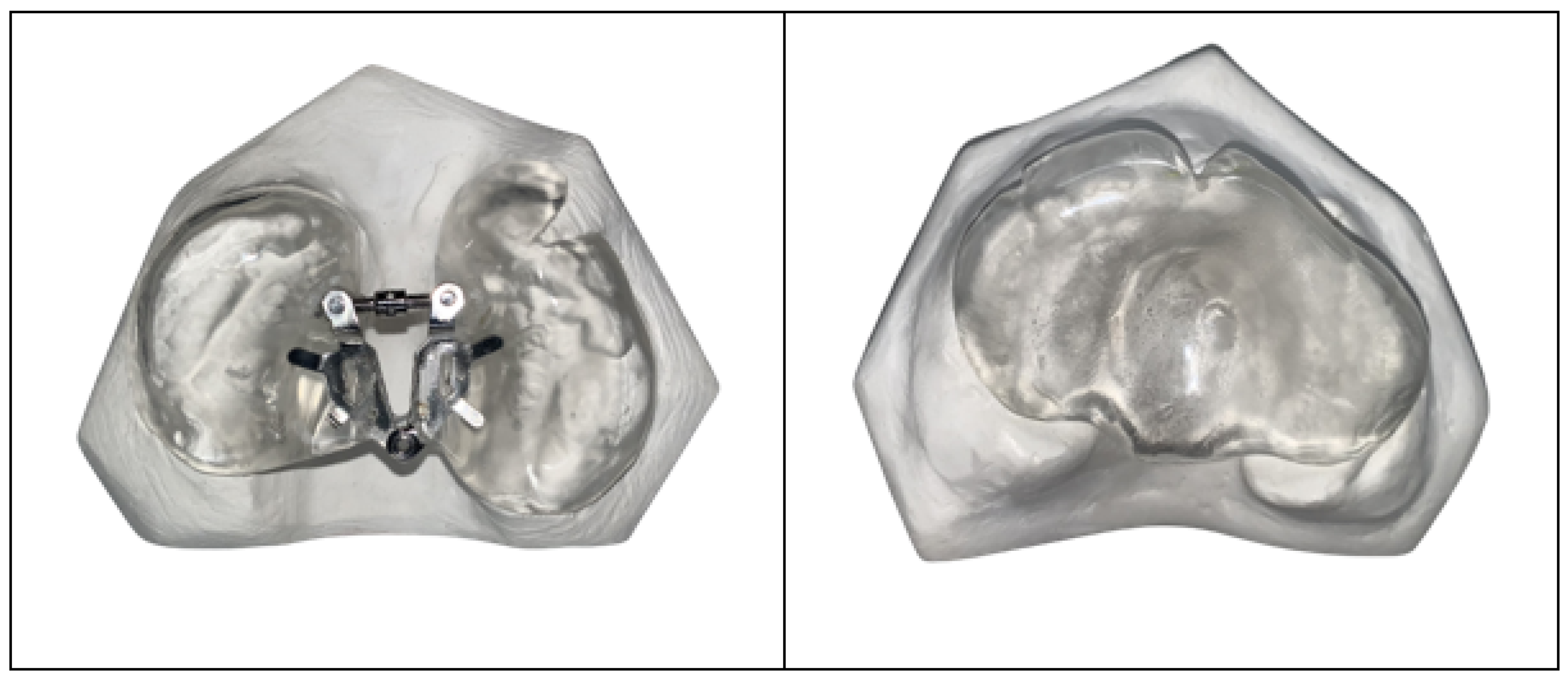
2.5. Fabrication of Maxillary Plates
2.6. Presurgical Infant Orthopedic Therapy with Maxillary Plates
2.7. Measurements
2.8. Statistical Analysis
3. Results
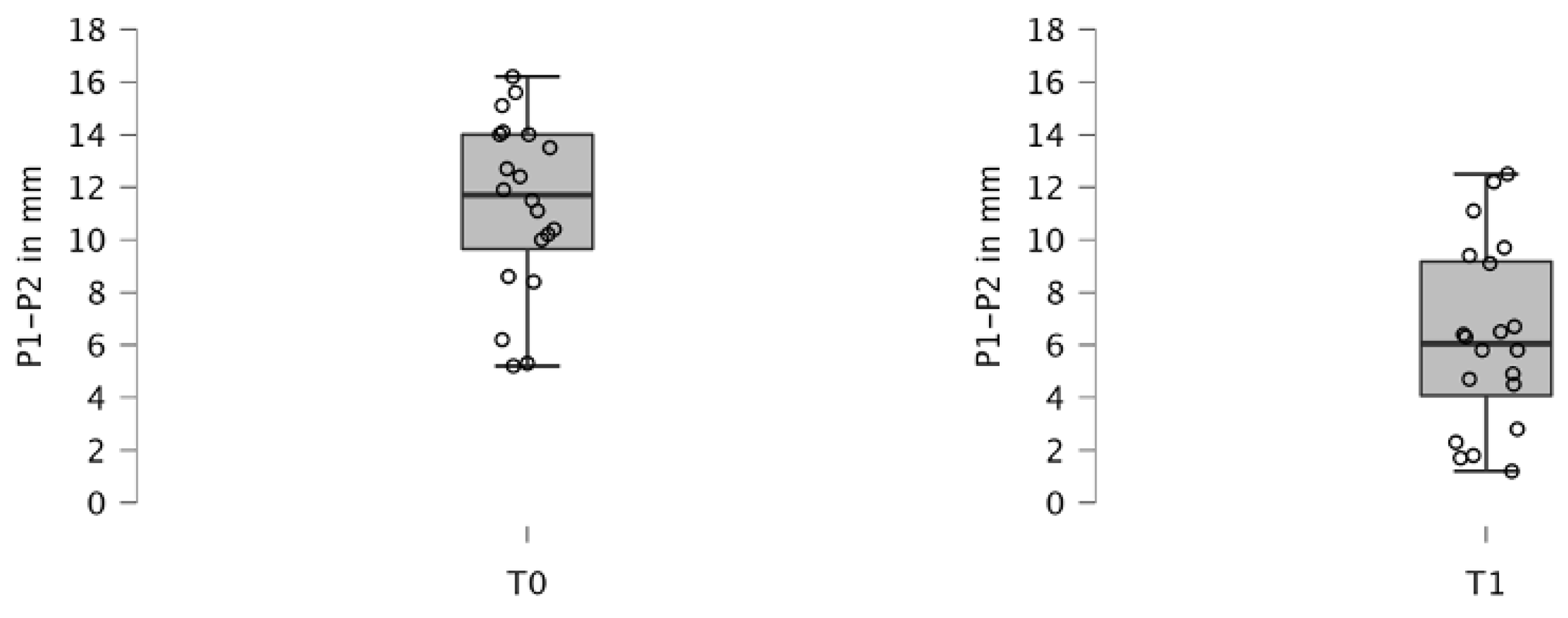
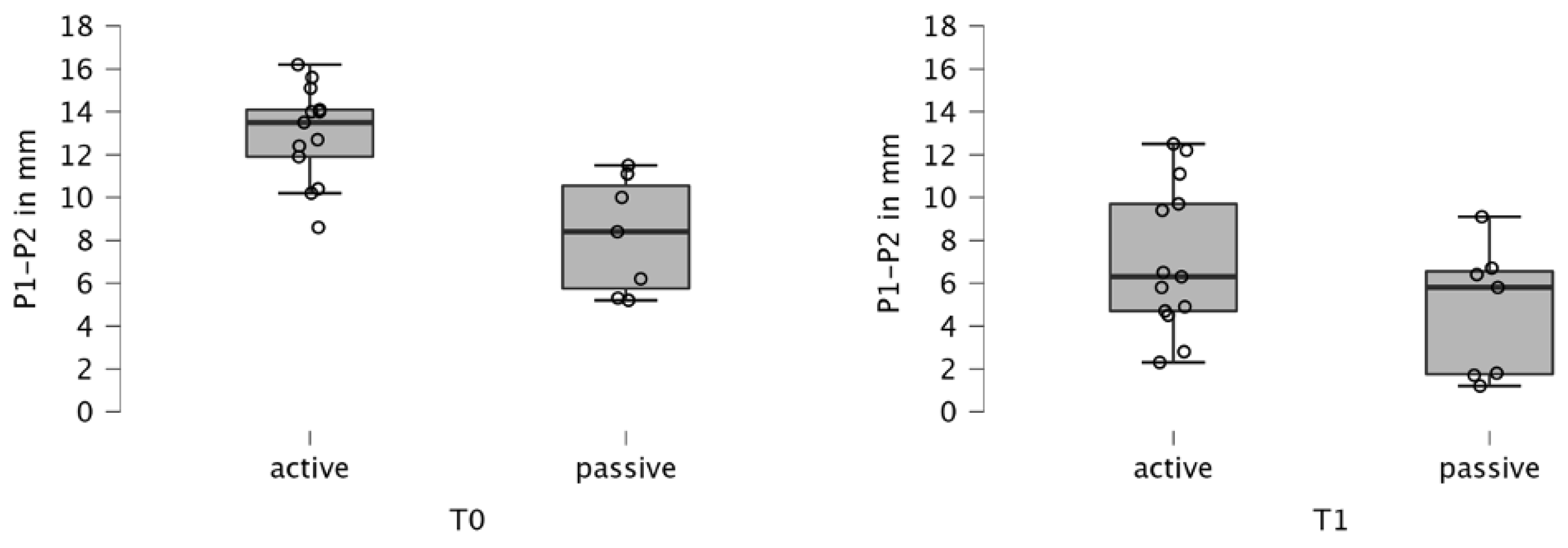
3.1. Cleft Width
3.2. Transversal Measurements
3.3. Sagittal Measurements
3.4. Segmental Arch Measurements
3.5. Angular Measurements
3.6. Manual Versus Digital Measurement
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UCLP | Unilateral Cleft Lip and Palate |
References
- Sader, R. Lippen-Kiefer-Gaumen-Segelspalten. Pädiatrie Up2date 2009, 4, 183–205. [Google Scholar] [CrossRef]
- Tian, H.; Feng, J.; Li, J.; Ho, T.V.; Yuan, Y.; Liu, Y.; Brindopke, F.; Figueiredo, J.C.; Magee, W., 3rd; Sanchez-Lara, P.A.; et al. Intraflagellar transport 88 (IFT88) is crucial for craniofacial development in mice and is a candidate gene for human cleft lip and palate. Hum. Mol. Genet. 2017, 26, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W. Global strategies to reduce the health care burden of craniofacial anomalies: Report of WHO meetings on international collaborative research on craniofacial anomalies. Cleft Palate Craniofac. J. 2004, 41, 238–243. [Google Scholar] [CrossRef]
- Suri, S.; Disthaporn, S.; Atenafu, E.G.; Fisher, D.M. Presurgical presentation of columellar features, nostril anatomy, and alveolar alignment in bilateral cleft lip and palate after infant orthopedics with and without nasoalveolar molding. Cleft Palate Craniofac. J. 2012, 49, 314–324. [Google Scholar] [CrossRef]
- Liao, Y.F.; Cole, T.J.; Mars, M. Hard palate repair timing and facial growth in unilateral cleft lip and palate: A longitudinal study. Cleft Palate Craniofac. J. 2006, 43, 547–556. [Google Scholar] [CrossRef]
- Brand, S.; Blechschmidt, A.; Müller, A.; Sader, R.; Schwenzer-Zimmerer, K.; Zeilhofer, H.F.; Holsboer-Trachsler, E. Psychosocial functioning and sleep patterns in children and adolescents with cleft lip and palate (CLP) compared with healthy controls. Cleft Palate Craniofac. J. 2009, 46, 124–135. [Google Scholar] [CrossRef]
- Hotz, M.M.; Gnoinski, W.M. Effects of early maxillary orthopaedics in coordination with delayed surgery for cleft lip and palate. J. Maxillofac. Surg. 1979, 7, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Schopf, P. Werkstoffe, festsitzende Apparaturen, kieferorthopädische Therapie, interdisziplinäre Aspekte. In Curriculum Kieferorthopädie/Peter Schopf; Quintessenz Verlags-GmbH: Berlin, Germany, 2008; Volume 2. [Google Scholar]
- Habel, A.; Sell, D.; Mars, M. Management of cleft lip and palate. Arch. Dis. Child. 1996, 74, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Gnoinski, W.; Nussbaumer, H. Joint treatment of patients with clefts by orthodontists and logopedists. SSO Schweiz Monatsschr Zahnheilkd 1976, 86, 375–386. [Google Scholar]
- Schwenzer, N.; Ehrenfeld, M. Mund-Kiefer-Gesichtschirurgie; Georg Thieme Verlag: New York, NY, USA, 2010. [Google Scholar]
- Kriens, O.; Bertzbach, P. Model analysis of the maxilla in newborn infants with unilateral cheilognathopalatoschisis. Fortschr. Kieferorthop. 1986, 47, 385–390. [Google Scholar] [CrossRef]
- Kozelj, V. Changes produced by presurgical orthopedic treatment before cheiloplasty in cleft lip and palate patients. Cleft Palate Craniofac. J. 1999, 36, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Hotz, M.; Gnoinski, W. Multidisziplinäre Betreuung von Patienten mit Lippen-Kiefer-Gaumenspalten: Vorläufige Ergebnisse. Inf. Orthodont. U. Kieferorthop. 1977, 9, 89–114. [Google Scholar]
- Bolter, H. Oberkiefer- Alveolarbogenmasse bei LKG-Spaltträgern. Nach der Geburt und mit 5 Jahren: Eine Standortbestimmung der primären Behandlung in Zürich. Doctoral Dissertation, Verlag nicht ermittelbar, Zürich, Switzerland, 1979. [Google Scholar]
- Opitz, C.; Kratzsch, H. Oberkieferdimension bei Patienten mit ein- und doppelseitiger Lippen-Kiefer-Gaumen-Spalte. J. Orofac. Orthop/Fortschr. Kieferorthop. 1997, 58, 110–123. [Google Scholar] [CrossRef]
- Dallaserra, M.; Pantoja, T.; Salazar, J.; Araya, I.; Yanine, N.; Villanueva, J. Effectiveness of pre-surgical orthopedics on patients with cleft lip and palate: A systematic review and meta-analysis. J. Stomatol. Oral. Maxillofac. Surg. 2022, 123, e506–e520. [Google Scholar] [CrossRef]
- Shen, C.; Yao, C.A.; Magee, W., 3rd; Chai, G.; Zhang, Y. Presurgical nasoalveolar molding for cleft lip and palate: The application of digitally designed molds. Plast Reconstr. Surg. 2015, 135, 1007e–1015e. [Google Scholar] [CrossRef]
- Ambrosio, E.C.P.; Sforza, C.; De Menezes, M.; Carrara, C.F.C.; Machado, M.; Oliveira, T.M. Post-surgical effects on the maxillary segments of children with oral clefts: New three-dimensional anthropometric analysis. J. Craniomaxillofac Surg. 2018, 46, 1511–1514. [Google Scholar] [CrossRef]
- Saad, M.S.; Fata, M.; Farouk, A.; Habib, A.M.A.; Gad, M.; Tayel, M.B.; Marei, M.K. Early Progressive Maxillary Changes with Nasoalveolar Molding: Randomized Controlled Clinical Trial. JDR Clin. Trans. Res. 2020, 5, 319–331. [Google Scholar] [CrossRef]
- Sabri, T.K. Dreidimensionale Auswertung des Wachstumsmechanismus bei Einseitiger Lippen-Kiefer-Gaumenspalte Nach Behandlung Mit der Modifizierten Trennplatte. Doctoral dissertation, Johann Wolfgang Goethe University Frankfurt am Main, Frankfurt, Germany, 2015. [Google Scholar]
- Mazaheri, M.; Harding, R.L.; Cooper, J.A.; Meier, J.A.; Jones, T.S. Changes in arch form and dimensions of cleft patients. Am. J. Orthod. 1971, 60, 19–32. [Google Scholar] [CrossRef]
- Ashley-Montague, M. The form and dimensions of the palate in the newborn. Int. J. Orthod. 1934, 20, 694–704. [Google Scholar]
- Sillman, J.H. Serial study of good occlusion from birth to 12 years of age. Am. J. Orthod. 1951, 37, 481–507. [Google Scholar] [CrossRef]
- García Abuabara, A.N.; Drescher, D. Development on the Maxillary of Patients with a Unilateral Total Cleft with the Use of a Orthopaedic Plate. Two-dimensional Cast Analysis. Rev. Colomb. Investig. Odontol. 2010, 1, 193–201. [Google Scholar]
- Burgaz, M.A.; Cakan, D.G.; Yılmaz, R.B.N. Three-dimensional evaluation of alveolar changes induced by nasoalveolar molding in infants with unilateral cleft lip and palate: A case-control study. Korean J. Orthod. 2019, 49, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Seckel, N.G.; van der Tweel, I.; Elema, G.A.; Specken, T.F. Landmark positioning on maxilla of cleft lip and palate infant--a reality? Cleft Palate Craniofac. J. 1995, 32, 434–441. [Google Scholar] [CrossRef]
- Kahl, B. Frühbehandlung von Kindern mit Lippen-Kiefer-Gaumen-Spalten—Kieferorthopädische Aspekte. Fortschritte Kieferorthopädie 1990, 51, 218–225. [Google Scholar] [CrossRef]
- Erkan, M.; Karaçay, S.; Atay, A.; Günay, Y. A modified feeding plate for a newborn with cleft palate. Cleft Palate Craniofac. J. 2013, 50, 109–112. [Google Scholar] [CrossRef]
- Nalabothu, P.; Benitez, B.K.; Dalstra, M.; Verna, C.; Mueller, A.A. Three-Dimensional Morphological Changes of the True Cleft under Passive Presurgical Orthopaedics in Unilateral Cleft Lip and Palate: A Retrospective Cohort Study. J. Clin. Med. 2020, 9, 962. [Google Scholar] [CrossRef]
- Grabowski, R.; Kopp, H.; Stahl, F.; Gundlach, K.K. Presurgical orthopaedic treatment of newborns with clefts--functional treatment with long-term effects. J. Craniomaxillofac. Surg. 2006, 34 (Suppl. S2), 34–44. [Google Scholar] [CrossRef]
- Martelli, D.R.; Machado, R.A.; Swerts, M.S.; Rodrigues, L.A.; Aquino, S.N.; Martelli Júnior, H. Non syndromic cleft lip and palate: Relationship between sex and clinical extension. Braz. J. Otorhinolaryngol. 2012, 78, 116–120. [Google Scholar] [CrossRef]
- Sharif, F.; Mahmood, F.; Azhar, M.J.; Asif, A.; Zahid, M.; Muhammad, N.; Rehman, I.U.; Neil, S.M. Incidence and management of cleft lip and palate in Pakistan. J. Pak. Med. Assoc. 2019, 69, 632–639. [Google Scholar]
- Kim, S.; Kim, W.J.; Oh, C.; Kim, J.C. Cleft lip and Palate Incidence Among the Live Births in the Republic of Korea. JKMS 2009, 17, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Neuschulz, J.; Schaefer, I.; Scheer, M.; Christ, H.; Braumann, B. Maxillary reaction patterns identified by three-dimensional analysis of casts from infants with unilateral cleft lip and palate. J. Orofac. Orthop. 2013, 74, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Braumann, B.; Rosenhayn, S.E.; Bourauel, C.; Jäger, A. Two- or three-dimensional cast analysis in patients with cleft lip and palate? J. Orofac. Orthop. 2001, 62, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Hoffmannova, E.; Moslerová, V.; Dupej, J.; Borský, J.; Bejdová, Š.; Velemínská, J. Three-dimensional development of the upper dental arch in unilateral cleft lip and palate patients after early neonatal cheiloplasty. Int. J. Pediatr. Otorhinolaryngol. 2018, 109, 1–6. [Google Scholar] [CrossRef]
- Ambrosio, E.C.P.; Sforza, C.; De Menezes, M.; Gibelli, D.; Codari, M.; Carrara, C.F.C.; Machado, M.; Oliveira, T.M. Longitudinal morphometric analysis of dental arch of children with cleft lip and palate: 3D stereophotogrammetry study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2018, 126, 463–468. [Google Scholar] [CrossRef]
- Garland, K.; Coyle, M.; Foley, T.; Matic, D. Ten-Year Cephalometric Comparison of Patients with Cleft Palate who Received Treatment with Active or Passive Pre-surgical Orthopedic Devices. Cleft Palate Craniofac. J. 2023, 60, 1359–1365. [Google Scholar] [CrossRef]
- Kornbluth, M.; Campbell, R.E.; Daskalogiannakis, J.; Ross, E.J.; Glick, P.H.; Russell, K.A.; Doucet, J.C.; Hathaway, R.R.; Long, R.E., Jr.; Sitzman, T.J. Active Presurgical Infant Orthopedics for Unilateral Cleft Lip and Palate: Intercenter Outcome Comparison of Latham, Modified McNeil, and Nasoalveolar Molding. Cleft Palate Craniofac. J. 2018, 55, 639–648. [Google Scholar] [CrossRef]
- Matic, D.B.; Power, S.M. The effects of gingivoperiosteoplasty following alveolar molding with a pin-retained Latham appliance versus secondary bone grafting on midfacial growth in patients with unilateral clefts. Plast. Reconstr. Surg. 2008, 122, 863–870. [Google Scholar] [CrossRef]
- Cruz, C. Presurgical Orthopedics Appliance: The Latham Technique. Oral. Maxillofac. Surg. Clin. N. Am. 2016, 28, 161–168. [Google Scholar] [CrossRef]
- Power, S.M.; Matic, D.B. Gingivoperiosteoplasty following alveolar molding with a Latham appliance versus secondary bone grafting: The effects on bone production and midfacial growth in patients with bilateral clefts. Plast. Reconstr. Surg. 2009, 124, 573–582. [Google Scholar] [CrossRef]
- Winnand, P.; Ooms, M.; Heitzer, M.; Schaffrath, K.; Pankert, T.; Hölzle, F.; Raith, S.; Modabber, A. Defining biomechanical principles in pre-surgical infant orthopedics in a real cleft finite element model. Int. J. Oral Maxillofac. Surg. 2024; In Press, Corrected Proof. [Google Scholar] [CrossRef]
- Kiya, K.; Oyama, T.; Sone, Y.; Ishii, N.; Hosokawa, K. A novel active intraoral appliance for presurgical orthopaedic treatment in patients with complete bilateral cleft lip and palate. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 632–637. [Google Scholar] [CrossRef]
- Oosterkamp, B.C.; van Oort, R.P.; Dijkstra, P.U.; Stellingsma, K.; Bierman, M.W.; de Bont, L.G. Effect of an intraoral retrusion plate on maxillary arch dimensions in complete bilateral cleft lip and palate patients. Cleft Palate Craniofac. J. 2005, 42, 239–244. [Google Scholar] [CrossRef]
- Budak, H.; Yilmaz, H.N. Evaluation of the Reliability of Facial Models Digitalized with Different Imaging Methods in Cleft Lip and Palate. Cleft Palate Craniofac. J. 2025, 10556656251314264. [Google Scholar] [CrossRef]
- Luu, N.S.; Nikolcheva, L.G.; Retrouvey, J.M.; Flores-Mir, C.; El-Bialy, T.; Carey, J.P.; Major, P.W. Linear measurements using virtual study models. Angle Orthod. 2012, 82, 1098–1106. [Google Scholar] [CrossRef]
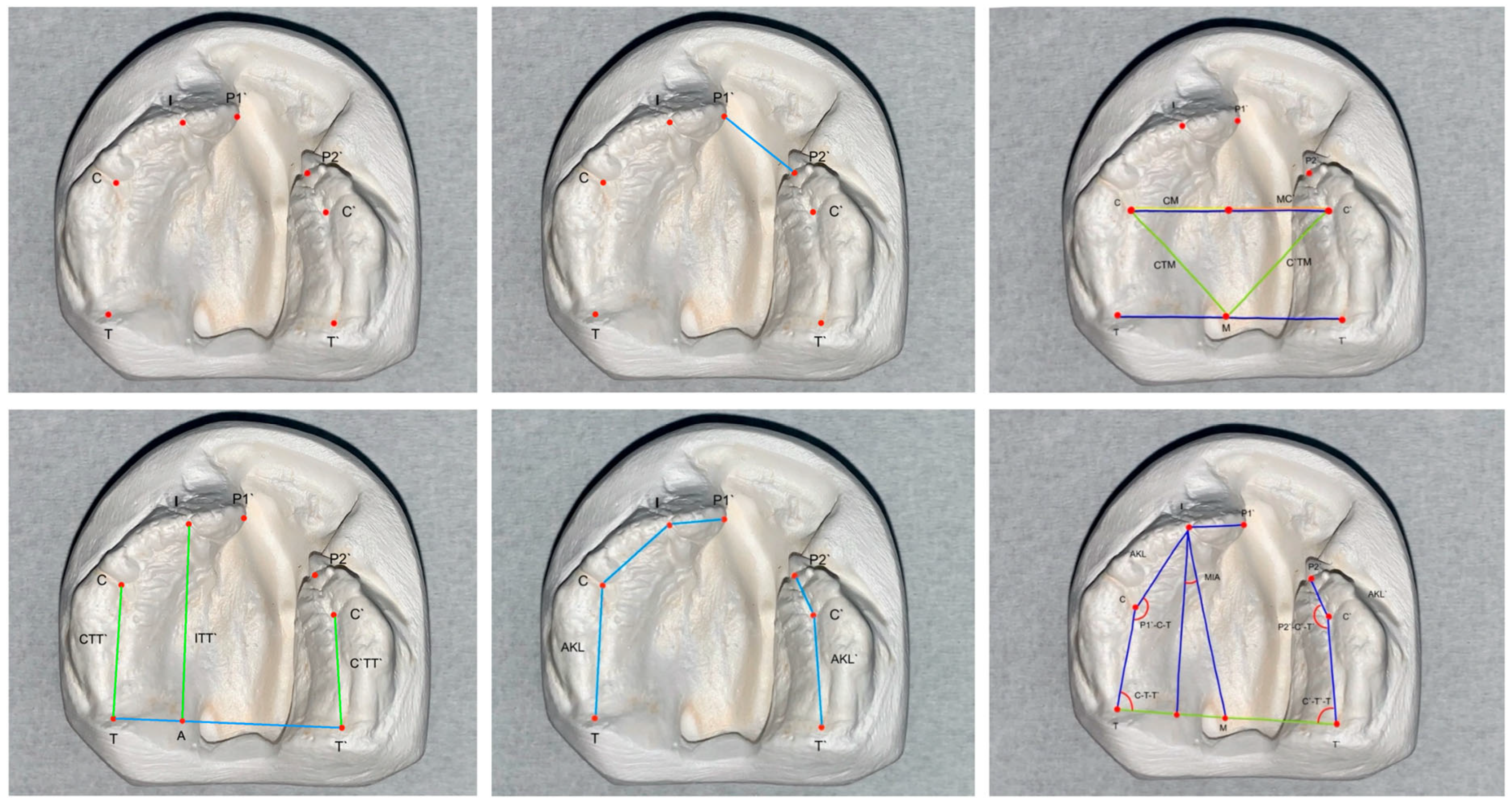
| Abbreviation | Landmark | Definition |
|---|---|---|
| I | Incisal Point | Intersection point of the alveolar ridge with the connecting line of the incisive papilla and the labial frenulum |
| P1 | Alveolar Gap Point Long | Outermost and foremost point of the alveolar ridge, long segment |
| P2 | Alveolar Gap Point Short | Outermost and foremost point of the alveolar ridge, short segment |
| C | C-Point Right | Lateral Sulcus Point Intersection point between the lateral sulcus and the alveolar ridge line on the right side |
| C’ | C-Point Left | Lateral Sulcus Point Intersection point between the lateral sulcus and the alveolar ridge line on the left side |
| T | Tubercle Point Right | Most distal point of the alveolar ridge on the right side |
| T’ | Tubercle Point Left | Most distal point of the alveolar ridge on the left side |
| M | Midpoint | Midpoint of the line segment TT’ |
| A | A-Point | Intersection point of the perpendicular from the TT’ line through point I |
| CT | CT-Point | Intersection point of the perpendicular from the TT’ line through point C |
| CIA | CIA-Point | Intersection point between CC’ line and IA line |
| Type of Stratification | Measurement Time | N | Mean (mm) | SD (mm) | p-Value | |
|---|---|---|---|---|---|---|
| Overall assessment | T0 | 20 | 11.320 | 3.296 | NA | |
| T1 | 20 | 6.270 | 3.475 | NA | ||
| T0–T1 | 20 | 5.050 | 2.210 | <0.001 *A | ||
| Type of appliance | AP | T0 | 14 | 12.9777 | 2.245 | NA |
| T1 | 14 | 7.131 | 3.474 | NA | ||
| T0–T1 | 14 | 5.846 | 2.334 | <0.001 *A 0.024 **B | ||
| PP | T0 | 6 | 8.243 | 2.707 | NA | |
| T1 | 6 | 4.671 | 3.085 | NA | ||
| T0–T1 | 6 | 3.571 | 0.804 | <0.001 *A 0.024 **B | ||
| Gender | M | T0 | 16 | 11.313 | 3.577 | NA |
| T1 | 16 | 5.969 | 3.690 | NA | ||
| T0–T1 | 16 | 5.344 | 2.264 | <0.001 *A 0.245 C | ||
| F | T0 | 4 | 11.350 | 2.198 | NA | |
| T1 | 4 | 7.475 | 2.445 | NA | ||
| T0–T1 | 4 | 3.875 | 1.735 | <0.001 *A 0.245 C | ||
| Cleft side | L | T0 | 11 | 10.345 | 3.707 | NA |
| T1 | 11 | 5.227 | 3.449 | NA | ||
| T0–T1 | 11 | 5.118 | 1.847 | <0.001 *A 0.884 D | ||
| R | T0 | 9 | 12.511 | 2.392 | NA | |
| T1 | 9 | 7.544 | 3.238 | NA | ||
| T0–T1 | 9 | 4.967 | 2.706 | <0.001 *A 0.884 D | ||
| Group | Time | CC’ | TT’ | CTM | C’T’M | CM | MC’ | AM |
|---|---|---|---|---|---|---|---|---|
| Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | ||
| CLP-AP | T0 | 26.10 ± 2.52 | 30.51 ± 2.40 | 19.08 ± 2.57 | 18.64 ± 1.97 | 12.92 ± 1.88 | 13.12 ± 1.99 | 0.74 ± 7.89 |
| T1 | 27.61 ± 4.19 | 31.42 ± 2.47 | 21.45 ± 3.45 | 19.77 ± 1.89 | 14.450 ± 2.75 | 13.07 ± 2.33 | 0.60 ± 6.94 | |
| T2 | 27.09 ± 3.07 | 34.21 ± 3.33 | 23.45 ± 3.11 | 22.81 ± 2.45 | 13.49 ± 1.43 | 13.46 ± 2.91 | 0.49 ± 3.05 | |
| T0–T1 | 1.51 ± 5.51 | 0.91 ± 2.57 | 2.37 ± 3.95 *A | 1.13 ± 2.09 | 1.00 ± 2.77 | −0.05 ± 2.42 | −0.14 ± 5.78 | |
| T1–T2 | −0.52 ± 5.08 | 2.79 ± 4.61 *B | 2.00 ± 2.71 *B | 3.04 ± 3.18 *B | −7.96 ± 3.69 | 0.39 ± 3.18 | −0.11 ± 5.69 | |
| T0–T2 | 0.99 ± 3.93 | 3.90 ± 3.99 *C | 4.37 ± 3.39 *C | 4.17 ± 3.77 *C | 0.57 ± 2.57 | 0.34 ± 3.08 | −1.90 ± 12.93 | |
| CLP-PP | T0 | 24.62 ± 2.84 | 32.58 ± 4.49 | 18.4 ± 2.67 | 19.47 ± 1.93 | 11.73 ± 1.41 | 12.83 ± 1.85 | −1.455 ± 5.28 |
| T1 | 25.82 ± 2.61 | 33.22 ± 4.25 | 20.38 ± 2.10 | 20.72 ± 1.73 | 12.50 ± 1.97 | 13.27 ± 1.47 | −0.12 ± 9.69 | |
| T2 | 25.55 ± 4.39 | 35.65 ± 3.54 | 24.55 ± 4.35 | 24.42 ± 2.72 | 12.07 ± 2.40 | 13.30 ± 2.88 | −1.30 ± 2.56 | |
| T0–T1 | 1.20 ± 2.84 | 0.63 ± 1.71 | 1.98 ± 1.76 *A | 1.25 ± 1.25 | 0.77 ± 2.72 | 0.43 ± 1.30 | 1.33 ± 7.34 | |
| T1–T2 | −0.27 ± 4.04 | 2.43 ± 2.97 | 4.17 ± 4.55 | 3.70 ± 3.48 | −0.43 ± 3.16 | 0.03 ± 1.73 | −1.18 ± 11.18 | |
| T0–T2 | 0.93 ± 1.96 | 3.07 ± 3.26 | 6.15 ± 3.67 *C | 4.95 ± 3.63 *C | 0.33 ± 1.99 | 0.47 ± 1.88 | 0.15 ± 6.62 | |
| CP | T0 | 20.18 ± 2.24 | 27.78 ± 3.86 | 17.13 ± 2.50 | 17.18 ± 2.08 | NA | NA | NA |
| T1 | 21.75 ± 3.62 | 27.91 ± 3.01 | 20.79 ± 3.57 | 20.63 ± 2.91 | NA | NA | NA | |
| T2 | 25.95 ± 2.39 | 33.45 ± 2.65 | 25.26 ± 2.07 | 25.354 | NA | NA | NA | |
| T0–T1 | 1.57 ± 2.46 | 0.13 ± 1.88 | 3.66 ± 2.86 *A | 3.45 ± 2.61 *A | NA | NA | NA | |
| T1–T2 | 4.19 ± 1.96 *B | 5.55 ± 3.62 *B | 4.47 ± 2.61 *B | 4.73 ± 2.36 *B | NA | NA | NA | |
| T0–T2 | 5.76 ± 2.02 *C | 5.67 ± 4.07 *C | 8.14 ± 2.19 *C | 8.17 ± 1.62 *C | NA | NA | NA |
| Group | Time | P1/P1‘-TT‘ | P2/P2‘-TT’ | I-TT’ (=I-A) | I-CC’ | C-TT’ | C‘-TT’ |
|---|---|---|---|---|---|---|---|
| Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | ||
| CLP-AP | T0 | 23.72 ± 3.31 | 18.27 ± 2.20 | 22.81 ± 2.62 | 8.66 ± 2.01 | 13.83 ± 3.31 | 13.21 ± 1.84 |
| T1 | 24.83 ± 3.60 | 21.58 ± 2.31 | 25.24 ± 2.84 | 9.35 ± 3.13 | 15.69 ± 2.57 | 15.58 ± 1.37 | |
| T2 | NA | NA | 26.48 ± 3.47 | 7.58 ± 3.17 | 19.73 ± 4.56 | 19.07 ± 4.56 | |
| T0–T1 | 1.11 ± 3.40 | 3.31 ± 2.66 *A | 1.90 ± 1.70 **A | 1.40 ± 1.17 | 1.10 ± 2.35 | 2.36 ± 2.55 *A | |
| T1–T2 | NA | NA | 1.24 ± 3.88 | −1.77 ± 3.10 | 4.04 ± 3.61 *B | 3.49 ± 4.37 *B | |
| T0–T2 | NA | NA | 3.67 ± 3.15 *C | −1.08 ± 3.79 | 5.90 ± 5.49 *B | 5.86 ± 5.19 *C | |
| CLP-PP | T0 | 22.20 ± 1.87 | 18.92 ± 1.73 | 20.98 ± 3.24 | 6.53 ± 1.80 | 14.07 ± 2.62 | 14.62 ± 2.04 |
| T1 | 25.67 ± 3.69 | 22.28 ± 2.20 | 26.78 ± 4.32 | 11.42 ± 2.43 | 16.20 ± 3.78 | 13.93 ± 2.21 | |
| T2 | NA | NA | 25.97 ± 2.55 | 7.52 ± 1.35 | 19.52 ± 3.74 | 17.83 ± 2.20 | |
| T0–T1 | 3.47 ± 5.34 | 3.37 ± 3.84 | 5.80 ± 7.37 | 4.88 ± 3.88 *A | 2.13 ± 6.36 | −0.72 ± 3.76 | |
| T1–T2 | NA | NA | −0.82 ± 5.27 | −3.90 ± 3.15 *B | 3.32 ± 3.11 | 4.20 ± 1.54 *B | |
| T0–T2 | NA | NA | 4.98 ± 2.94 *C | 0.98 ± 1.99 | 5.45 ± 5.84 | 3.48 ± 3.37 | |
| CP | T0 | NA | NA | 20.00 ± 2.72 | 6.11 ± 1.53 | 13.65 ± 2.17 | 14.09 ± 2.55 |
| T1 | NA | NA | 24.05 ± 2.59 | 14.63 ± 6.03 | 17.56 ± 3.18 | 18.01 ± 3.07 | |
| T2 | NA | NA | 28.88 ± 3.46 | 7.35 ± 2.27 | 21.59 ± 2.29 | 21.85 ± 2.15 | |
| T0–T1 | NA | NA | 4.05 ± 2.94 *A | 8.52 ± 5.49 *A | 3.92 ± 3.05 *A | 3.92 ± 3.55 *A | |
| T1–T2 | NA | NA | 4.84 ± 3.64 *B | −7.27 ± 5.10 *B | 4.03 ± 3.12 *B | 3.84 ± 3.05 *B | |
| T0–T2 | NA | NA | 8.88 ± 3.37 *C | 1.25 ± 2.43 | 7.95 ± 2.58 *C | 7.75 ± 2.45 *C |
| Group | Time | P1/P1’-I | I-C | C-T | AKL (P1-I-C-T) | P2/P2’-C’ | C‘-T‘ | AKL’ (P2-C’-T’) |
|---|---|---|---|---|---|---|---|---|
| Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | Mean/Median ± SD/IQR (mm) | ||
| CLP-AP | T0 | 7.29 ± 2.03 | 10.64 ± 2.03 | 14.10 ± 3.58 | 28.53 ± 6.15 | 6.80 ± 1.40 | 13.59 ± 1.64 | 22.86 ± 5.35 |
| T1 | 8.89 ± 2.16 | 13.09 ± 4.09 | 16.00 ± 2.63 | 35.52 ± 7.21 | 8.98 ± 1.92 | 15.90 ± 1.41 | 25.47 ± 3.76 | |
| T2 | NA | 14.97 ± 3.54 | 20.28 ± 4.52 | 35.09 ± 3.98 | NA | 19.64 ± 4.77 | 31.05 ± 6.39 | |
| T0–T1 | 1.60 ± 2.28 *A | 1.89 ± 4.37 | 0.80 ± 2.45 | 6.99 ± 4.91 *A | 2.18 ± 2.23 *A | 2.31 ± 2.20 *A | 2.61 ± 5.81 | |
| T1–T2 | NA | 1.89 ± 4.37 *B | 4.28 ± 3.63 *B | −0.43 ± 9.45 | NA | 4.80 ± 4.27 **B | 5.58 ± 7.60 *B | |
| T0–T2 | NA | 4.34 ± 3.93 *C | 6.18 ± 5.95 *C | 6.56 ± 8.53 *C | NA | 6.75 ± 7.03 **C | 8.19 ± 6.83 *C | |
| CLP-PP | T0 | 6.62 ± 2.06 | 9.87 ± 2.77 | 14.88 ± 2.57 | 30.18 ± 4.51 | 7.23 ± 1.40 | 15.07 ± 1.99 | 22.22 ± 3.77 |
| T1 | 11.27 ± 2.36 | 14.53 ± 2.93 | 16.77 ± 3.69 | 32.18 ± 5.66 | 11.40 ± 1.53 | 14.35 ± 2.24 | 30.22 ± 6.37 | |
| T2 | NA | 15.63 ± 2.46 | 20.15 ± 4.07 | 35.50 ± 3.94 | NA | 18.55 ± 2.19 | 31.40 ± 3.78 | |
| T0–T1 | 3.15 ± 2.78 **A | 4.67 ± 3.28 *A | 1.88 ± 6.11 | 2.00 ± 6.85 | 4.17 ± 1.56 *A | −0.72 ± 3.76 | 6.65 ± 2.85 **A | |
| T1–T2 | NA | 1.10 ± 3.60 | 3.38 ± 3.07 *B | 3.32 ± 7.74 | NA | 4.20 ±1.54 *B | 1.18 ± 7.16 | |
| T0–T2 | NA | 5.77 ± 3.02 *C | 5.27 ± 6.21 | 5.32 ± 6.22 | NA | 3.48 ± 3.37 | 9.18 ± 2.48 *C | |
| CP | T0 | NA | NA | 14.33 ± 1.84 | NA | NA | 14.75 ± 2.28 | NA |
| T1 | NA | NA | 18.00 ± 3.07 | NA | NA | 17.81 ± 3.12 | NA | |
| T2 | NA | NA | 21.99 ± 2.28 | NA | NA | 22.20 ± 2.09 | NA | |
| T0–T1 | NA | NA | 3.67 ± 2.85 *A | NA | NA | 3.06 ± 3.39 *A | NA | |
| T1–T2 | NA | NA | 3.99 ± 3.18 *B | NA | NA | 4.39 ± 3.78 *B | NA | |
| T0–T2 | NA | NA | 7.66 ± 2.52 *C | NA | NA | 7.45 ± 2.54 *C | NA |
| Group | Time | MIA | C-T-T’ | P1/P1’-C-T | C’-T-T’ | P2/P2’-C’-T’ |
|---|---|---|---|---|---|---|
| Mean/Median ± SD/IQR (°) | Mean/Median ± SD/IQR (°) | Mean/Median ± SD/IQR (°) | Mean/Median ± SD/IQR (°) | Mean/Median ± SD/IQR (°) | ||
| CLP-AP | T0 | 17.51 ± 5.43 | 80.99 ± 8.46 | 129.50 ± 10.24 | 79.61 ± 7.35 | 153.59 ± 11.60 |
| T1 | 12.59 ± 5.83 | 80.74 ± 8.28 | 124.61 ± 10.91 | 79.42 ± 5.17 | 151.21 ± 11.62 | |
| T2 | 5.63 ± 4.37 | 77.91 ± 6.23 | NA | 79.86 ± 9.08 | NA | |
| T0–T1 | −4.93 ± 5.92 *A | −0.26 ± 9.85 | −4.89 ± 15.90 | −0.19 ± 9.53 | −2.39 ± 17.00 | |
| T1–T2 | −6.96 ± 6.74 *B | −2.82 ± 9.52 | NA | −0.65 ± 6.70 | NA | |
| T0–T2 | −11.89 ± 7.23 *C | −3.08 ± 9.58 | NA | −2.10 ± 6.60 | NA | |
| CLP-PP | T0 | 10.50 ± 3.78 | 73.53 ± 8.32 | 132.55 ± 10.42 | 76.98 ± 5.16 | 147.08 ± 14.04 |
| T1 | 12.67 ± 5.40 | 75.98 ± 16.44 | 125.95 ± 24.54 | 76.80 ± 9.96 | 147.35 ± 17.29 | |
| T2 | 5.62 ± 1.60 | 76.35 ± 9.02 | NA | 76.35 ± 9.02 | NA | |
| T0–T1 | 2.17 ± 8.81 | 2.45 ± 22.12 | −6.60 ± 24.07 | −0.18 ± 14.18 *A | 0.27 ± 9.90 | |
| T1–T2 | −7.05 ± 5.39 *B | 0.65 ± 13.89 | NA | −0.45 ± 12.33 | NA | |
| T0–T2 | −4.88 ± 3.98 *C | 3.10 ± 10.14 | NA | −0.63 ± 10.28 | NA | |
| CP | T0 | NA | NA | NA | NA | NA |
| T1 | NA | NA | NA | NA | NA | |
| T2 | NA | NA | NA | NA | NA | |
| T0–T1 | NA | NA | NA | NA | NA | |
| T1–T2 | NA | NA | NA | NA | NA | |
| T0–T2 | NA | NA | NA | NA | NA |
| Distance/Angle | N | ICC (95% CI) | ||
|---|---|---|---|---|
| T0 | T1 | T2 | ||
| P1-P2 | 9 | 0.994 [0.914, 0.998] | 0.507 [0.105, 0.769] | NA |
| P1’-P2’ | 11 | 0.967 [0.920, 0.987] | 0.940 [0.857, 0.976] | NA |
| CC’ | 20 | 0.982 [0.954, 0.993] | 0.986 [0.946, 0.995] | 0.991 [0.978, 0.996] |
| TT’ | 20 | 0.984 [0.960, 0.994] | 0.970 [0.910, 0.989] | 0.996 [0.991, 0.999] |
| CM | 20 | 0.789 [0.367, 0.924] | 0.797 [0.562, 0.914] | 0.989 [0.972, 0.996] |
| C’M | 20 | 0.869 [0.588, 0.953] | 0.949 [0.879, 0.979] | 0.336 [0.107, 0.669] |
| AM | 20 | 0.995 [0.987, 0.998] | 0.997 [0.993, 0.999] | 0.972 [0.932, 0.989] |
| P1-TT’ | 9 | 0.981 [0.948, 0.993] | 0.980 [0.951, 0.992] | NA |
| P2-TT’ | 9 | 0.954 [0.889, 0.981] | 0.972 [0.933, 0.989] | NA |
| P1’-TT’ | 11 | 0.972 [0.932, 0.989] | 0.991 [0.978, 0.996] | NA |
| P2’-TT’ | 11 | 0.776 [0.299, 0.921] | 0.947 [0.872, 0.978] | NA |
| I-TT’ | 20 | 0.931 [0.712, 0.977] | 0.976 [0.942, 0.990] | 0.987 [0.968, 0.995] |
| I-CC’ | 20 | 0.974 [0.937, 0.990] | 0.870 [0.707, 0.946] | 0.990 [0.977, 0.996] |
| C-TT’ | 20 | 0.971 [0.871, 0.990] | 0.989 [0.971, 0.996] | 0.996 [0.991, 0.999] |
| C’-TT’ | 20 | 0.955 [0.794, 0.986] | 0.918 [0.803, 0.967] | 0.956 [0.891, 0.982] |
| P1-I | 9 | 0.969 [0.924, 0.987] | 0.974 [0.901, 0.991] | NA |
| P1’-I | 11 | 0.770 [0.513, 0.902] | 0.861 [0.679, 0.943] | NA |
| I-C | 9 | 0.924 [0.822, 0.969] | 0.811 [0.550, 0.923] | NA |
| I-C’ | 11 | 0.362 [−0.07, 0.685] | 0.996 [0.991, 0.999] | NA |
| P1-C | 9 | 0.948 [0.876, 0.979] | 0.917 [0.758, 0.969] | NA |
| P2-C’ | 9 | 0.801 [0.570, 0.915] | 0.951 [0.882, 0.980] | NA |
| P1’-C | 11 | 0.366 [−0.07, 0.688] | 0.964 [0.911, 0.985] | NA |
| P2’-C’ | 11 | 0.175 [−0.274, 0.563] | 0.977 [0.930, 0.991] | NA |
| C-T | 20 | 0.974 [0.925, 0.990] | 0.989 [0.973, 0.996] | 0.997 [0.991, 0.999] |
| C’-T’ | 20 | 0.935 [0.821, 0.974] | 0.913 [0.797, 0.964] | 0.957 [0.897, 0.983] |
| AKL (P1-I-C-T) | 9 | 0.813 [0.227, 0.941] | 0.774 [0.395, 0.914] | 0.517 [0.124, 0.774] |
| AKL (P1’-I-C-T) | 11 | 0.186 [−0.264, 0.571] | 0 [−0.243, 0.330] | 0.980 [0.952, 0.992] |
| AKL’ (P2-C’-T’) | 9 | 0 [−0.09, 0.175] | 0 [−0.088, 0.169] | 0.699 [0.388, 0.868] |
| AKL’ (P2’-C’-T’) | 11 | 0.082 [−0.113, 0.364] | 0.0898 [−0.105, 0.371] | 0.584 [0.209, 0.811] |
| MIA | 20 | 0.882 [0.731, 0.951] | 0.789 [0.547, 0.910] | 0.897 [0.753, 0.958] |
| C-T-T’ | 20 | 0.986 [0.964, 0.994] | 0.982 [0.954, 0.992] | 0.977 [0.946, 0.991] |
| C’-T’-T’ | 20 | 0.892 [0.719, 0.958] | 0.917 [0.806, 0.966] | 0.985 [0.962, 0.993] |
| P1-C-T | 9 | 0.934 [0.553, 0.981] | 0.616 [0.162, 0.839] | NA |
| P2-C’-T’ | 9 | 0.908 [0.788, 0.962] | 0.910 [0.788, 0.963] | NA |
| P1’-C-T | 11 | 0.598 [0.115, 0.834] | 0.960 [0.854, 0.986] | NA |
| P2’-C’-T’ | 11 | 0.115 [−0.331, 0.521] | 0.920 [0.748, 0.970] | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bühling, S.; Selge, H.M.; Eslami, S.; Seifert, L.B.; Sayahpour, B.; Plein, N.; Sader, R.; Kopp, S. Impact of Active and Passive Maxillary Plates on Cleft Width Morphology in Unilateral Cleft Lip and Palate: A Prospective Intervention Study. Children 2025, 12, 714. https://doi.org/10.3390/children12060714
Bühling S, Selge HM, Eslami S, Seifert LB, Sayahpour B, Plein N, Sader R, Kopp S. Impact of Active and Passive Maxillary Plates on Cleft Width Morphology in Unilateral Cleft Lip and Palate: A Prospective Intervention Study. Children. 2025; 12(6):714. https://doi.org/10.3390/children12060714
Chicago/Turabian StyleBühling, Sarah, Helena Mariella Selge, Sara Eslami, Lukas Benedikt Seifert, Babak Sayahpour, Nicolas Plein, Robert Sader, and Stefan Kopp. 2025. "Impact of Active and Passive Maxillary Plates on Cleft Width Morphology in Unilateral Cleft Lip and Palate: A Prospective Intervention Study" Children 12, no. 6: 714. https://doi.org/10.3390/children12060714
APA StyleBühling, S., Selge, H. M., Eslami, S., Seifert, L. B., Sayahpour, B., Plein, N., Sader, R., & Kopp, S. (2025). Impact of Active and Passive Maxillary Plates on Cleft Width Morphology in Unilateral Cleft Lip and Palate: A Prospective Intervention Study. Children, 12(6), 714. https://doi.org/10.3390/children12060714






