Abstract
Background/Objectives: Aneurysmal bone cysts (ABCs) are rare bone tumors that can occur in the skull, leading to extensive bone destruction and compression of surrounding tissues. Due to the rarity of these lesions, there are limited data available in the literature, which primarily consists of case reports. We aimed to collect and analyze the available data to summarize the current state of knowledge on this rare pathology, while also conducting a statistical analysis to identify potential risk factors and management strategies. Methods: A review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, covering studies published from January 1950 to December 2023. A total of 60 articles and 74 case reports were included. Results: The mean age at diagnosis was 14.8 ± 12.5 years, with slightly higher male gender predominance. Regarding the different skull bones, a statistically significant higher growth trend of ABCs was found at the parietal bone in the male population (p = 0.025). At the occipital bone, a significant correlation was observed with the age of incidence for symptomatic lesions (p = 0.007) and development from fibrous dysplasia (p = 0.019). Secondary lesions showed a higher frequency of complications within the first months post-surgery (p = 0.041). Conclusions: No significant correlation was found between ABCs and fibrous dysplasia (FD) or head trauma. Male patients with FD showed a higher tendency to develop an aneurysmal cyst at the occipital bone at an older age and a higher tendency for growth in ABCs at the parietal bone. However, to date, no molecular or genetic correlation with male hormones has been reported in the literature. Surgery remains the only effective treatment, but complications should be carefully considered, particularly in patients with pre-existing pathological conditions.
1. Introduction
Aneurysmal bone cysts (ABCs) are rare, benign, vascular bone tumors most commonly diagnosed during the first two decades of life [1]. These complex lesions consist of blood-filled cystic spaces separated by fibrous stroma containing inflammatory cells, numerous capillaries, and multinucleated giant cells/osteoclasts [2]. The most typical location for an ABC is the metaphysis of long bones; therefore, lesions occurring in the calvaria are exceedingly rare, accounting for only 3–6% of all such vascular bone lesions [3].
Although benign, ABCs can lead to extensive bone destruction and compression of surrounding tissues due to their expansile nature, resulting in symptoms such as pain, swelling, deformity, pathological fractures, and neurological deficits. Most patients are treated with surgical curettage, but recurrence is common and may require additional surgical intervention.
To date, various risk factors in the development of ABCs have been investigated in the literature, with fibrous dysplasia and head trauma emerging as the most likely contributors to the pathogenesis of the disease.
We therefore aimed to collect and synthesize the available data to provide an up-to-date overview of this rare pathology, while also performing a statistical analysis to identify potential risk factors and optimal management strategies.
2. Materials and Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [4]. Additionally, a single case report from our institution was included in the case history.
2.1. Objectives
The primary objective of this study is to investigate the existence of a correlation between the major risk factors currently implicated in the etiopathogenesis of ABC—namely, head trauma and fibrous dysplasia. Secondary objectives include exploring potential correlations between patient demographic data and the formation and progression of ABC lesions, particularly with respect to the bone of origin and their management.
2.2. Literature Search
All full-text, English-language manuscripts reporting relevant case reports of aneurysmal bone cysts (ABCs) were screened using the PubMed/MEDLINE, Embase, Cochrane Library, Scopus, and Web of Science databases, covering the period from February 1965 to December 2023. Search terms included key phrases and Boolean operators, such as “Aneurysmal bone“ OR “Aneurysmal cyst” AND “Fibrous dysplasia” OR “Skull bone” OR “Case report” OR “Trauma”. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) diagram illustrates the steps involved in screening and analyzing the articles selected for this review.
2.3. Study Selection
Articles were initially screened based on title, abstract, and full text to assess their eligibility, and any discrepancies were resolved by consensus. Studies were included if they met the following predefined inclusion criteria: they had to be case reports or case series written in English, refer specifically to aneurysmal bone cysts (ABCs) and not solely to fibrous dysplasia (FD), and report at least one relevant clinical outcome (it was not necessary for all outcomes of interest to be included).
Studies were excluded if they were not in English, not published in peer-reviewed journals, unrelated to aneurysmal bone cysts of the skull bones, or if they involved animal models, cadaveric specimens, or purely histological analyses. With regard to “skull bones”, only lesions affecting the neurocranium—namely, the frontal, parietal, temporal, ethmoid, sphenoid, and occipital bones—were included. Studies involving lesions of the maxilla, mandible, or zygomatic bones were excluded. Likewise, cases of ABCs extending into the orbital space or originating in the neurocranium but extending beyond the cranial vault were excluded.
Further exclusions included studies with unavailable full texts, insufficient demographic or treatment data, aggregated data, or anatomical locations outside the head. Duplicate reports of previously published cases were also excluded to avoid double counting.
2.4. Outcomes
A total of 586 manuscripts were identified during the initial phase of the literature search, of which 471 were excluded based on title and abstract screening. All remaining reports were successfully retrieved. Of the 115 full-text articles assessed for eligibility, the following were excluded: 13 written in languages other than English, 1 animal study, 1 radiological study, and 1 that did not include any case report or case series. An additional 39 articles were excluded due to the anatomical location not being relevant to the neurocranium.
This process resulted in 60 articles meeting the inclusion criteria (Figure 1). The included articles are listed in Table 1, while the excluded articles are detailed in Table 2.
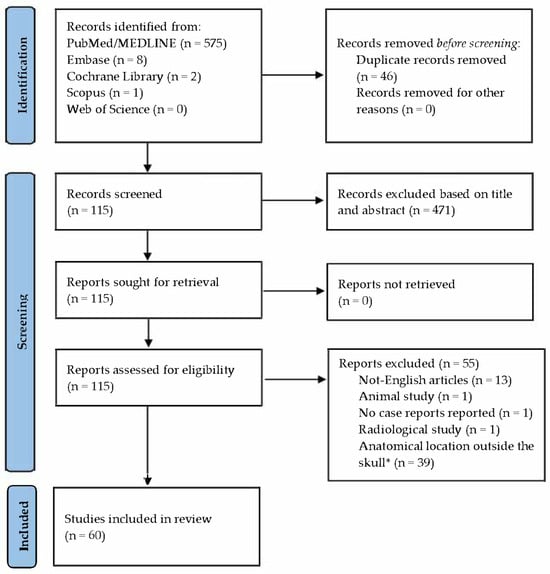
Figure 1.
Articles’ selection following the PRISMA guidelines. Source: https://www.bmj.com/content/372/bmj.n71 (accessed on 15 April 2025). * The term “Skull” refers only to the neurocranium, and, therefore, to the bones forming it.

Table 1.
All included articles. * MC: multi-country. N/A: not available. FD: fibrous dysplasia.

Table 2.
Excluded reports.
The total number of subjects analyzed was 71, as some studies reported multiple ABC cases. These were treated as individual cases in the statistical analysis.
2.5. Analyzed Data
The collected data were categorized into three main groups:
- Patient demographics, including gender, age, presence of known genetic disorders, and history of previous head trauma;
- ABC characteristics, including lesion location, size (measured in millimeters), growth trend, symptoms and their relation to the lesion, and etiopathogenesis;
- Management characteristics, including type of treatment, type of surgery, use of preoperative embolization, short- and long-term complications, duration of follow-up (in months), and presence of recurrence.
History of head trauma was defined as any injury to the calvaria that occurred before the onset of the ABC. Cases in which head trauma led to the diagnosis due to lesion rupture and subsequent hemorrhage were not considered as having a trauma-related etiological factor.
As previously mentioned, only ABCs located in neurocranial bones were included, specifically the frontal, parietal, temporal, occipital (including condyles), sphenoid (greater and lesser wings, sella turcica), and ethmoid (cribriform plate) bones.
Growth trend was assessed based on clinical history, particularly any reported enlargement of the lesion during outpatient follow-up.
In terms of etiopathogenesis, we focused on distinguishing between primary ABCs and secondary ABCs, the latter arising from pre-existing fibrous dysplasia (FD) or other congenital/malformative lesions.
Treatment was classified as either conservative management or surgery, with or without adjunctive pharmacotherapy. Surgical procedures were categorized as Gross Total Resection (GTR), Subtotal Resection (STR), or biopsy. In cases where a preoperative biopsy was followed by a GTR, only the GTR was considered for final analysis.
Short-term complications were defined as any adverse events occurring within the first month post-surgery. Complications occurring beyond this period were classified as long-term.
2.6. Statistical Analysis
The normality of data distribution was assessed using the Shapiro–Wilk test, which revealed significant deviations from normality (p < 0.05) across all datasets. As a result, non-parametric tests were employed for all subsequent analyses. Specifically, the Mann–Whitney U test was used for comparing continuous variables. The significance of frequency distributions in contingency tables was evaluated using the chi-square (χ2) test. Only statistically relevant results were reported in detail.
3. Case Report
Our patient was a 14-year-old male who presented with acute pain and swelling in his left arm. His medical history included short stature unresponsive to recombinant Growth Hormone (GH) therapy, a traumatic vertebral fracture, and a previous diagnosis of relapsing Acute Lymphoblastic Leukemia (ALL) at the age of 6. He had also recently experienced headaches. Angiographic studies revealed a subclavian artery thrombosis, for which Warfarin therapy was initiated, resulting in clinical improvement.
Physical examination revealed a supernumerary nipple, limb shortening, and bony prominences in the extremities. A full-body X-ray identified multiple exostoses. Genetic testing confirmed a diagnosis of Hereditary Multiple Exostosis (HME) due to an EXT1 gene variant inherited from his mother.
During hospitalization, the patient experienced an acute episode of headache, dizziness, visual disturbances, and a fall. Brain MRI revealed an expansile extra-axial lesion in the right occipital condyle with blood-fluid levels (Figure 2), consistent with a suspected aneurysmal bone cyst (ABC) causing compression of the sigmoid sinus and small ischemic areas in the right cerebellum. Angio-MRI confirmed venous drainage via the left transverse and sigmoid sinuses. Stenosis of the right vertebral artery (VA) and posterior cerebral artery (PCA) was also observed. A cervical spine CT scan revealed small osteochondromas on the cervical vertebrae.
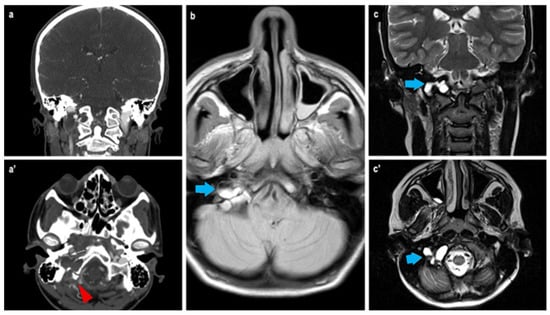
Figure 2.
Radiological findings at the CT scan (a,a’): it is possible to appreciate the bony erosion caused by the extracranial expansive lesion at the right occipital condyle (white asterisk, red arrowhead) with direct contact to the sigmoid sinus. At MRI (b,c,c’), the presence of the characteristic fluid–fluid interface of aneurysmal cyst is clearly visible (blue arrows).
Given the vascular features of the lesion, an angiographic study was conducted. Antiplatelet and anticoagulant therapy were administered preoperatively. During the procedure, reduced blood flow in the left PCA and a floating thrombus in the right VA suggested a vascular dissection (Figure 3). Due to vascular spasm, the placement of a flow diverter stent was not feasible. Instead, the artery was occluded, sparing the right posterior inferior cerebellar artery (PICA), and coils were used to fill the dissected segment. Heparin was administered during the procedure. The neurological outcome was stable postoperatively. Angio-MRI indicated possible vasospasm in the posterior cerebral circulation, prompting continuous heparin infusion.
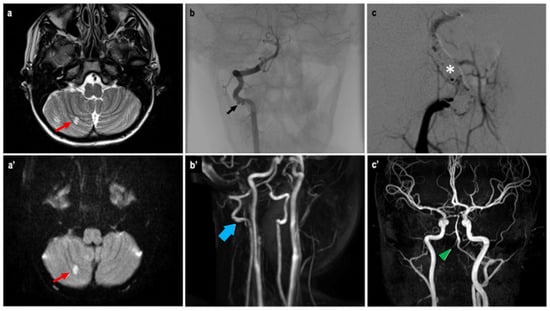
Figure 3.
Angio-MRI T2w (a) and DWI (a’) sequences showing a hyperintense area (red arrow) of the right cerebellar hemisphere, a possible ischemic consequence of the suspected vasospasm of the posterior cerebral circulation. Angiographic study (b) confirmed a floating thrombus at the right VA previously described as a VA stenosis at the Angio-MRI (b’). A flow-diverter placement was not possible, thus requiring a coiling (white asterisk) of the VA sparing the PICA (c). The control Angio-MRI (c’) showed the exclusion of the right VA after the endovascular procedure (green arrowhead).
Due to suspected instability at the CranioCervical Junction (CCJ), a multidisciplinary conference recommended an OccipitoCervical Fixation (OCF) combined with removal of the right occipital condyle. The patient underwent a 4-h surgery with complete en bloc resection of the condyle, which was found to be filled with a dark, hemorrhagic cystic lesion. No major intraoperative bleeding occurred. Fixation included C0-C1-C2, with C1 lateral mass screws, C2 pedicle screws, and an occipital plate.
Histopathological examination revealed numerous multinucleated giant cells lining the cyst wall, new bone matrix deposition (Figure 4), a loosely arranged proliferation of spindle cells without significant atypia (Figure 5), and mitotic figures (Figure 6). The final diagnosis was consistent with an aneurysmal bone cyst.
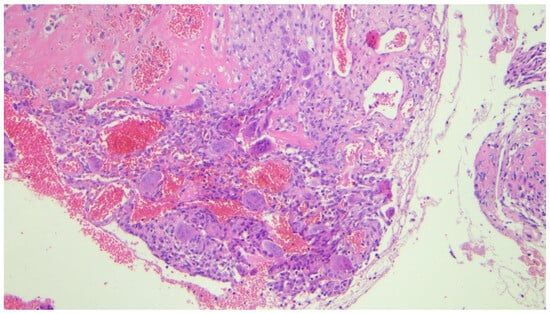
Figure 4.
Photomicrograph (hematoxylin-eosin, ×20 magnification) showing numerous multinucleated giant cells filling the wall of the cystic lesion associated with deposition of new bone matrix (upper left).
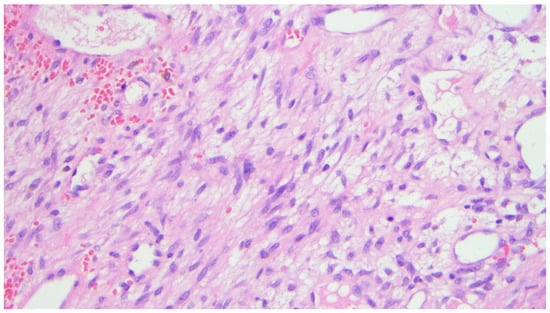
Figure 5.
Photomicrograph (hematoxylin-eosin, ×20 magnification) showing associated loose proliferation of spindle cells without significant atypia.
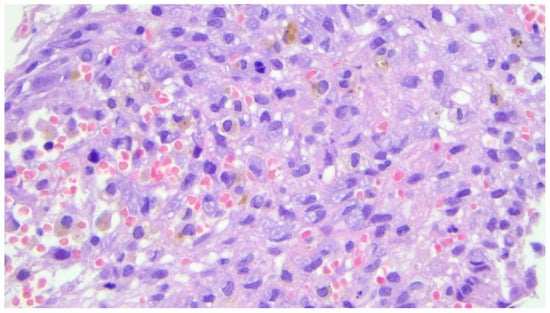
Figure 6.
Photomicrograph (hematoxylin and eosin, ×40 magnification) showing the presence of mitosis, which is a common morphologic finding in aneurysmal cysts of the bone.
The postoperative course was uneventful, and the patient was discharged after 14 days in good clinical condition. Radiological follow-up at one year demonstrated optimal fusion of the instrumented segments and no evidence of tumor recurrence. The patient was transitioned to antiplatelet therapy with Aspirin, and Warfarin was resumed.
The patient is currently alive and asymptomatic.
4. Results
A total of 74 patients affected by aneurysmal bone cysts (ABCs) were identified in the literature from January 1950 to December 2023, together with our own case report. The mean age at diagnosis was 14.8 ± 12.5 years (range: 2 months to 62 years), with a slightly male gender predominance of 56.8% (Table 3).

Table 3.
Summary of the total collected data. The mean age is in years. Mean follow-up is in months. No gender predominance was seen. Genetic disorder was considered any condition genetically diagnosed. Fibrous dysplasia was considered only in the skull bone. Growth tendency refers to the increase in the lesion’s volume reported by the patient or documented by imaging. Any symptoms related to the cyst were considered, like tenderness, swelling, pain, or neurological deficit. Surgery was divided into Gross Total Resection (GTR), Subtotal Resection (STR), and biopsy. * Lesion size is calculated as the major diameter in millimeters.
The majority of ABCs occurred in the occipital bone (43.2%), followed by the temporal (21.6%), frontal (14.9%), parietal (8.1%), sphenoid (6.8%), and ethmoid (5.4%) bones.
Two cases (2.7%) involved a known genetic disorder: McCune–Albright syndrome [47] and HME (our case report). Thirteen cases (17.6%) showed signs of fibrous dysplasia (FD) of the skull bones on CT scan, of which eight eventually developed into aneurysmal cysts. In two cases (2.7%), the ABC arose from a pre-existing osteoblastoma [59,61], and in one case (1.4%), from a capillary venous malformation [56].
Ten patients (13.5%) reported a history of head trauma prior to the onset of the ABC, while thirty-nine (52.7%) showed a tendency toward progressive growth during follow-up. Fifty-two patients (70.3%) were symptomatic, presenting with compression-related symptoms such as tenderness, swelling, pain, or neurological deficits.
Surgical treatment was performed in 70 cases (94.6%), of which 67 underwent Gross Total Resection (GTR). Among the remaining patients,
- Two were managed conservatively: one refused surgery and was treated with bisphosphonate therapy (alendronate monosodium trihydrate) [34]; the other had surgery postponed due to the COVID-19 pandemic and experienced spontaneous lesion regression [57].
- One underwent an open biopsy, with residual pathological tissue left due to adherence to the clivus and cerebellar structures. This case was managed with corticosteroids and a hormonal blocker [33].
- One underwent a Subtotal Resection (STR) followed by radiation therapy and interferon alfa-2a treatment [37].
Seven patients (9.5%) received preoperative embolization.
Regarding complications, nine patients (12.2%) experienced early postoperative issues, including
- Wound infection [41];
- Headaches and nuchal pressure [33];
- Early recurrence [29];
- Cerebrospinal fluid leakage [54];
- Conductive hearing loss and facial nerve deficit [56];
- Visual deterioration [16];
- Hemifacial numbness [62];
- Cerebral sinus thrombosis [6];
- Cardiac arrest with fatal outcome [7].
No late complications were reported. The mean follow-up period was 24.7 ± 23.2 months, with five recurrences reported at 1 week [29], 2.5 months [22], 4 months [53], and 12 months [52,59], respectively (mean recurrence interval: 0.6 ± 2.5 months).
Statistical analysis was performed, and the statistically significant results are reported in the following paragraphs.
4.1. Parietal and Occipital Bone Localization
A statistically significant higher growth trend of aneurysmal bone cysts was observed in lesions located in the parietal bone (p = 0.025, Table 4). In contrast, lesions located in the occipital bone were found to have a significantly higher incidence of symptoms and a higher likelihood of developing from fibrous dysplasia in older patients, with p-values of 0.007 and 0.019, respectively. Both of these findings were observed exclusively in the male population.

Table 4.
Contingency table showing a statistically significant higher frequency in growth trend of aneurysmal cysts at the parietal bone in the male population alone.
Conversely, no statistically significant correlation was found between fibrous dysplasia and the onset of ABCs (p = 0.07), nor between a history of trauma and the development of an ABC (p > 0.05).
4.2. Early Complications and Etiopathogenesis
A statistically significant difference in the frequency of early complications was found between patients with primary ABCs and those with secondary lesions (regardless of whether they originated from fibrous dysplasia or other genetic syndromes) (p = 0.041, Table 5, Figure 7). This finding indicates that secondary lesions are more likely to develop early complications within the first month after surgery, regardless of their origin.

Table 5.
Contingency table showing a statistically significant difference in frequency of the onset of early complications between patients with a primitive ABC and those with a secondary lesion.
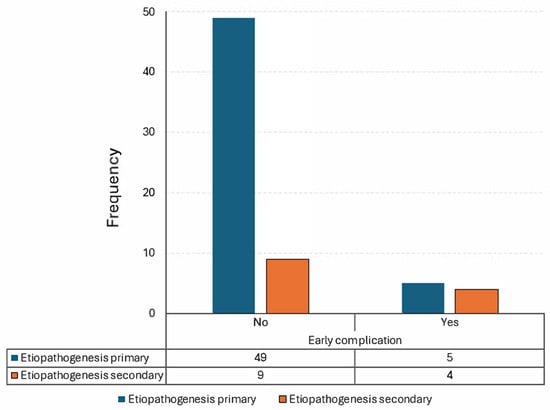
Figure 7.
Visual representation of Table 5.
5. Discussion
Originally described by Jaffe and Lichtenstein in 1942 as “solitary unicameral bone cysts” [118], these lesions were formally named aneurysmal bone cysts (ABCs) in 1944 [119]. ABCs are rare, benign, vascular bone tumors, most frequently diagnosed within the first two decades of life [1].
They are histologically complex lesions, composed of blood-filled cystic spaces separated by fibrous stroma containing inflammatory cells, numerous capillaries, and multinucleated giant cells/osteoclasts [2].
The typical location for an ABC is the metaphysis of long bones, although approximately 20% occur in the spine [120]. They account for 1–5% of all primary pediatric bone tumors [121], with a slightly higher incidence in females, as reported in the literature. ABCs involving the calvaria are extremely rare, representing only 3–6% of all ABCs [3].
All cranial bones may potentially be involved, with the jaw—particularly the mandible—being the most commonly affected according to the available literature [122]. However, our study focused exclusively on the neurocranium, where we found the occipital bone (including condylar lesions) to be the most frequently involved site. Notably, patients with occipital lesions tended to become symptomatic at a later age compared to other skull locations—a finding that may suggest slower growth rates, though this was not statistically demonstrated. Moreover, patients with fibrous dysplasia (FD) in the occipital bone were more likely to develop ABCs later in life than those without FD. This may be related to the distinct genetic pathway of FD, which is caused by somatic activating mutations in the GNAS gene, located on chromosome 20q13.3 [122]. FD leads to the replacement of normal bone with fibro-osseous tissue, typically lacking hematopoietic marrow [123], and additional genetic alterations may be required for the development of a vascular lesion such as an ABC, thus necessitating more time for the transformation.
The parietal bone showed a statistically significant higher frequency of growing ABCs, which could be explained by the anatomical and functional context. Lesions in this area may cause early symptoms due to involvement of the temporalis muscle, making them easier to detect. The earlier the diagnosis, the higher the chance of observing lesion growth during follow-up.
Interestingly, both of the above findings—occipital and parietal bone associations—were observed exclusively in the male population. However, no definitive gender predisposition has been confirmed in the literature [124], and to date, no molecular or genetic studies have explored a potential role of male hormones. Future research into the influence of sex hormones on ABC pathophysiology is therefore warranted.
Despite being benign, ABCs can lead to significant bone destruction and mass effect due to their expansile nature, producing symptoms such as pain, swelling, deformity, pathological fractures, and neurological deficits.
Imaging modalities used to assess ABCs include CT scans, which typically show lytic lesions with a honeycomb or eggshell ballooning appearance, and MRI, which reveals characteristic fluid-fluid levels on T2-weighted sequences and a heterogeneous appearance on T1-weighted images [125].
Surgical curettage remains the most common treatment, though recurrence is frequent, often requiring repeat interventions. In cases where lesions are incompletely resectable or inoperable, selective arterial embolization may be the first-line treatment. Other minimally invasive options include radiofrequency ablation, percutaneous injection of demineralized bone matrix, and autologous bone marrow concentrate grafting [126].
In the presented case, embolization was not feasible due to vertebral artery dissection, though the intentional closure of the artery may have contributed to the bloodless surgical field during resection of the right occipital condyle. The rationale for condyle removal was based on CranioCervical Junction (CCJ) instability, with potential for lesion rupture and associated complications.
Despite the availability of numerous surgical options, the clinical course of aneurysmal bone cysts (ABCs) remains unpredictable in some cases, and local recurrences may occur. Recurrence rates have been reported to be as high as 60% in long bones [127], approximately 12.8% in the spine [128], and vary widely in the skull, with estimates ranging from 10% to 50% [129]. In our case series, 5 out of 73 patients (6.9%) experienced a recurrence of the ABC lesion during the follow-up period (the cohort was reduced to 73 from 74 due to one intraoperative death). However, the considerable variability in follow-up duration limited the statistical significance of this finding.
Regarding complication rates, these are generally considered low for curettage alone [130]. The most common complication in long bones is recurrence itself, while long-term complications may include chronic pain, limb-length discrepancies, infection, and heterotopic ossification, although these are considered rare events [131]. At the spinal level, the most common complications are persistent neurological deficits (6.6%), spinal deformity (5.5%), and ongoing pain (2.6%), with an overall low mortality rate of 1.5% [128]. For skull bones specifically, limited data are available in the literature regarding the incidence of complications. In our series, we observed a significant association between early postoperative complications and secondary ABCs, likely reflecting a more complex clinical scenario with increased morbidity and mortality risks.
The etiology of ABCs remains uncertain, with several competing hypotheses. Primary ABCs are widely thought to be reactive lesions, though recent cytogenetic analyses suggest they may be neoplastic. Trauma has been proposed as a possible trigger, but major reviews by Tillman et al. [132] and Ruiter et al. [133] found no supporting evidence—a conclusion echoed by our data (p > 0.05).
Another proposed mechanism involves altered osseous circulation, which could occur in patients with increased thrombotic tendency, such as those with Acute Lymphoblastic Leukemia (ALL). Abuzayed et al. discussed this theory in a 2010 case report [40], though the low incidence of ABCs in ALL patients argues against it.
Secondary ABCs, on the other hand, may arise from preexisting lesions such as FD, a rare, benign but chronically progressive bone disorder characterized by the replacement of normal bone with fibrous tissue [134]. FD may be monostotic or polyostotic, and in some cases is associated with endocrinopathies and café-au-lait spots, as seen in McCune-Albright syndrome.
In recent years, molecular studies have revealed a complex genetic landscape underlying aneurysmal bone cysts (ABCs). The most common alteration is a translocation involving the USP6 gene (also known as TRE17) on chromosome 17p13, most frequently with CDH11 as the fusion partner [135,136,137]. This translocation leads to USP6 overexpression, which drives tumorigenesis and local bone destruction. As previously mentioned, fibrous dysplasia is associated with mutations in the GNAS gene, which encodes the alpha subunit of the stimulatory G protein. A possible hypothesis linking GNAS mutations to USP6 translocations may involve an increased replication rate in affected cells, predisposing them to duplication errors such as gene translocations. However, this hypothesis remains purely speculative, as no supporting studies currently exist.
Other translocations, such as MYH9-USP6, have also been identified [138]. Additionally, somatic mutations in genes related to angiogenesis (e.g., IDH1, IDH2) and osteoclast activity (e.g., PLCG2) have been observed, shedding light on the vascular and osteolytic characteristics of ABCs [139,140].
Our 14-year-old patient was affected by Hereditary Multiple Exostoses (HME), an autosomal dominant disorder marked by multiple osteochondromas. While not observed in our case, the literature reports cases of chondrosarcoma arising in HME patients, sometimes associated with ABCs in long bones. Whether altered bone circulation, osteolytic activity of chondrosarcoma, or both contribute to ABC formation remains unclear. However, ABCs may coexist with a variety of underlying lesions, suggesting the existence of a yet unidentified common molecular or cellular pathway.
6. Conclusions
In conclusion, we demonstrated that no significant correlation exists between fibrous dysplasia (FD) and aneurysmal bone cyst (ABC) formation, nor between head trauma and the development of ABCs. However, male patients with FD show a slightly higher tendency to develop ABCs at an older age, specifically in the occipital bone, compared to healthy individuals. Additionally, these patients exhibit a higher tendency for ABCs to grow in the parietal bone. To date, and to the best of our knowledge, no molecular or genetic correlation with male hormones has been reported in the literature. Surgery remains the primary treatment for ABCs, although complications should be carefully considered, especially in the presence of pre-existing pathological conditions.
7. Limitations
Our study has several limitations that must be acknowledged. First, aneurysmal bone cysts are rare lesions, and their occurrence in the skull is even less common. This rarity inherently limits the size and representativeness of available case series, including our own, which compromises the statistical power and generalizability of the findings. Second, the majority of published literature on skull ABCs consists of isolated case reports or small case series, often lacking standardized methodology, consistent outcome measures, or long-term follow-up. As such, our ability to draw robust comparisons or establish evidence-based conclusions is restricted.
The retrospective nature of our data collection introduces inherent limitations, including information and selection biases. Medical records, imaging studies, and surgical reports vary in completeness and quality, and the lack of prospective protocols may have led to underreporting or inconsistent documentation of relevant variables such as lesion size, surgical margins, and long-term outcomes.
Moreover, the small number of patients in our series not only limits statistical significance for several variables but also impairs our capacity to perform multivariate analysis to control for potential confounding factors. Some findings, while suggestive, should be interpreted with caution due to the limited sample size.
Another major limitation is the current lack of understanding of the genetic and molecular mechanisms underlying ABC development, especially in secondary forms or in association with other bone pathologies such as fibrous dysplasia. Although we proposed a speculative hypothesis regarding a possible link between GNAS mutations and USP6 translocations, as well as a possible interaction with male hormones, this remains unvalidated and requires experimental support. The absence of molecular data in many of the cases further impedes a mechanistic interpretation of the disease process.
Finally, heterogeneity in diagnostic criteria, treatment approaches, and follow-up duration across the included cases further complicates efforts to identify consistent patterns or risk factors. Differences in imaging modalities, surgical expertise, and institutional protocols may have influenced outcomes in ways that are difficult to quantify retrospectively.
In conclusion, although our study represents one of the more comprehensive reviews of skull ABCs to date, its findings should be considered exploratory. To validate our observations and clarify the biological behavior of these lesions, future prospective studies with larger, multicentric patient cohorts, standardized clinical protocols, and integrated genetic analyses are essential.
Author Contributions
Conceptualization, M.P. and F.P.; methodology, L.B., P.C. and A.M.; formal analysis, P.C.; investigation, L.B., F.P., S.C., E.L.B. and A.B.; writing—original draft preparation, L.B.; writing—review and editing, L.B. and P.C.; supervision, M.P., G.P., D.G. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This is an observational study. The Research Ethics Committee at our institution has confirmed that no ethical approval is required.
Informed Consent Statement
Written informed consent, both for the surgical procedure and for publication, was obtained from the patients’ parents.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Leonardo Bradaschia, upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ABC | aneurysmal bone cysts |
| FD | fibrous dysplasia |
| GH | Growth Hormone |
| ALL | Acute Lymphoblastic Leukemia |
| HME | Hereditary Multiple Exostosis |
| PCA | Posterior Cerebral Artery |
| PICA | Posterior Inferior Cerebellar Artery |
| CCJ | CranioCervical Junction |
| OCF | OccipitoCervical Fixation |
| GTR | Gross Total Resection |
| STR | Sub Total Resection |
| MRI | Magnetic Resonance Imagining |
| MC | multi-country |
| CT | computed tomography |
| DWI | Diffusion-Weighted Imaging |
References
- Mankin, H.J.; Hornicek, F.J.; Ortiz-Cruz, E.; Villafuerte, J.; Gebhardt, M.C. Aneurysmal bone cyst: A review of 150 patients. J. Clin. Oncol. 2005, 23, 6756–6762. [Google Scholar] [CrossRef] [PubMed]
- Saccomanni, B. Aneurysmal bone cyst of spine: A review of literature. Arch. Orthop. Trauma Surgery 2008, 128, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.C.; Hockley, A.D. Aneurysmal bone cysts of the cranium in children: Report of three cases and brief review of the literature. J. Neurosurg. Pediatr. 2007, 106, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhende, Y.M.; Kothare, S.N. Aneurysmal bone cyst; a case report. Ind. Med. Gaz. 1950, 85, 544–546. [Google Scholar] [PubMed] [PubMed Central]
- Blundell, J.E. Aneurysmal bone cysts. A Report of six cases, including a fatal case associated with a non-ossifying fibroma. Australas. Radiol. 1965, 9, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Odeku, E.L.; Mainwaring, A.R. Unusual aneurysmal bone cyst: A case report. J. Neurosurg. 1965, 22, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.E.; Iraci, G.; Benedetti, A.; Melanotte, P.L. Aneurysmal bone cyst as an intracranial space-occupying lesion. Case report. J. Neurosurg. 1966, 25, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Cacdac, M.A.; Malis, L.I.; Anderson, P.J. Aneurysmal parietal bone cyst. Case report. J. Neurosurg. 1972, 37, 237–241. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.M.; Kirkham, T.H. Aneurysmal bone cyst of the orbit with unusual angiographic features. Am. J. Roentgenol. 1976, 126, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Chalapati Rao, K.V.; Rao, B.S.; Reddy, C.P.; Sundareshwar, B.; Reddy, C.R. Aneurysmal bone cyst of the skull. Case report. J. Neurosurg. 1977, 47, 633–636. [Google Scholar] [PubMed]
- Mufti, S.T. Aneurysmal bone cyst of the skull. Case report. J. Neurosurg. 1978, 49, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Luccarelli, G.; Fornari, M.; Savoiardo, M. Angiography and computerized tomography in the diagnosis of aneurysmal bone cyst of the skull: Case report. J. Neurosurg. 1980, 53, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Keuskamp, P.A.; Horoupian, D.S.; Fein, J.M. Aneurysmal bone cyst of the temporal bone presenting as a spontaneous intracerebral hemorrhage: Case report. Neurosurgery 1980, 7, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Komjátszegi, S. Aneurysmal bone cyst of the skull. J. Neurosurg. 1981, 55, 497. [Google Scholar] [PubMed]
- Kimmelman, C.P.; Potsic, W.P.; Schut, L. Aneurysmal bone cyst of the sphenoid in a child. Ann. Otol. Rhinol. Laryngol. 1982, 91, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.L.; Papsidero, M.J.; Batsakis, J.G.; Krause, C.J. Aneurysmal bone cyst of the ethmoid. Head Neck Surg. 1982, 5, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Sanerkin, N.G.; Mott, M.G.; Roylance, J. An unusual intraosseous lesion with fibroblastic, osteoclastic, osteoblastic, aneurysmal and fibromyxoid elements. “Solid” variant of aneurysmal bone cyst. Cancer 1983, 51, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Bilge, T.; Coban, O.; Ozden, B.; Turantan, I.; Türker, K.; Bahar, S. Aneurysmal bone cysts of the occipital bone. Surg. Neurol. 1983, 20, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ameli, N.O.; Abbassioun, K.; Azod, A.; Saleh, H. Aneurysmal bone cyst of the skull. Can. J. Neurol. Sci. 1984, 11, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Calliauw, L.; Roels, H.; Caemaert, J. Aneurysmal bone cysts in the cranial vault and base of skull. Surg. Neurol. 1985, 23, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Guilburd, J.N.; Borovich, B.; Goldsher, D.; Mendelson, H.; Kerner, H. Occipital aneurysmal bone cyst: CT features. J. Comput. Assist. Tomogr. 1987, 11, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.J.; Brunelle, F. Computerised tomography in the evaluation of expansile lesions arising from the skull vault in childhood—A report of 5 cases. Pediatr. Radiol. 1988, 18, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, Z.H. Aneurysmal bone cyst associated with fibrous dysplasia of the skull. Neurochirurgia 1989, 32, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.V.; Yokoyama, C.; Moseley, I.F.; Wright, J.E. Aneurysmal bone cyst of the sphenoid with orbital involvement. Br. J. Ophthalmol. 1990, 74, 505–508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dávid, K.; Horváth, Z.; Horváth, A.; Illés, T. Aneurysmal bone cyst of the occipital bone: Case report. Surg. Neurol. 1993, 40, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.Y.; Ragland, R.L.; Weaver, J.P.; Smith, T.W.; Knorr, J.R.; Weyreuther, M. Epidural mass related to calvarial aneurysmal bone cyst: Computed tomographic and magnetic resonance demonstration. J. Neuroimaging 1995, 5, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Chateil, J.F.; Dousset, V.; Meyer, P.; Pedespan, J.M.; San-Galli, F.; Rivel, J.; Caillé, J.M.; Diard, F. Cranial aneurysmal bone cysts presenting with raised intracranial pressure: Report of two cases. Neuroradiology 1997, 39, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Park, A.H.; Phillips, J.; Forte, V. Aneurysmal bone cyst of the temporal bone. Otolaryngol. Head Neck Surg. 1999, 120, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Petro, M.L.; Lancon, J.A. Occipital aneurysmal bone cyst. Pediatr. Neurosurg. 2001, 34, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Roncaroli, F.; Consales, A.; Galassi, E.; Bernardi, B.; Valeri, B. Occipital aneurysmal bone cyst secondary to eosinophilic granuloma. Pediatr. Neurosurg. 2001, 35, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Itshayek, E.; Spector, S.; Gomori, M.; Segal, R. Fibrous dysplasia in combination with aneurysmal bone cyst of the occipital bone and the clivus: Case report and review of the literature. Neurosurgery 2002, 51, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Mattei, T.A.; Mattei, J.A.; Ramina, R.; Aguiar, P.H. Fibrous dysplasia in combination with aneurysmal bone cyst presenting as a subarachnoid haemorrhage. Neurol. Sci. 2005, 26, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Iseri, P.K.; Efendi, H.; Demirci, A.; Komsuoglu, S. Fibrous dysplasia of the cranial bones: A case report and review of the literature. Yale J. Biol. Med. 2005, 78, 141–145. [Google Scholar] [PubMed] [PubMed Central]
- Lin, S.P.; Fang, Y.C.; Chu, D.C.; Chang, Y.C.; Hsu, C.I. Characteristics of cranial aneurysmal bone cyst on computed tomography and magnetic resonance imaging. J. Formos. Med. Assoc. 2007, 106, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Dong, Y.; Sun, K.; Lu, Y. A huge occipital osteoblastoma accompanied with aneurysmal bone cyst in the posterior cranial fossa. Clin. Neurol. Neurosurg. 2008, 110, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Segall, L.; Cohen-Kerem, R.; Ngan, B.Y.; Forte, V. Aneurysmal bone cysts of the head and neck in pediatric patients: A case series. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, C.V.; Rao, B.R.; Nair, S.; Radhakrishnan, V.V.; Kesavadas, C. Intracranial intradural aneurysmal bone cyst: A unique case. Pediatr. Neurosurg. 2009, 45, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, J.H.; Han, S.H.; Kang, H.I. Fibrous Dysplasia with Aneurysmal Bone Cyst Presenting as Painful Solitary Skull lesion. J. Korean Neurosurg. Soc. 2010, 48, 551–554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abuzayed, B.; Dashti, R.; Turk, O.; Kaynar, M.Y. Aneurysmal frontal bone cyst in a child with history of acute lymphoblastic leukemia: A case of rare location and history. J. Pediatr. Hematol. Oncol. 2010, 32, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, C. Langerhans cell histiocytosis masquerading as aneurysmal bone cyst. J. Clin. Oncol. 2011, 29, e688–e690. [Google Scholar] [CrossRef] [PubMed]
- Genizi, J.; Srugo, I.; Attias, D.; Ben-Sira, L.; Braun, J.; Bamberger, E.S.; Margalit, N.; Constantini, S. Giant pediatric aneurysmal bone cysts of the occipital bone: Case report and review of the literature. Pediatr. Neurol. 2011, 45, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Umredkar, A.; Srinivasa, R. Giant pediatric aneurysmal bone cyst of the occipital bone: Case report and review of the literature. Neurol. India 2012, 60, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.R.; Petteys, R.J.; Rossi, C.T.; Keating, R.F.; Magge, S.N. Large occipital aneurysmal bone cyst causing obstructive hydrocephalus in a pediatric patient. J. Neurosurg. Pediatr. 2012, 10, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Garber, S.T.; Riva-Cambrin, J.K. Occipital aneurysmal bone cyst rupture following head trauma: Case report. J. Neurosurg. Pediatr. 2015, 15, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Kalina, P.; Wetjen, N. Aneurysmal Bone Cyst of the Occipital Bone. J. Pediatr. 2015, 167, 496–496.e2. [Google Scholar] [CrossRef] [PubMed]
- Urgun, K.; Yılmaz, B.; Toktaş, Z.O.; Akakın, A.; Konya, D.; Demir, M.K.; Kılıç, T. Craniospinal Polyostotic Fibrous Dysplasia, Aneurysmal Bone Cysts, and Chiari Type 1 Malformation Coexistence in a Patient with McCune-Albright Syndrome. Pediatr. Neurosurg. 2016, 51, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Boari, N.; Gagliardi, F.; Giudice, L.; Mortini, P. Atlanto-occipital dislocation due to aneurysmal bone cyst of the occipital condyle. Acta Neurochir. Wien. 2016, 158, 1637–1638. [Google Scholar] [CrossRef] [PubMed]
- Mete, M.; Duransoy, Y.K.; Çinar, C.; Ovali, G.; Temiz, P.; Selcuki, M. Giant occipital aneurysmal bone cyst caused to hydrocephalus in a child. Childs Nerv. Syst. 2017, 33, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, C.; Bipin, C.; Rabiul, K.; Ananth Kumar, B.; Nazmin, A.; Raushan Kumar, C.; Ranjit Kumar, C. Aneurysmal Bone Cyst of the Occipital Bone. EC Neurol. 2018, 10, 1047–1051. [Google Scholar]
- Alves Filho, A.C.; Porfírio, A.S.; Ribeiro, W.C.S.; de Oliveira Fonseca, D.; Malta, M.V.; Camelo, R.C.; Camelo, R.M. Aneurysmal Bone Cyst of the Skull Base—Case Report. Arq. Bras. Neurocir. 2019, 38, 51–55. [Google Scholar] [CrossRef]
- Brohi, S.R.; Dilber, M.; Mallah, F.A. Secondary Aneurysmal Bone Cyst of Base of Skull Associated with Chondroblastoma. J. Coll. Physicians Surg. Pak. 2019, 29, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.H.; Jiang, F.Y.; Radatz, M.W.R.; Sinha, S.; Zaki, H. Skull base aneurysmal bone cyst presenting with hydrocephalus: Progressive residuum obliterated by Gamma Knife stereotactic radiosurgery in a pediatric patient. J. Neurosurg. Pediatr. 2020, 26, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Gotecha, S.; Punia, P.; Chugh, A.; Patil, A.; Kashyap, D.; Raghu, V.; Chhabra, S.; Patel, A.; Kotecha, M. A Rare Case of an Aneurysmal Bone Cyst of the Temporal Bone. Asian J. Neurosurg. 2020, 15, 699–702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farouk, A.G.; Mohammed, B.; Umar, U.H.; Wala, S.; Ahidjo, A. Giant pediatric aneurysmal bone cyst of the occipital bone: A case report and review of the literature. Med. J. Islam. World Acad. Sci. 2020, 28, 24–31. [Google Scholar] [CrossRef]
- Canzano, F.; Giombelli, E.; Cerasti, D.; Corradi, D.; Falcioni, M. Capillary Venous Malformation With Secondary Aneurysmal Bone Cyst of Temporal Bone. J. Int. Adv. Otol. 2021, 17, 471–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borni, M.; Kolsi, F.; Cherif, I.; Boudawara, M.Z. Spontaneous rapid regression of a juvenile primary aneurysmal bone cyst of the skull: A case report and literature review. Radiol. Case Rep. 2022, 17, 1634–1639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woldow, A.; Foy, V.M. Aneurysmal bone cyst of the skull: A case report. SAGE Open Med. Case Rep. 2022, 10, 2050313X221117727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- An, P.; Gao, P.; Liu, J.; Feng, G. Epithelioid Osteoblastoma Combined with Aneurysmal Bone Cyst Originating from the Right Temporal Bone and the Greater Wing of the Sphenoid Bone. Balkan Med. J. 2023, 40, 300–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koketsu, Y.; Tanei, T.; Kuwabara, K.; Hasegawa, T.; Kato, T.; Maesawa, S.; Nishimura, Y.; Araki, Y.; Saito, R. Secondary aneurysmal bone cyst of the frontal bone with fibrous dysplasia showing rapid expansion: A case report. Nagoya J. Med. Sci. 2023, 85, 395–401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsabbagh, B.M.; Alessa, A.; Aljohani, H.; Alhammad, O. Large skull osteoblastoma presented as aneurysmal bone cyst (ABC). Neurosciences 2023, 28, 277–280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, C.F.; Yang, C.C.; Liu, S.Y.; Shen, C.C. Middle cranial fossa tumor presenting as chronic otitis media: Rare case of aneurysmal bone cyst. Int. J. Surg. Case Rep. 2023, 112, 108996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mousavinejad, S.A.; Eraghi, M.M.; Tavasol, H.H.; Salarinezhad, Z.; Ansari, M.; Ebrahimzadeh, K.; Kazemi, R.; Sharifi, G.; Samadian, M.; Rezaei, O. A rare case of aneurysmal bone cyst of the frontal bone, and an overlook of literature. Interdiscip. Neurosurg. 2023, 34, 101859. [Google Scholar] [CrossRef]
- Oki, S.; Shima, T.; Uozumi, T.; Kodama, T. Aneurysmal bone cyst of the skull-A case report-(Author’s transl). No Shinkei Geka 1978, 6, 1197–1201. (In Japanese) [Google Scholar] [PubMed]
- Mima, S.; Taguchi, Y.; Sekino, H.; Inomata, I. Case of aneurysmal bone cyst of the skull. No Shinkei Geka 1984, 12, 825–831. (In Japanese) [Google Scholar] [PubMed]
- Ikeda, H.; Niizuma, H.; Suzuki, J.; Nagamine, Y.; Kameyama, M. Chemical embolization using estrogen for aneurysmal bone cyst of the skull: Case report. No Shinkei Geka 1985, 13, 203–208. (In Japanese) [Google Scholar] [PubMed]
- Tokarz, F.; Jankowski, R.; Zukiel, R.; Nowak, S. Torbiele tetniakowate czaski i kregosłupa leczone operacyjnie [Aneurysmal bone cyst of the skull and vertebral column treated operatively]. Neurol. Neurochir. Pol. 1993, 27, 533–540. (In Polish) [Google Scholar] [PubMed]
- Arnaldsson, O.S.; Ragnarsson, T. Aneurysmal bone cyst, following a skull trauma. A case report. Laeknabladid 1995, 81, 799–802. (In Icelandic) [Google Scholar] [PubMed]
- Clavel Escribano, M.; Robles Balibrea, A.; Clavel Laria, P.; Robles Cano, V. Quiste óseo aneurismático del hueso frontal. Caso clínico [Frontal bone aneurysmal cyst. Case report]. Neurocirugia 2001, 12, 166–169. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, T.; Matsuura, H.; Kamada, H.; Yamamoto, Y.; Itoh, K. A case of calvarial aneurysmal bone cyst: Transcranial contrast sonographic examination with pulse inversion harmonic imaging method. No To Shinkei 2002, 54, 1075–1080. (In Japanese) [Google Scholar] [PubMed]
- Mazlout, O.; Dfouni, N.; Ladeb, M.F.; Becker, M. Kyste osseux anévrismal de l’ethmoïde [Aneurysmal bone cyst of the ethmoid bone]. J. Radiol. 2005, 86, 948–950. (In French) [Google Scholar] [CrossRef] [PubMed]
- Broc-Haro, G.G.; Rodríguez-Valencia, F.; Manrique-Guzmán, S. Quiste óseo aneurismático de cráneo con resolución espontánea. Reporte de un caso [Spontaneous regression of aneurysmal bone cyst of the skull. Case report]. Cir. Cir. 2007, 75, 49–51. (In Spanish) [Google Scholar] [PubMed]
- Wendt, S.; Flügel, W.; Spuler, A.; Mairinger, T.; Hoch, H.; Bloching, M. Aneurysmatische Knochenzyste der Rhinobasis bei einem 3-jährigen Kind: Seltene Lokalisation einer benignen Knochenläsion [Aneurysmal bone cyst of the ethmoid sinus and skull base in a 3-year old child: A rare location of a benign bone lesion]. HNO 2010, 58, 57–62. (In German) [Google Scholar] [CrossRef] [PubMed]
- Abdoulkader, F.; Aammou, K.; Siwane, A.; Essodegui, F. Une localisation rare au sphénoïde d’un kyste osseux anévrysmal [Rare localization of aneurysmal bone cyst in the sphenoid bone]. Pan Afr. Med. J. 2017, 27, 231. (In French) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, M.; Zhu, X.Y.; Wang, L.H.; Zhang, T.; Zhang, X.L.; Rao, X.S. Primary osteosarcoma of the skull with aneurysmal bone cyst like change: Report of a case. Zhonghua Bing Li Xue Za Zhi 2021, 50, 1382–1384. (In Chinese) [Google Scholar] [PubMed]
- Ao, J.W.; Chen, Q.R. Aneurysmal bone cyst of mandible: Report of a case. Zhonghua Bing Li Xue Za Zhi 2022, 51, 564–566. (In Chinese) [Google Scholar] [PubMed]
- Bull, I.; MBell, C.; Storli, S.H. Maxillary Aneurysmal Bone Cyst in a Young Dog-A Case Report. J. Vet. Dent. 2024, 41, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.N.; Talwar, D.; Kell, D.; Arkader, A. Predicting Blood Loss in Aneurysmal Bone Cyst Surgery. J. Pediatr. Orthop. 2025, 45, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hino, N.; Ohtsuka, K.; Hashimoto, M.; Sakata, M. Radiographic features of an aneurysmal bone cyst of the orbit. Ophthalmologica 1998, 212, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Yarington, C.T., Jr.; Abbott, J.; Raines, D. Aneurysmal bone cyst of the maxilla; association with giant cell reparative granuloma. Arch. Otolaryngol. 1964, 80, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Fite, J.D.; Schwartz, J.F.; Calhoun, F.P., Jr. Aneurysmal bone cyst of the orbit (a clinicopathologic case report). Trans. Am. Acad. Ophthalmol. Otolaryngol. 1968, 72, 614–618. [Google Scholar] [PubMed]
- Eveson, J.W.; Moos, K.F.; MacDonald, D.G. Aneurysmal bone cyst of the zygomatic arch. Br. J. Oral Surg. 1978, 15, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Rasi, H.B.; Swamy, P.T.; Alpert, L.I.; Bochetto, J.; Giovanniello, J. Aneurysmal bone cyst of zygoma. N. Y. State J. Med. 1978, 78, 1937–1941. [Google Scholar] [PubMed]
- Matt, B.H. Aneurysmal bone cyst of the maxilla: Case report and review of the literature. Int. J. Pediatr. Otorhinolaryngol. 1993, 25, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Winters, V.; Schraepen, T.; Geusens, E.; Vanwijck, R.; Broeckx, J. Aneurysmal bone cyst of the zygomatic arch. J. Belge. Radiol. 1998, 81, 7–8. [Google Scholar] [PubMed]
- Senol, U.; Karaali, K.; Akyüz, M.; Gelen, T.; Tuncer, R.; Lüleci, E. Aneurysmal bone cyst of the orbit. AJNR Am. J. Neuroradiol. 2002, 23, 319–321. [Google Scholar] [PubMed] [PubMed Central]
- Sánchez, A.P.; Diaz-Lopez, E.O.; Rojas, S.K.; Neri, H.A.; Valle, P.L.; Pine, S.S. Aneurysmal bone cyst of the maxilla. Craniofac. Surg. 2004, 15, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, V.; Rubini, C.; Fioroni, M.; Piattelli, A. Solid aneurysmal bone cyst of the mandible. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Smolka, W.; Lieger, O.; Balmer, M.C.; Brekenfeld, C.; Iizuka, T.; Smolka, K. Aneurysmal bone cyst of the tuberculum articulare of the temporomandibular joint: A case report. Quintessence Int. 2008, 39, 679–683. [Google Scholar] [PubMed]
- Pelo, S.; Gasparini, G.; Boniello, R.; Moro, A.; Amoroso, P.F. Aneurysmal bone cyst located in the mandibular condyle. Head Face Med. 2009, 5, 8. [Google Scholar] [PubMed] [PubMed Central]
- Roychoudhury, A.; Rustagi, A.; Bhatt, K.; Bhutia, O.; Seith, A. Aneurysmal bone cyst of the mandible: Report of 3 cases. J. Oral Maxillofac. Surg. 2009, 67, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Bozbuğa, M.; Süslü, H.T. Aneurysmal bone cyst of the sphenoid bone extending into the ethmoid sinus, nasal cavity and orbita in a child. Turk. Neurosurg. 2009, 19, 172–176. [Google Scholar] [PubMed]
- Sun, Z.J.; Sun, H.L.; Yang, R.L.; Zwahlen, R.A.; Zhao, Y.F. Aneurysmal bone cysts of the jaws. Int. J. Surg. Pathol. 2009, 17, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Breuer, C.; Paul, H.; Zimmermann, A.; Braunstein, S.; Schaper, J.; Mayatepek, E.; Oh, J. Mandibular aneurysmal bone cyst in a child misdiagnosed as acute osteomyelitis: A case report and a review of the literature. Eur. J. Pediatr. 2010, 169, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.J.; Choi, S.C.; Kwon, Y.D.; Drew, S.J. Aneurysmal bone cyst causing a pathologic fracture of the mandibular condyle. J. Oral Maxillofac. Surg. 2011, 69, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Cho, K.S.; Choi, K.U.; Roh, H.J. Aggressive aneurysmal bone cyst of the maxilla confused with telangiectatic osteosarcoma. Auris Nasus Larynx 2012, 39, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Kumar, R.; Bal, A.; Panda, N.K. Aneurysmal bone cyst of maxilla with ectopic molar tooth—a case report. Otolaryngol. Pol. 2013, 67, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Simsek, G.; Saka, C.; Sonbay, D.N.; Akin, I.; Koybasioglu, F. Aneurysmal bone cyst in the middle turbinate: A case report. Ear Nose Throat J. 2013, 92, E47. [Google Scholar] [CrossRef] [PubMed]
- Lerant, G.; Ivanyi, E.; Toth, E.; Levai, A.; Godeny, M. Aneurysmal bone cyst of the zygomatic arch: A case report. Clin. Imaging 2013, 37, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.; Cresswell, M.; Sharma, R.; Maheshwar, A. Aneurysmal bone cyst of the ethmoid bone. BMJ Case Rep. 2014, 2014, bcr2013202319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.Y.; Ko, Y.I.; Kwon, H.; Jung, S.N. Aneurysmal bone cyst of the zygomatic bone. J. Craniofac. Surg. 2014, 25, e148–e149. [Google Scholar] [CrossRef] [PubMed]
- Neuschl, M.; Reinert, S.; Gülicher, D.; Neuschl, J.; Hoffmann, J. Aneurysmal bone cyst of the ascending ramus mandible. A case report. J. Craniomaxillofac. Surg. 2014, 42, e36–e38. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Sharma, R.; Muralidharan, C.G. Sagittal split ramus osteotomy for aneurysmal bone cyst of the mandibular condyle. J. Craniofac. Surg. 2015, 26, e38–e39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Zhang, M.; Zhao, X.X. Aneurysmal bone cyst of the orbit. Chin. Med. J. 2015, 128, 562–563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Guo, C.; Guo, R.; Meng, J. A Giant Aneurysmal Bone Cyst in the Mandibular Condyle. J. Craniofac. Surg. 2017, 28, e148–e151. [Google Scholar] [CrossRef] [PubMed]
- Toescu, S.M.; Alalade, A.F.; Steele, L.; Bhargava, D.; Hunter, R. Frontal skull osteoblastoma with aneurysmal bone cyst-like changes associated with trauma during pregnancy: A case report. Acta Neurochir. 2017, 159, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Arocho-Quinones, E.V.; Self, S.; Suchi, M.; Zwagerman, N.T.; Lew, S.M. Spheno-Orbital Aneurysmal Bone Cyst in a 10-Month-Old Infant. World Neurosurg. 2018, 117, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Asi, K.W.; Abdelmeguid, A.; Bell, D.; Hanna, E.Y. Massive aneurysmal bone cyst of the skull base treated with denosumab. Head Neck 2018, 40, E107–E113. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, M.; Pardhe, N.; Vijay, P. Aneurysmal Bone Cyst of Mandible with Classical Histopathological Presentation. J. Coll. Physicians Surg. Pak. 2018, 28, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Rațiu, C.; Ilea, A.; Gal, F.A.; Ruxanda, F.; Boşca, B.A.; Miclăuș, V. Mandibular aneurysmal bone cyst in an elderly patient: Case report. Gerodontology 2018, 35, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Choby, G.; Van Gompel, J.J.; Link, M.J.; Van Abel, K.M. Aneurysmal Bone Cysts of the Paranasal Sinuses: The Mayo Clinic Experience and Review of the Literature. Laryngoscope 2021, 131, E2525–E2533. [Google Scholar] [CrossRef] [PubMed]
- Foo, M.I.; Nicol, K.; Murakami, J.W. Skull base chondroblastoma with aneurysmal bone cyst-like changes treated with percutaneous radiofrequency ablation and doxycycline sclerotherapy: Illustrative case. J. Neurosurg. Case Lessons 2022, 4, CASE22436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yahaya, J.J.; Morgan, E.D.; Abraham, Z.S.; Othieno, E. Aneurysmal bone cyst of the mandible: A rare case report and literature review. Ann. Med. Surg. 2023, 85, 5133–5137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mo, J.T.; Darrow, M.A.; Sharma, J.D. Langerhans cell histiocytosis with aneurysmal bone cyst-like changes: A case-based literature review. Childs Nerv. Syst. 2023, 39, 3057–3064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Phan, T.; Tong, J.; Krivanek, M.; Graf, N.; Dexter, M.; Tumuluri, K. Aneurysmal Bone Cyst of the Orbit With USP6 Gene Rearrangement. Ophthalmic Plast. Reconstr. Surg. 2023, 39, 206–210. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Hayat, F.; Amasa, S.; Ammar Haider, M.; Akram Asbeutah, S.; AlDallal, U.; Barrie, U.; Ismail, M. Rare Aneurysmal Bone Cyst Presentation in the Orbit: A Systematic Review of the Literature with an Illustrative Case Report. World Neurosurg. 2024, 191, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Han, S.I.; Lim, S.C. Intraosseous hemangioma with aneurysmal bone cyst-like changes of the hyoid bone: Case report and literature review. Medicine 2024, 103, e37137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaffe, H.L.; Lichtenstein, L. Solitary unicameral bone cyst: With emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch. Surg. 1942, 44, 1004–1025. [Google Scholar] [CrossRef]
- Lichtenstein, L. Aneurysmal Bone Cyst: Pathological Entity Commonly Mistaken for Giant-Cell Tumor and Occasionally for Hemangioma and Osteogenic Sarcoma. Cancer 1950, 3, 279–289. [Google Scholar] [CrossRef]
- Lau, A.W.; Pringle, L.M.; Quick, L.; Riquelme, D.N.; Ye, Y.; Oliveira, A.M.; Chou, M.M. TRE17/ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation. J. Biol. Chem. 2010, 285, 37111–37120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cottalorda, J.; Bourelle, S. Modern concepts of primary aneurysmal bone cyst. Arch. Orthop. Trauma Surg. 2007, 127, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, L.S.; Shenker, A.; Gejman, P.V.; Merino, M.J.; Friedman, E.; Spiegel, A.M. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N. Engl. J. Med. 1991, 325, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Riminucci, M.; Saggio, I.; Robey, P.G.; Bianco, P. Fibrous dysplasia as a stem cell disease. J. Bone Miner. Res. 2006, 21, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.; Dekhou, A.; Osto, M.; Agemy, J.; Chaaban, A.; Yuhan, B.; Thorpe, E. Aneurysmal Bone Cysts of the Craniofacial Origin: A Systematic Review. OTO Open 2021, 5, 2473974X211052950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caro, P.A.; Mandell, G.A.; Stanton, R.P. Aneurysmal bone cyst of the spine in children. MRI imaging at 0.5 tesla. Pediatr. Radiol. 1991, 21, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Muratori, F.; Mondanelli, N.; Rizzo, A.R.; Beltrami, G.; Giannotti, S.; Capanna, R.; Campanacci, D.A. Aneurysmal Bone Cyst: A Review of Management. Surg. Technol. Int. 2019, 35, 325–335. [Google Scholar] [PubMed]
- Hauschild, O.; Lüdemann, M.; Engelhardt, M.; Baumhoer, D.; Baumann, T.; Elger, T.; Südkamp, N.P.; Herget, G.W. Aneurysmal bone cyst (ABC): Treatment options and proposal of a follow-up regime. Acta Orthop. Belg. 2016, 82, 474–483. [Google Scholar] [PubMed]
- Parker, J.; Soltani, S.; Boissiere, L.; Obeid, I.; Gille, O.; Kieser, D.C. Spinal Aneurysmal Bone Cysts (ABCs): Optimal Management. Orthop. Res. Rev. 2019, 1, 159–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yildiz, C.; Erler, K.; Atesalp, A.S.; Basbozkurt, M. Benign bone tumors in children. Curr. Opin. Pediatr. 2003, 15, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Strohm, J.A.; Strohm, P.C.; Kühle, J.; Schmal, H.; Zwingmann, J. Management of juvenile and aneurysmal bone cysts: A systematic literature review with meta-analysis. Eur. J. Trauma Emerg. Surg. 2023, 49, 361–372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Döring, K.; Puchner, S.; Vertesich, K.; Funovics, P.T.; Hobusch, G.; Sulzbacher, I.; Chiari, C.; Windhager, R. Results in the surgical treatment of aneurysmal bone cysts-A retrospective data analysis. Orthop. Traumatol. Surg. Res. 2022, 108, 103095. [Google Scholar] [CrossRef] [PubMed]
- Tillman, B.P.; Dahlin, D.C.; Lipscomb, P.R.; Stewart, J.R. Aneurysmal bone cyst: An analysis of ninety-five cases. Mayo Clin. Proc. 1968, 43, 478–495. [Google Scholar] [PubMed]
- Ruiter, D.J.; van Rijssel, T.G.; van der Velde, E.A. Aneurysmal bone cysts: A clinicopathological study of 105 cases. Cancer 1977, 39, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.T.; Jiang, S.; Cen, Y. Fibrous dysplasia of skull. J. Craniofac. Surg. 2010, 21, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Perez-Atayde, A.R.; Inwards, C.Y.; Medeiros, F.; Derr, V.; Hsi, B.L.; Gebhardt, M.C.; Rosenberg, A.E.; Fletcher, J.A. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am. J. Pathol. 2004, 165, 1773–1780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliveira, A.M.; Hsi, B.L.; Weremowicz, S.; Rosenberg, A.E.; Dal Cin, P.; Joseph, N.; Bridge, J.A.; Perez-Atayde, A.R.; Fletcher, J.A. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004, 64, 1920–1923. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Chou, M.M.; Perez-Atayde, A.R.; Rosenberg, A.E. Aneurysmal bone cyst: A neoplasm driven by upregulation of the USP6 oncogene. J. Clin. Oncol. 2006, 24, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Yoshida, A.; Guo, T.; Chang, N.E.; Zhang, L.; Agaram, N.P.; Qin, L.X.; Brennan, M.F.; Singer, S.; Maki, R.G. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. 2009, 69, 7175–7179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panoutsakopoulos, G.; Pandis, N.; Kyriazoglou, I.; Gustafson, P.; Mertens, F.; Mandahl, N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes. Chromosomes Cancer 1999, 26, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Tarpey, P.S.; Presneau, N.; Scheipl, S.; Pillay, N.; Van Loo, P.; Wedge, D.C.; Cooke, S.L.; Gundem, G.; Davies, H.; et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 2013, 45, 1479–1482, Erratum in Nat. Genet. 2014, 46, 316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).