Reading and White Matter Development: A Systematic Review of Neuroplastic Changes in Literacy
Abstract
1. Introduction
- which white matter pathways are consistently implicated across studies;
- how reading-related brain changes differ across developmental stages (e.g., preschool vs. adolescence);
- how these patterns vary between typically developing children and those with reading difficulties such as dyslexia.
1.1. Reading Habits
1.2. Reading Skills
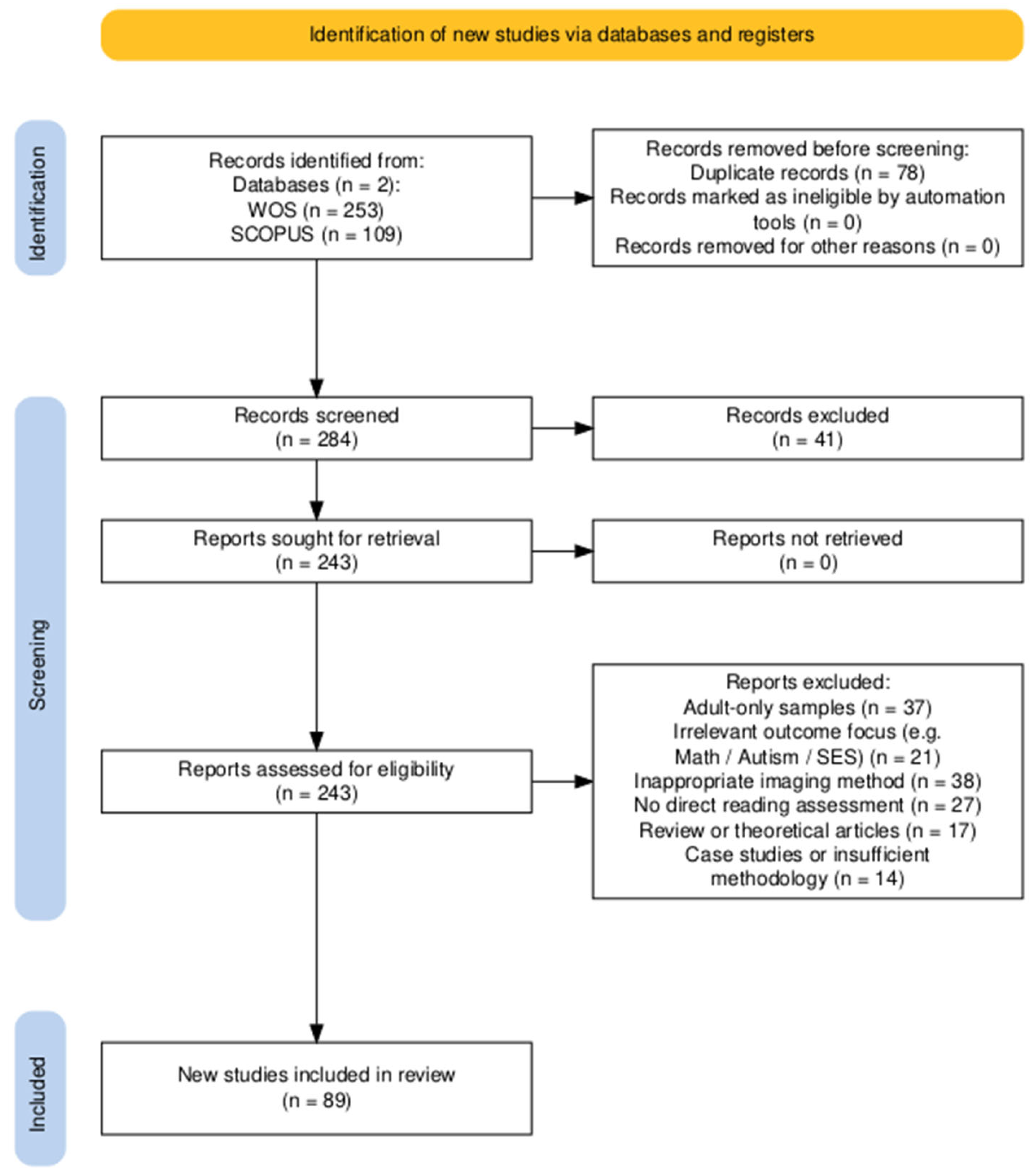
2. Methods
2.1. Review Framework and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
2.4. Study Selection Process
2.5. Study Grouping
3. Results
| Authors | Study Group | N: | Measures | Results | |
|---|---|---|---|---|---|
| 1 | Deutsch et al., 2005 [49] | Fourteen children aged 7–13 years | 14 | FA and CI in white matter tracts; reading, spelling, and rapid naming skills | Lower FA and CI in left temporo-parietal pathways linked to poorer reading, spelling, and naming, supporting white matter’s role. |
| 2 | Beaulieu et al., 2005 [34] | Children aged 8–12 years | 32 | DTI (FA in left temporo-parietal white matter); word identification (WRMT-R) | Higher FA in left temporo-parietal white matter correlates with better reading (Word ID), with the strongest correlation in the posterior internal capsule. |
| 3 | Niogi and McCandliss, 2006 [50] | Thirty-one children (6.5–10.3 years), including RD (reading disability) and non-impaired groups | 31 | FA in left SCR, CS, ACR; word ID, word attack, lateralization index | Higher FA in left SCR and CS correlated with better reading skills. RD group showed right lateralization, linked to poorer reading scores |
| 4 | Leonard et al., 2006 [51] | Children with DD, SLI, and mixed profiles (ages 11–16) | 22 | Structural MRI; anatomical risk index based on volumetric asymmetry, reading and comprehension assessments | Children with more symmetrical brain structures had severe comprehension deficits (SLI-like), while those with more leftward asymmetry had decoding impairments but preserved comprehension (DD-like). Anatomical profile predicted reading/differentiation patterns. |
| 5 | Dougherty et al., 2007 [35] | Children aged 7–12 with a range of reading abilities | 49 | DTI; FA and radial diffusivity in seven corpus callosum segments; phonological awareness (CTOPP), reading measures (WJ-III) | Higher phonological awareness was significantly associated with increased radial diffusivity and lower FA in the temporal-callosal segment. Suggests better readers have fewer but larger axons, indicating reduced interhemispheric connectivity may support reading. |
| 6 | Cao et al., 2008 [52] | Children with and without reading difficulties (8.9–14.11 years) | 24 | fMRI, Dynamic Causal Modeling (DCM), rhyming task | Children with reading difficulties showed weaker modulatory connectivity between left fusiform and parietal regions, especially on trials with conflicting phonological and orthographic cues. Reading skill was correlated with several connectivity patterns only in typical readers. |
| 7 | Odegard et al., 2009 [53] | Children with and without dyslexia (10–14 y/o) | 17 | DTI (FA); WIAT-II (real word), DST (pseudoword decoding) | FA in left superior corona radiata and other tracts correlated with decoding; overlap found for real and pseudoword skills. Negative correlation in corpus callosum. |
| 8 | Andrews et al., 2010 [33] | Preterm (mean age 11.9 ± 1.8 years) and term children (mean age 12.7 ± 2.5 years) | 28 | DTI (FA in corpus callosum and temporo-parietal region;, reading skills (WJ-III subtests) | Preterm children had lower reading scores and FA values in corpus callosum. FA in the body of corpus callosum correlated significantly with reading scores. |
| 9 | Rimrodt et al., 2010 [31] | Children with and without dyslexia, aged 7–16 | 31 | DTI (FA, PDD); TOWRE (reading fluency), WJ-III (word ID, word attack), tractography of perisylvian network | Lower FA in LIFG and temporo-parietal WM in dyslexia; FA positively correlated with reading fluency; differences linked to fiber orientation and tract overlap. |
| 10 | Frye et al., 2011 [54] | Adolescents (~16 y), born at term and preterm | 32 | DTI (FA, RD, SLF volume); letter–word ID, phoneme reversal, word attack | FA and RD in left SLF significantly correlated with reading measures; SLF volume linked to reading only in preterm-born group. |
| 11 | Yeatman et al., 2011 [40] | Children aged 7–11 years | 55 | DTI (FA, RD in AF); phonological awareness, memory, and word reading | Higher FA in the left AF correlated with better phonological awareness and memory, with left-lateralized volume predicting reading skills. |
| 12 | Raschle et al., 2011 [55] | Pre-reading children with (FHD+) and without (FHD−) familial risk for dyslexia | 20 | VBM, RAN; family history, gray matter volume | Reduced GMV in left occipitotemporal and parietotemporal regions in children at risk; positive correlation between GMV and rapid automatized naming. |
| 13 | Feldman et al., 2012 [56] | Forty-two children (23 preterm, 19 full-term), aged 9–16 years | 42 | FA in corpus callosum, AF, SLF; verbal IQ, syntactic comprehension, decoding, and reading comprehension | Preterm group showed FA correlations with language and reading in ventral and dorsal tracts; no such links in full-term group. |
| 14 | Saygin et al., 2013 [13] | Pre-readers and early readers (Kindergarten children, aged ~5 years) | 40 | Diffusion MRI (FA, volume in AF, ILF, SLFp); CTOPP, WRMT-R, and RAN | Phonological awareness correlated with FA and volume in left AF; no link to RAN or letter knowledge. |
| 15 | Gullick and Booth, 2015 [37] | Children aged 8–14 years | 47 | DTI (FA in AF), fMRI (pSTS activity), word reading (rhyme judgment) | Better reading skills and cross-modal task performance linked to higher FA in left arcuate fasciculus, with pSTS activity predicting AF integrity. |
| 16 | Myers et al., 2014 [57] | 38 children aged 5–6 years at baseline, followed until Grade 3 | 38 | WM volume changes; reading skills | Left dorsal WM growth (such as, AF) predicted Grade 3 reading outcomes, beyond familial, env., and pre-literacy factors. |
| 17 | Horowitz-Kraus et al., 2014 [58] | Typically developing adolescents aged 15–19 years | 21 | DTI (FA in arcuate fasciculus and inferior longitudinal fasciculus); TOWRE-II (sight word efficiency), WJ-III (passage comprehension) | Right ILF was associated with word reading efficiency; left ILF and bilateral AF, especially right AF, were associated with reading comprehension. Findings support the Simple View of Reading, indicating distinct white matter correlates for word- and sentence-level reading. |
| 18 | Broce et al., 2015 [59] | Typically developing children (ages 5–8) | 19 | DWI (FA in arcuate fasciculus [segments] and frontal aslant tract); CELF-4 receptive language, | FA in bilateral AF predicted expressive and receptive language; FA in left AF increased with age; left FAT length predicted receptive language scores. |
| 19 | Skeide et al., 2015 [32] | Children aged 9–12 years | 50 | Phonological awareness, spelling DTI (FA of arcuate fasciculus), rs-fMRI | Genetic variant rs11100040 was associated with functional and structural connectivity (arcuate fasciculus FA) between phonological regions. Structural connectivity was linked to phonological awareness, which in turn predicted spelling and dyslexia risk scores. |
| 20 | Travis et al., 2015 [26] | Forty-five children and adolescents (9–17 years) | 45 | FA in cerebellar peduncles (SCP, MCP, ICP); decoding, reading comprehension | FA in MCP positively correlated with decoding and comprehension; left SCP and ICP showed negative correlations. |
| 21 | Richards et al., 2015 [30] | Grades 4–9: dysgraphia (n = 14), dyslexia (n = 17), controls (n = 9) | 40 | DTI (FA, RA, AD); fMRI during writing; spelling, alphabet, planning, resting state | WM integrity (FA, RA, AD) higher in controls; dyslexia and dysgraphia showed distinct WM–fMRI connectivity patterns during writing tasks. |
| 22 | Gullick et al., 2016 [38] | Typically developing children, SES stratified | 42 | DTI (FA in ILF, SLF, CST); real-word reading scores, SES | High SES: left ILF/SLF FA correlated with reading. Low SES: right ILF FA correlated with reading, suggesting divergent neural strategies. |
| 23 | Travis et al., 2016 [60] | Forty-two children (5.8–6.8 years), 31 Readers, 11 Pre-readers | 42 | FA in white matter; phonological awareness, language, and pseudoword decoding | Readers had higher FA in left aSLF and right UF; aSLF-L linked to phonological awareness, UF-R to language skills. |
| 24 | Borst et al., 2016 [61] | Typically developing children aged 9–10 years | 16 | Anatomical MRI (sulcal morphology of left and right OTS); oral reading (Alouette-R test) | Left OTS sulcal pattern predicted reading accuracy; interrupted OTS linked to better word reading. |
| 25 | Travis et al., 2017 [62] | Six-year-old children (readers and pre-readers) | 42 | Cross-sectional, FA in white matter tracts (e.g., left anterior SLF, right UF); reading skills, phonological awareness, language skills | Readers had higher FA compared to pre-readers; FA significantly correlated with phonological awareness and language skills. |
| 26 | De Moura et al., 2016 [48] | Forty children aged 8–12 years: 17 poor readers, 23 good readers | 40 | FA in AF, ILF, cingulum; word-level reading ability | Poor readers had lower FA in bilateral white matter tracts, indicating reduced fiber coherence. |
| 27 | Mürner-Lavanchy et al., 2018 [63] | Very preterm (VPT) and term-born children, age 7 | 178 | DTI (FA, RD, MD), NODDI (axon density), TBSS, arcuate fasciculus tractography; language tests (semantics, grammar, phonological awareness) | Higher FA and axon density, and lower RD, AD, MD were associated with better performance in semantics, grammar, and phonological awareness in both groups. |
| 28 | Sun et al., 2017 [64] | Children with variable reading skills | 66 | ROBO1 genotyping, DTI (FA in corpus callosum); word-list reading. | ROBO1 polymorphisms influence reading via FA in the genu of the corpus callosum; genu FA mediates gene-to-reading effect. |
| 29 | Arrington et al., 2017 [24] | School-aged children with typical or poor decoding skills | 76 | Reading accuracy, fluency, comprehension, and white matter integrity (FA values) | White matter integrity in SLF, ILF, and UF correlated with reading; poor readers showed distinct tract reliance. |
| 30 | Horowitz-Kraus et al., 2017 [65] | Adolescents with mood or behavioral disorders and controls (11–17 y) | 39 | DTI (FA); CTOPP (phonemic awareness), WJ-III (orthographic processing, reading comprehension) | Reading skills correlated with FA in AF, ILF, SLF, IFOF; mood disorders showed lower comprehension and phonological scores and altered WM–reading associations. |
| 31 | Wang et al., 2017 [66] | Seventy-eight children (5–12 years) | 78 | FA, RD, and AD in AF, SLF, ILF; reading fluency, phonological awareness, and familial risk | FHD+ children had lower FA and atypical left AF lateralization; faster FA growth in right SLF aided compensation in good readers. |
| 32 | Su et al., 2018 [41] | Forty Chinese children (18 with dyslexia, 22 controls), mean age 11.1 years | 40 | FA in left AF (dorsal) and left ILF (ventral), phonological, morphological, and orthographic processing skills | Reduced FA in left AF linked to phonological deficits and in left ILF to morphological deficits, showing dual-pathway disruption. |
| 33 | Su et al., 2018 [67] | Chinese children (longitudinal, ages 4–14) | 79 | Vocabulary development (ages 4–10); DTI at age 14 (FA in arcuate fasciculus) | Children with consistently poor vocabulary growth showed significantly reduced FA in the left arcuate fasciculus, particularly in the posterior and direct segments. Vocabulary growth rate was a significant predictor of FA, independent of initial vocabulary level. |
| 34 | Lou et al., 2019 [46] | Children with developmental dyslexia (n = 26) and age-matched controls (n = 31) | 57 | Net. metrics (clustering, efficiency), literacy skills (reading, phonemes) | Dyslexic children had reduced left occipito-temporo-parietal connectivity, correlating with literacy skills beyond known abnormalities. |
| 35 | Banfi et al., 2019 [68] | Children with dyslexia, isolated spelling deficits, and typical peers (Grade 3) | 69 | DTI (FA via AFQ); SLRT-II (word and pseudoword reading), spelling test, PA, RAN, IQ | Dyslexia group showed higher FA in bilateral ILF and right SLF; FA in right ILF negatively correlated with reading; left AF FA correlated with spelling in SD group. |
| 36 | Huber et al., 2019 [69] | Children aged 7–12 with varied reading skills | 53 | DTI, WMTI, NODDI, Woodcock–Johnson basic reading composite | AWF and ICVF in posterior corpus callosum correlated significantly with reading skill; results robust after controlling for age and motion. |
| 37 | Dubner et al., 2019 [70] | Preterm with inflammation (PT+), preterm without inflammation (PT−), full-term (FT) | 78 | FA and MD in 7 corpus callosum segments; WRMT-III (reading), WASI-II (IQ), BRIEF (executive function) | Reading correlated with occipital FA (r = 0.32, p < 0.01); PT+ group had lower FA and higher MD in multiple callosal segments compared to PT and FT groups. |
| 38 | Broce et al., 2019 [71] | Typically developing children (5–8 y/o) | 60 | DTI (FA in AF, ILF, IFOF, VOF); phonological awareness; decoding | FA in AF, ILF, and VOF predicted early literacy skills; VOF newly identified as relevant for early reading development. |
| 39 | Del Tufo et al., 2019 [72] | Children with early expressive language delay | 340 | Reading/listening comprehension tests (e.g., QRI, WJ-PC), expressive language milestones, FA of left ILF (DTI) | Later expressive language milestones predicted poorer comprehension. Left ILF moderated the relationship. Early intervention reduced the risk of poor comprehension by 39% in at-risk children. |
| 40 | Vanderauwera et al., 2019 [73] | Adolescents aged 13–14, wide range of reading skills | 34 | DTI (FA, AD, RD), word and pseudoword fluency tests (Een-minuut-Test, Klepel), SES via paternal education | FA in left long AF and UF positively associated with word reading; SES also linked to FA and reading skills, suggesting environmental effects. |
| 41 | De Vos et al., 2020 [74] | Typically developing pre-readers | 59 | DTI (FA in left AF); auditory steady-state response, phonological awareness tasks | Rightward lateralization of 4Hz syllable-rate processing associated with higher FA in left AF; both predicted better phonological processing. |
| 42 | Beaulieu et al., 2020 [75] | Children and adolescents (aged 10–18 years) | 20 | myelin water fraction (MWF) imaging; standardized reading tests | Lower MWF in poor readers in corpus callosum, thalamus, and internal capsule; MWF positively correlated with reading scores. |
| 43 | Geeraert et al., 2020 [76] | Typically developing children aged 6–16 years | 46 | DTI, neurite orientation dispersion and NODDI, magnetization transfer imaging | White matter microstructure developed with age but showed no direct link to reading skills. |
| 44 | Hutton et al., 2020 [77] | Preschoolers aged 3–5, typical development | 47 | DTI (FA, AD, RD, MD); StimQ-P2 READ (HLE), EVT-2, GRTR, TRH, CTOPP-2 RAN | Higher HLE scores associated with lower AD, RD, MD in AF, ILF, UF; book reading quantity linked to higher FA and better emergent literacy skills. |
| 45 | Bruckert et al., 2020 [78] | Twenty-three children, aged 8 years (mean age = 8.2, 12 male) | 23 | FA and R1 in SCP, MCP, and ICP; word reading efficiency | Reading efficiency negatively correlated with FA in SCPs; no R1 link, suggesting non-myelin factors influence FA-reading associations. |
| 46 | Zhao et al., 2021 [79] | Ninety-six children aged 8–12 years, | 96 | FA in AF, SLF, corpus callosum, cerebellar tracts; phonological and reading fluency | Dyslexia linked to reduced left AF and SLF connectivity; better reading correlated with stronger corpus callosum and cerebellar pathways. |
| 47 | Lou et al., 2021 [80] | Children with and without reading disabilities | 64 | DTI, whole-brain connectome; feeder connection strength, word reading efficiency, phonemic decoding | Feeder connections between hubs and non-hubs significantly correlated with word reading efficiency and phonemic decoding; effects stronger in girls. |
| 48 | Van Der Auwera et al., 2021 [81] | Children with and without dyslexia; three time points (pre-, early-, and advanced-reading stages) | 52 | DTI; word and pseudoword reading (Grades 2–5), phonological awareness | FA in left arcuate fasciculus was lower in pre-readers who developed dyslexia and predicted later word and pseudoword reading skills. |
| 49 | Zuk et al., 2021 [82] | At-risk children followed from Kindergarten to Grade 2 | 74 | DTI (FA in posterior right SLF); TOWRE-2, WRMT-III, SES, phonological awareness, speech accuracy | Higher FA in right posterior SLF predicted better decoding in at-risk children. SES, speech accuracy, and PA also predicted successful reading outcomes. |
| 50 | Wang et al., 2021 [83] | Typically developing children (n = 22) and children with reading difficulties (n = 24), aged ~9 years | 46 | Diffusion spectrum imaging; reading comprehension test, Chinese character recognition test | RD group showed reduced white matter integrity (GFA, NQA); reading comprehension and character recognition linked to corpus callosum indices. |
| 51 | Liu et al., 2021 [79] | Children aged 9–14 with and without dyslexia | 57 | DTI-based graph analysis (FA in right fusiform gyrus); word and pseudoword reading accuracy, spelling | In children with dyslexia, higher FA in the right fusiform gyrus was negatively correlated with reading accuracy, suggesting maladaptive compensation. |
| 52 | Koirala et al., 2021 [84] | Children aged 6–16 (typical and struggling readers) | 412 | DWI (NODDI: ODI, NDI); single word reading, phonological processing | Lower ODI and NDI associated with better reading and phonological processing; phonological processing mediated the WM–reading relationship. |
| 53 | Yu et al., 2022 [85] | Adolescents with PAE (FAS/PFAS, HE), controls | 74 | DTI (ILF lateralization, FA), fMRI; phonological processing, reading | FAS/PFAS group showed rightward ILF lateralization and increased right hemisphere activation; HE group showed weaker left ILF–reading correlations. |
| 54 | Gao et al., 2022 [36] | Chinese–English bilingual children, aged 8.2–12 | 40 | DTI (FA in left/right arcuate fasciculus); English and Chinese reading tests, phonological awareness, visual–spatial ability | English reading was associated with FA in left AF (especially caudal nodes, correlated with phonological awareness); Chinese reading was associated with FA in right AF (correlated with visual-spatial ability). Findings support both language-universal and language-specific white matter mechanisms. |
| 55 | Liu et al., 2022 [27] | Fifty-seven children (9–14 years), including 26 with dyslexia and 31 matched controls | 57 | FA values in right fusiform gyrus (FFG); reading accuracy, pseudoword reading, spelling accuracy | Higher FA in the right FFG negatively correlated with word and pseudoword reading accuracy in dyslexic children, suggesting maladaptive compensation. |
| 56 | Meisler and Gabrieli, 2022 [39] | Children and adolescents (6–18 years) | 983 | Diffusion MRI (FDC, FD, FC metrics); word-reading efficiency test (TOWRE) | Higher FDC in left temporo-parietal white matter correlated with better reading; no differences between reading-disabled and typical groups. |
| 57 | Meisler and Gabrieli, 2022 [86] | Six hundred and eighty-six children aged 5–18 years, with and without reading disabilities | 686 | FA values in white matter tracts; TOWRE composite scores (SWE and PDE) | No significant FA differences in groups. Positive FA associations with nonword reading in older children (9+), particularly in right SLF and left ICP. |
| 58 | Brignoni-Pérez et al., 2021 [45] | Children born full-term (FT) and preterm (PT), average age: 8 | 79 | Oral reading index, FA from dMRI, R1 metric from qT1 relaxometry | FT: Reading correlated with FA in dorsal tracts; PT: reading correlated with R1 in dorsal and ventral tracts. |
| 59 | Ostertag et al., 2023 [87] | Children with and without prenatal alcohol exposure, age 5 | 57 | DTI (FA, AD in arcuate fasciculus); NEPSY-II (speeded naming, phonological processing), vocabulary | In PAE children, greater FA in right AF predicted better speeded naming; higher AD in left AF was linked to better phonological processing. No such associations were found in controls. Indicates altered white matter–language function relationships in PAE. |
| 60 | Harriott et al., 2023 [88] | Children with NF1 (M = 12.5 years) | 28 | Word reading, phonological awareness, visuospatial skills; MRI (T2/FLAIR UBO volume); | Total UBO volume significantly predicted word reading and phonological awareness, even when controlling for age, sex, scanner, and PIQ. |
| 61 | Zhao et al., 2023 [42] | Children with developmental dyslexia and age-matched controls | 57 | high-angular diffusion imaging (HARDI), spherical deconvolution tractography, HMOA in AF segments; reading accuracy (words, nonwords, meaningless text) | Lateralization index (LI) of AFAS correlated with nonword and meaningless text reading; LI of AFLS correlated with word reading. Findings suggest segment-specific compensatory lateralization in dyslexia. |
| 62 | Vandecruys et al., 2024 [89] | Typically developing preschoolers (mean age ≈ 5.6 years) | 56 | DWI; phonological awareness, letter knowledge; tracts: bilateral IFOF, ILF, anterior and direct arcuate fasciculus | Bilateral IFOF microstructure was associated with both reading and math precursors; associations were shared, not specific to reading alone. |
| 63 | Ghasoub et al., 2024 [90] | Typically developing children (ages 2–6) | 81 | Diffusion MRI; graph-theory analysis, NEPSY-II phonological processing, and speeded naming | Phonological processing scores positively associated with efficiency and clustering in reading-language structural networks, especially right hemisphere. |
| 64 | Cross et al., 2023 [91] | Sixty-five children aged 8–14 years | 65 | DTI (FA in AF, ILF, IFOF, uncinate fasciculus); single word reading, decoding, comprehension, rapid naming tasks | Higher FA in left AF associated with better decoding efficiency; higher FA in left IFOF positively linked with reading comprehension; greater FA in right ILF and bilateral uncinate fasciculus negatively correlated with reading comprehension and rapid naming skills |
| Authors | Study Group | N: | Measures | Results | |
|---|---|---|---|---|---|
| 1 | Hoeft et al., 2011 [95] | Children with and without dyslexia (6–12 y/o) | 45 | DTI (right SLF); fMRI during phonological task; reading assessments over 2.5 years | WM organization in right SLF predicted long-term reading gains in dyslexia; behavioral predictors alone were not sufficient. |
| 2 | Yeatman et al., 2012 [4] | 55 children, aged 7–15 years (39 with at least 3 measurements) | 55 | FA in left AF and ILF; standardized reading scores | Above-average readers had low initial FA that increased over time; below-average readers had high initial FA that declined, reflecting differing developmental trajectories. |
| 3 | Gullick and Booth, 2015 [37] | Children aged 8–14 years | 30 | Diffusion MRI (FA in AF); reading assessments (real-word reading, pseudoword reading) | Higher FA in the direct segment of the AF at baseline was predictive of greater improvements in reading skills over a three-year period. |
| 4 | Kraft et al., 2016 [96] | Pre-reading children with and without family risk of DD; followed into Grade 1 or 2 | 53 | Quantitative T1 MRI (left anterior AF); behavioral literacy precursors (phonological representations, RAN); reading/spelling tests (SLRT-II, ELFE, DERET) | Increased T1 intensity (↓ myelin) in left anterior AF in risk group. Neuroanatomical predictor model (incl. AF) predicted DD better (80%) than behavioral-only model (63%). T1 of left anterior AF was significant predictor of later DD. |
| 5 | Takeuchi et al., 2016 [2] | Healthy Japanese children | 296 | FA; verbal comprehension scores (WAIS-III, WISC-III) | Stronger reading habits increased FA in left AF, IFOF, PCR. |
| 6 | Vandermosten et al., 2017 [44] | Pre-readers (Kindergarten children aged ~5–6) | 71 | Diffusion MRI (FA of IFOF and AF); cognitive reading precursors (PA, RAN), parental reading data | Paternal reading skills mediate early childhood reading outcomes via left IFOF, SES impacts white matter structure. |
| 7 | Vanderauwera et al., 2018 [97] | 61 children: pre-reading stage (5–6 years) and early reading stage (7–8 years) | 61 | FA in ventral pathways (IFOF, ILF, UF); phonological awareness, orthographic knowledge | Left IFOF supported orthographic knowledge at early stages of reading. No ventral pathways supported phonological processes. |
| 8 | Huber et al., 2018 [98] | Struggling grade-school readers (ages 7–12) | 24 | Diffusion MRI (white matter metrics); reading assessments (Woodcock–Johnson, TOWRE) | Significant improvement in reading skills and widespread white matter changes over 8-week intervention. |
| 9 | Richards et al., 2018 [99] | Dyslexia (n = 20), OWL LD (n = 6), dysgraphia (n = 10), typical readers (n = 6); Grades 4–9 | 42 | DTI (AD, MD), fMRI clustering coefficient; word, sentence, and text-level silent reading tasks pre/post 18 computerized lessons | WM–GM correlations emerged post-instruction only: (1) AD in left superior frontal ↔ right IFG (word reading); (2) MD in left superior corona radiata ↔ left MFG (sentence); (3) MD in left anterior corona radiata ↔ right MFG (text). Reading gains observed behaviorally. |
| 10 | Bruckert et al., 2019 [100] | Seventy-one children: 34 preterm (PT) and 37 full-term (FT), aged 6–8 years | 71 | FA in AF, SLF, ICP; phonological awareness, language, reading fluency | FA in dorsal and cerebellar pathways predicted reading outcomes in FT but not in PT children, suggesting distinct neural adaptations in PT group. |
| 11 | Lebel et al., 2018 [47] | Children (9.5 ± 1.3 years) | 70 | FA and MD in white matter tracts; reading fluency, phonological decoding, and sight word reading | FA increased with age in non-impaired readers, absent in dysfluent readers. Faster MD decreases in dysfluent inaccurate readers suggest compensatory changes (e.g., corona radiata, uncinate). |
| 12 | Borchers et al., 2019 [101] | Typically developing children aged 6–8 years | 37 | Diffusion MRI (FA in left and right SLF, left Arcuate, left ICP); cognitive assessments (language, phonological awareness) | White matter properties (FA) at age 6 in specific pathways (SLF, Arcuate, ICP) predict reading outcomes at age 8, beyond pre-literacy skills and demographic factors. |
| 13 | Su et al., 2020 [16] | Seventy-nine children; language and reading assessments from aged 1–14 years; | 79 | FA in AF, IFOF; early family factors (SES, literacy exposure), vocabulary growth rate | Earlier literacy exposure and SES correlated with higher FA in left AF and IFOF. Vocabulary growth rate predicted FA in left AF-posterior and AF-direct. |
| 14 | Partanen et al., 2020 [102] | 87 children: 46 dyslexic, 41 average readers; aged 8–12 years | 87 | FA and MD in white matter tracts; voxel-based analysis of gray matter, reading fluency | Changes in white matter microstructure (lower MD) in right-hemisphere predicted reading fluency improvement. Gray matter showed no significant associations |
| 15 | Phan et al., 2021 [103] | Children aged 6–10 (typical and dyslexic readers) | 41 | Longitudinal T1 MRI (PBVC + scaling); reading assessments (word reading, pseudoword reading) | Cortical volume in the left reading network (temporo-parietal regions) increased during early reading stages and stabilized later. Dyslexic readers showed compensatory mechanisms in the right-hemisphere (right pars opercularis and isthmus cingulate). |
| 16 | Brignoni-Pérez et al., 2021 [45] | Very preterm infants (24–31 weeks GA), randomized to maternal speech vs. control | 42 | DTI (FA, MD) at 36 weeks PMA and 12 months AA; language via MacArthur–Bates CDI at 12–18 months AA | Infants exposed to increased maternal speech showed enhanced white matter development and higher language scores. |
| 17 | Zhou et al., 2022 [92] | Preschool children aged 2–7 years | 73 | DTI (FA, MD); phonological processing raw score, rapid naming scores | Increased FA in internal capsule and IFOF correlated with phonological processing ability; mediation analysis showed age-related changes in FA supported phonological skill development. |
| 18 | Beelen et al., 2022 [104] | Children aged 8–11 (grades 2 and 5) | 43 | Structural MRI (left fusiform gyrus size); reading skills (word and pseudoword reading tasks) | Early reading skills (grade 2) predicted an increase in the size of the left fusiform gyrus by grade 5. However, the size of the left fusiform gyrus in grade 2 did not predict later reading skills, indicating behavior-driven brain plasticity. |
| 19 | Manning et al., 2022 [94] | Preschool children aged 3.5–4.5 years | 35 | Diffusion MRI (FA, RD, AD in SLF), resting-state fMRI (functional connectivity); pre-reading skills (NEPSY-II speeded naming, phonological processing) | Structural features in SLF and functional connectivity in fronto-parietal networks at 3.5 years predicted better pre-reading skills (phonological processing, speeded naming) at 4.5 years. |
| 20 | Weiss et al., 2022 [43] | Fifty-three children; subset of 20 underwent MRI at 2 years | 53 | Parent–child conversational turns; FA and MPF in AF, SLF; letter knowledge and phonological awareness | Parent and conversational turns at 14 months correlated with literacy skills at 5 years. AF myelination mediated the relationship between early language input and literacy. |
| 21 | Davison et al., 2023 [105] | Children aged 5–10 (PLING cohort) | 77 | DTI (FA); left/right AF and SLF; lateralization index, parent-reported shared reading, standardized reading and language tests (TOWRE-2, GORT-5, PPVT-4, CELF-5) | More shared reading in Kindergarten was linked to greater FA and left-lateralized SLF, which predicted better word reading fluency in Grade 2. |
| 22 | Ghasoub et al., 2024 [106] | Children aged 3–7 with and without prenatal alcohol exposure (PAE); repeated DTI scans | 135 | DTI (graph theory metrics in reading network), NEPSY-II phonological processing and speeded naming | Children with PAE had lower pre-reading scores and lower graph theory metrics (global efficiency, nodal degree). PAE moderated brain–behavior associations: stronger links between phonological processing and network efficiency in PAE group. |
| 23 | Lou et al., 2024 [107] | Children aged 8–14 with and without reading disabilities | 64 | Diffusion-weighted MRI, connectome-based graph theory analysis of left thalamus; TOWRE-PDE (phonemic decoding), RAN (rapid automatized naming), SWE (sight word efficiency), reading comprehension | Transmission cost of the left thalamus (in reading network) was positively correlated with phonemic decoding. Local efficiency and clustering coefficient were negatively correlated with RAN. Stronger pulvinar and mediodorsal thalamic nucleus connections to temporal areas were negatively associated with decoding. Results were replicated in a validation sample. |
| 24 | Vandecruys et al., 2024 [93] | Preschool children (Mage ≈ 5.5 years); schooling vs. non-schooling groups | 67 | Longitudinal DTI; FA and MD in AF, ILF, IFOF, CC; word reading, letter knowledge (DMT) | Behavioral reading skills improved significantly in the schooling group, but white matter changes were driven by age-related maturation, not schooling. |
| 25 | Roy et al., 2024 [108] | Children and young adults from diverse demographic backgrounds | 14,249 | Reading skills (e.g., TOWRE scores), white matter integrity (FA values), socio-economic factors | Dynamic relationship between reading skill gains and white matter changes over time. No evidence of static white matter properties predicting reading skills across cross-sectional datasets. |
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Cunningham, A.E.; Stanovich, K.E. Assessing print exposure and orthographic processing skill in children: A quick measure of reading experience. J. Educ. Psychol. 1990, 82, 733. [Google Scholar] [CrossRef]
- Takeuchi, H.; Taki, Y.; Hashizume, H.; Asano, K.; Asano, M.; Sassa, Y.; Kawashima, R. Impact of reading habit on white matter structure: Cross-sectional and longitudinal analyses. Neuroimage 2016, 133, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, M.; Dougherty, R.F.; Wandell, B.A. White matter pathways in reading. Curr. Opin. Neurobiol. 2007, 17, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, J.D.; Dougherty, R.F.; Ben-Shachar, M.; Wandell, B.A. Development of white matter and reading skills. Proc. Natl. Acad. Sci. USA 2012, 109, e3045–e3053. [Google Scholar] [CrossRef]
- Clark, C.; Rumbold, K. Reading for Pleasure: A Research Overview; National Literacy Trust: London, UK, 2006. [Google Scholar]
- Dunst, C.J.; Simkus, A.; Hamby, D.W. Relationship between age of onset and frequency of reading and infants’ and toddlers’ early language and literacy development. Cent. Early Lit. Learn. Rev. 2012, 5, 4. [Google Scholar]
- Bennett, K.K.; Weigel, D.J.; Martin, S.S. Children’s acquisition of early literacy skills: Examining family contributions. Early Child. Res. Q. 2002, 17, 95–317. [Google Scholar] [CrossRef]
- Evans, M.A.; Shaw, D.; Bell, M. Home literacy activities and their influence on early literacy skills. Can. J. Exp. Psychol. 2000, 54, 65. [Google Scholar] [CrossRef]
- Mol, S.E.; Bus, A.G.; de Jong, M.T. Interactive Book Reading in Early Education: A Tool to Stimulate Print Knowledge as Well as Oral Language. Rev. Educ. Res. 2009, 79, 979–1007. [Google Scholar] [CrossRef]
- Pugh, K.R.; Landi, N.; Preston, J.L.; Mencl, W.E.; Austin, A.C.; Sibley, D.; Frost, S.J. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain Lang. 2013, 125, 173–183. [Google Scholar] [CrossRef]
- Rollans, C.; Cheema, K.; Georgiou, G.K.; Cummine, J. Pathways of the inferior frontal occipital fasciculus in overt speech and reading. Neuroscience 2017, 364, 93–106. [Google Scholar] [CrossRef]
- Wang, K.; Li, X.; Huang, R.; Ding, J.; Song, L.; Han, Z. The left inferior longitudinal fasciculus supports orthographic processing: Evidence from a lesion-behavior mapping analysis. Brain Lang. 2020, 201, 104721. [Google Scholar] [CrossRef] [PubMed]
- Saygin, Z.M.; Norton, E.S.; Osher, D.E.; Beach, S.D.; Cyr, A.B.; Ozernov-Palchik, O.; Gabrieli, J.D. Tracking the roots of reading ability: White matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J. Neurosci. 2013, 33, 13251–13258. [Google Scholar] [CrossRef] [PubMed]
- Johnsson-Smaragdi, U.; Jönsson, A. Book Reading in Leisure Time: Long-Term changes in young peoples’ book reading habits. Scand. J. Educ. Res. 2006, 50, 519–540. [Google Scholar] [CrossRef]
- Jabbar, A.; Mahmood, K.; Warraich, N.F. Influence of Family Factors on Children’s Reading Habits: A Review of Literature. Bull. Educ. Res. 2021, 43, 121–144. [Google Scholar]
- Su, M.; Schotten, M.T.; Zhao, J.; Song, S.; Zhou, W.; Gong, G. Influences of the early family environment and long-term vocabulary development on the structure of white matter pathways: A longitudinal investigation. Dev. Cogn. Neurosci. 2020, 42, 100767. [Google Scholar] [CrossRef]
- Mol, S.E.; Bus, A.G. To read or not to read: A meta-analysis of print exposure from infancy to early adulthood. Psychol. Bull. 2011, 137, 267–296. [Google Scholar] [CrossRef]
- Salem, A.A.G.G.; Ferreira da Silva, P.; Felizardo, D.; Holz, M.R.; Fonseca, R.P. Does the frequency of reading and writing habits contribute to executive functions, intelligence, and learning in adolescents with healthy development? Appl. Neuropsychol. Child 2023, 12, 34–44. [Google Scholar] [CrossRef]
- Dylman, A.S.; Blomqvist, E.; Champoux-Larsson, M.F. Reading habits and emotional vocabulary in adolescents. Educ. Psychol. 2020, 40, 681–694. [Google Scholar] [CrossRef]
- Smith, R.; Snow, P.; Serry, T.; Hammond, L. The role of background knowledge in reading comprehension: A critical review. Read. Psychol. 2021, 42, 214–240. [Google Scholar] [CrossRef]
- Giazitzidou, S.; Mouzaki, A.; Padeliadu, S. Pathways from morphological awareness to reading fluency: The mediating role of phonological awareness and vocabulary. Read. Writ. 2024, 37, 1109–1131. [Google Scholar] [CrossRef]
- Zugarramurdi, C.; Fernández, L.; Lallier, M.; Valle-Lisboa, J.C.; Carreiras, M. Mind the orthography: Revisiting the contribution of prereading phonological awareness to reading acquisition. Dev. Psychol. 2022, 58, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.L.; Farkas, G.; Hillemeier, M.M.; Hammer, C.S.; Maczuga, S. 24-month-old children with larger oral vocabularies display greater academic and behavioral functioning at kindergarten entry. Child Dev. 2015, 86, 1351–1370. [Google Scholar] [CrossRef] [PubMed]
- Arrington, C.N.; Kulesz, P.A.; Juranek, J.; Cirino, P.T.; Fletcher, J.M. White matter microstructure integrity in relation to reading proficiency☆. Brain Lang. 2017, 174, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.; Huynh, T.; Ostevik, A.V.; Cheema, K.; Sweneya, S.; Craig, J.; Cummine, J. Phonological, orthographic and morphological skills are related to structural properties of ventral and motor white matter pathways in skilled and impaired readers. Appl. Neuropsychol. Adult 2024, 1–12. [Google Scholar] [CrossRef]
- Travis, K.E.; Leitner, Y.; Feldman, H.M.; Ben-Shachar, M. Cerebellar white matter pathways are associated with reading skills in children and adolescents. Hum. Brain Mapp. 2015, 36, 1536–1553. [Google Scholar] [CrossRef]
- Liu, T.; Thiebaut de Schotten, M.; Altarelli, I.; Ramus, F.; Zhao, J. Neural dissociation of visual attention span and phonological deficits in developmental dyslexia: A hub-based white matter network analysis. Hum. Brain Mapp. 2022, 43, 5210–5219. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shapiro, E.G.; Lipton, M.E.; Krivit, W. White matter dysfunction and its neuropsychological correlates: A longitudinal study of a case of metachromatic leukodystrophy treated with bone marrow transplant. J. Clin. Exp. Neuropsychol. 1992, 14, 610–624. [Google Scholar] [CrossRef]
- Richards, T.L.; Grabowski, T.J.; Boord, P.; Yagle, K.; Askren, M.; Mestre, Z.; Berninger, V. Contrasting brain patterns of writing-related DTI parameters, fMRI connectivity, and DTI–fMRI connectivity correlations in children with and without dysgraphia or dyslexia. NeuroImage Clin. 2015, 8, 408–421. [Google Scholar] [CrossRef]
- Rimrodt, S.L.; Peterson, D.J.; Denckla, M.B.; Kaufmann, W.E.; Cutting, L.E. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex 2010, 46, 739–749. [Google Scholar] [CrossRef]
- Skeide, M.A.; Kirsten, H.; Kraft, I.; Schaadt, G.; Müller, B.; Neef, N.; Friederici, A.D. Genetic dyslexia risk variant is related to neural connectivity patterns underlying phonological awareness in children. Neuroimage 2015, 118, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.S.; Ben-Shachar, M.; Yeatman, J.D.; Flom, L.L.; Luna, B.; Feldman, H.M. Reading performance correlates with white-matter properties in preterm and term children. Dev. Med. Child Neurol. 2010, 52, e94–e100. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C.; Plewes, C.; Paulson, L.A.; Roy, D.; Snook, L.; Concha, L.; Phillips, L. Imaging brain connectivity in children with diverse reading ability. Neuroimage 2005, 25, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, R.F.; Ben-Shachar, M.; Deutsch, G.K.; Hernandez, A.; Fox, G.R.; Wandell, B.A. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc. Natl. Acad. Sci. USA 2007, 104, 8556–8561. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, X.; Bai, Z.; Liu, X.; Zhang, M.; Li, H.; Booth, J.R. Left and right arcuate fasciculi are uniquely related to word reading skills in Chinese-English bilingual children. Neurobiol. Lang. 2022, 3, 109–131. [Google Scholar] [CrossRef]
- Gullick, M.M.; Booth, J.R. The direct segment of the arcuate fasciculus is predictive of longitudinal reading change. Dev. Cogn. Neurosci. 2015, 13, 68–74. [Google Scholar] [CrossRef]
- Gullick, M.M.; Demir-Lira, Ö.E.; Booth, J.R. Reading skill–fractional anisotropy relationships in visuospatial tracts diverge depending on socioeconomic status. Dev. Sci. 2016, 19, 673–685. [Google Scholar] [CrossRef]
- Meisler, S.L.; Gabrieli, J.D. Fiber-specific structural properties relate to reading skills in children and adolescents. Elife 2022, 11, e82088. [Google Scholar] [CrossRef]
- Yeatman, J.D.; Dougherty, R.F.; Rykhlevskaia, E.; Sherbondy, A.J.; Deutsch, G.K.; Wandell, B.A.; Ben-Shachar, M. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J. Cogn. Neurosci. 2011, 23, 3304–3317. [Google Scholar] [CrossRef]
- Su, M.; Zhao, J.; de Schotten, M.T.; Zhou, W.; Gong, G.; Ramus, F.; Shu, H. Alterations in white matter pathways underlying phonological and morphological processing in Chinese developmental dyslexia. Dev. Cogn. Neurosci. 2018, 31, 11–19. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Song, Z.; de Schotten, M.T.; Altarelli, I.; Ramus, F. Adaptive compensation of arcuate fasciculus lateralization in developmental dyslexia. Cortex 2023, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Weiss, Y.; Huber, E.; Ferjan Ramírez, N.; Corrigan, N.M.; Yarnykh, V.L.; Kuhl, P.K. Language input in late infancy scaffolds emergent literacy skills and predicts reading related white matter development. Front. Hum. Neurosci. 2022, 16, 922552. [Google Scholar] [CrossRef] [PubMed]
- Vandermosten, M.; Cuynen, L.; Vanderauwera, J.; Wouters, J.; Ghesquiere, P. White matter pathways mediate parental effects on children’s reading precursors. Brain Lang. 2017, 173, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Brignoni-Pérez, E.; Morales, M.C.; Marchman, V.A.; Scala, M.; Feldman, H.M.; Yeom, K.; Travis, K.E. Listening to Mom in the NICU: Effects of increased maternal speech exposure on language outcomes and white matter development in infants born very preterm. Trials 2021, 22, 444. [Google Scholar] [CrossRef]
- Lou, C.; Duan, X.; Altarelli, I.; Sweeney, J.A.; Ramus, F.; Zhao, J. White matter network connectivity deficits in developmental dyslexia. Hum. Brain Mapp. 2019, 40, 505–516. [Google Scholar] [CrossRef]
- Walton, M.; Dewey, D.; Lebel, C. Brain white matter structure and language ability in preschool-aged children. Brain Lang. 2018, 176, 19–25. [Google Scholar] [CrossRef]
- De Moura, L.M.; Cogo-Moreira, H.; De Ávila, C.R.B.; Pan, P.M.; Gadelha, A.; Moriyama, T.; Jackowski, A.P. Children with poor reading skills at the word level show reduced fractional anisotropy in white matter tracts of both hemispheres. Brain Connect. 2016, 6, 519–523. [Google Scholar] [CrossRef]
- Deutsch, G.K.; Dougherty, R.F.; Bammer, R.; Siok, W.T.; Gabrieli, J.D.; Wandell, B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex 2005, 41, 354–363. [Google Scholar] [CrossRef]
- Niogi, S.N.; McCandliss, B.D. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia 2006, 44, 2178–2188. [Google Scholar] [CrossRef]
- Leonard, C.; Eckert, M.; Given, B.; Virginia, B.; Eden, G. Individual differences in anatomy predict reading and oral language impairments in children. Brain 2006, 129, 3329–3342. [Google Scholar] [CrossRef]
- Cao, F.; Bitan, T.; Booth, J.R. Effective brain connectivity in children with reading difficulties during phonological processing. Brain Lang. 2008, 107, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Odegard, T.N.; Farris, E.A.; Ring, J.; McColl, R.; Black, J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia 2009, 47, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Liederman, J.; Hasan, K.M.; Lincoln, A.; Malmberg, B.; McLean III, J.; Papanicolaou, A. Diffusion tensor quantification of the relations between microstructural and macrostructural indices of white matter and reading. Hum. Brain Mapp. 2011, 32, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Raschle, N.M.; Chang, M.; Gaab, N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage 2011, 57, 742–749. [Google Scholar] [CrossRef]
- Feldman, H.M.; Lee, E.S.; Yeatman, J.D.; Yeom, K.W. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia 2012, 50, 3348–3362. [Google Scholar] [CrossRef]
- Myers, C.A.; Vandermosten, M.; Farris, E.A.; Hancock, R.; Gimenez, P.; Black, J.M.; Hoeft, F. Structural changes in white matter are uniquely related to children’s reading development. Psychol. Sci. 2014, 25, 1870. [Google Scholar] [CrossRef]
- Horowitz-Kraus, T.; Wang, Y.; Plante, E.; Holland, S.K. Involvement of the right hemisphere in reading comprehension: A DTI study. Brain Res. 2014, 1582, 34–44. [Google Scholar] [CrossRef]
- Broce, I.; Bernal, B.; Altman, N.; Tremblay, P.; Dick, A.S. Fiber tracking of the frontal aslant tract and subcomponents of the arcuate fasciculus in 5–8-year-olds: Relation to speech and language function. Brain Lang. 2015, 149, 66–76. [Google Scholar] [CrossRef]
- Travis, K.E.; Ben-Shachar, M.; Myall, N.J.; Feldman, H.M. Variations in the neurobiology of reading in children and adolescents born full term and preterm. NeuroImage Clin. 2016, 11, 555–565. [Google Scholar] [CrossRef]
- Borst, G.; Cachia, A.; Tissier, C.; Ahr, E.; Simon, G.; Houdé, O. Early cerebral constraints on reading skills in school-age children: An MRI study. Mind Brain Educ. 2016, 10, 47–54. [Google Scholar] [CrossRef]
- Travis, K.E.; Adams, J.N.; Kovachy, V.N.; Ben-Shachar, M.; Feldman, H.M. White matter properties differ in 6-year old Readers and Pre-readers. Brain Struct. Funct. 2017, 222, 1685–1703. [Google Scholar] [CrossRef] [PubMed]
- Mürner-Lavanchy, I.M.; Kelly, C.E.; Reidy, N.; Doyle, L.W.; Lee, K.J.; Inder, T.; Anderson, P.J. White matter microstructure is associated with language in children born very preterm. NeuroImage Clin. 2018, 20, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Song, S.; Liang, X.; Xie, Y.; Zhao, C.; Zhang, Y.; Gong, G. ROBO1 polymorphisms, callosal connectivity, and reading skills. Hum. Brain Mapp. 2017, 38, 2616–2626. [Google Scholar] [CrossRef] [PubMed]
- Horowitz-Kraus, T.; Holland, S.K.; Versace, A.L.; Bertocci, M.A.; Bebko, G.; Almeida, J.R.; Phillips, M.L. Reading related white matter structures in adolescents are influenced more by dysregulation of emotion than behavior. NeuroImage Clin. 2017, 15, 732–740. [Google Scholar] [CrossRef]
- Wang, Y.; Mauer, M.V.; Raney, T.; Peysakhovich, B.; Becker, B.L.; Sliva, D.D.; Gaab, N. Development of tract-specific white matter pathways during early reading development in at-risk children and typical controls. Cereb. Cortex 2017, 27, 2469–2485. [Google Scholar] [CrossRef]
- Su, M.; Thiebaut de Schotten, M.; Zhao, J.; Song, S.; Zhou, W.; Gong, G.; Shu, H. Vocabulary growth rate from preschool to school-age years is reflected in the connectivity of the arcuate fasciculus in 14-year-old children. Dev. Sci. 2018, 21, e12647. [Google Scholar] [CrossRef]
- Banfi, C.; Koschutnig, K.; Moll, K.; Schulte-Körne, G.; Fink, A.; Landerl, K. White matter alterations and tract lateralization in children with dyslexia and isolated spelling deficits. Hum. Brain Mapp. 2019, 40, 765–776. [Google Scholar] [CrossRef]
- Huber, E.; Henriques, R.N.; Owen, J.P.; Rokem, A.; Yeatman, J.D. Applying microstructural models to understand the role of white matter in cognitive development. Dev. Cogn. Neurosci. 2019, 36, 100624. [Google Scholar] [CrossRef]
- Dubner, S.E.; Dodson, C.K.; Marchman, V.A.; Ben-Shachar, M.; Feldman, H.M.; Travis, K.E. White matter microstructure and cognitive outcomes in relation to neonatal inflammation in 6-year-old children born preterm. NeuroImage Clin. 2019, 23, 101832. [Google Scholar] [CrossRef]
- Broce, I.J.; Bernal, B.; Altman, N.; Bradley, C.; Baez, N.; Cabrera, L.; Dick, A.S. Fiber pathways supporting early literacy development in 5–8-year-old children. Brain Cogn. 2019, 134, 80–89. [Google Scholar] [CrossRef]
- Del Tufo, S.N.; Earle, F.S.; Cutting, L.E. The impact of expressive language development and the left inferior longitudinal fasciculus on listening and reading comprehension. J. Neurodev. Disord. 2019, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, J.; van Setten, E.R.; Maurits, N.M.; Maassen, B.A. The interplay of socio-economic status represented by paternal educational level, white matter structure and reading. PLoS ONE 2019, 14, e0215560. [Google Scholar] [CrossRef] [PubMed]
- De Vos, A.; Vanderauwera, J.; Vanvooren, S.; Vandermosten, M.; Ghesquiere, P.; Wouters, J. The relation between neurofunctional and neurostructural determinants of phonological processing in pre-readers. Dev. Cogn. Neurosci. 2020, 46, 100874. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C.; Yip, E.; Low, P.B.; Mädler, B.; Lebel, C.A.; Siegel, L.; Laule, C. Myelin water imaging demonstrates lower brain myelination in children and adolescents with poor reading ability. Front. Hum. Neurosci. 2020, 14, 568395. [Google Scholar] [CrossRef]
- Geeraert, B.L.; Chamberland, M.; Lebel, R.M.; Lebel, C. Multimodal principal component analysis to identify major features of white matter structure and links to reading. PLoS ONE 2020, 15, e0233244. [Google Scholar] [CrossRef]
- Hutton, J.S.; Dudley, J.; Horowitz-Kraus, T.; DeWitt, T.; Holland, S.K. Associations between home literacy environment, brain white matter integrity and cognitive abilities in preschool-age children. Acta Paediatr. 2020, 109, 1376–1386. [Google Scholar] [CrossRef]
- Bruckert, L.; Travis, K.E.; Mezer, A.A.; Ben-Shachar, M.; Feldman, H.M. Associations of reading efficiency with white matter properties of the cerebellar peduncles in children. Cerebellum 2020, 19, 771–777. [Google Scholar] [CrossRef]
- Liu, T.; Thiebaut de Schotten, M.; Altarelli, I.; Ramus, F.; Zhao, J. Maladaptive compensation of right fusiform gyrus in developmental dyslexia: A hub-based white matter network analysis. Cortex 2021, 145, 57–66. [Google Scholar] [CrossRef]
- Lou, C.; Cross, A.M.; Peters, L.; Ansari, D.; Joanisse, M.F. Rich-club structure contributes to individual variance of reading skills via feeder connections in children with reading disabilities. Dev. Cogn. Neurosci. 2021, 49, 100957. [Google Scholar] [CrossRef]
- Van Der Auwera, S.; Vandermosten, M.; Wouters, J.; Ghesquière, P.; Vanderauwera, J. A three-time point longitudinal investigation of the arcuate fasciculus throughout reading acquisition in children developing dyslexia. NeuroImage 2021, 237, 118087. [Google Scholar] [CrossRef]
- Zuk, J.; Dunstan, J.; Norton, E.; Yu, X.; Ozernov-Palchik, O.; Wang, Y.; Gaab, N. Multifactorial pathways facilitate resilience among kindergarteners at risk for dyslexia: A longitudinal behavioral and neuroimaging study. Dev. Sci. 2021, 24, e12983. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Y.H.; Wang, H.L.S.; Liu, Y.C.; Chang, Y.P.E.; Weng, J.C. Investigating the white matter correlates of reading performance: Evidence from Chinese childr en with reading difficulties. PLoS ONE 2021, 16, e0248434. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Perdue, M.V.; Su, X.; Grigorenko, E.L.; Landi, N. Neurite density and arborization is associated with reading skill and phonological processing in children. NeuroImage 2021, 241, 118426. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Dunstan, J.; Jacobson, S.W.; Molteno, C.D.; Lindinger, N.M.; Turesky, T.K.; Gaab, N. Distinctive neural correlates of phonological and reading impairment in fetal alcohol-exposed adolescents with and without facial dysmorphology. Neuropsychologia 2022, 169, 108188. [Google Scholar] [CrossRef]
- Meisler, S.L.; Gabrieli, J.D. A large-scale investigation of white matter microstructural associations with reading ability. NeuroImage 2022, 249, 118909. [Google Scholar] [CrossRef]
- Ostertag, C.; Reynolds, J.E.; Kar, P.; Dewey, D.; Gibbard, W.B.; Tortorelli, C.; Lebel, C. Arcuate fasciculus and pre-reading language development in children with prenatal alcohol exposure. Front. Neurosci. 2023, 17, 1174165. [Google Scholar] [CrossRef]
- Harriott, E.M.; Nguyen, T.Q.; Landman, B.A.; Barquero, L.A.; Cutting, L.E. Using a semi-automated approach to quantify Unidentified Bright Objects in Neurofibromatosis type 1 and linkages to cognitive and academic outcomes. Magn. Reson. Imaging 2023, 98, 17–25. [Google Scholar] [CrossRef]
- Vandecruys, F.; Vandermosten, M.; De Smedt, B. The inferior fronto-occipital fasciculus correlates with early precursors of mathematics and reading before the start of formal schooling. Cortex 2024, 174, 149–163. [Google Scholar] [CrossRef]
- Ghasoub, M.; Perdue, M.; Long, X.; Donnici, C.; Dewey, D.; Lebel, C. Structural neural connectivity correlates with pre-reading abilities in preschool children. Dev. Cogn. Neurosci. 2024, 65, 101332. [Google Scholar] [CrossRef]
- Cross, A.M.; Lammert, J.M.; Peters, L.; Frijters, J.C.; Ansari, D.; Steinbach, K.A.; Joanisse, M.F. White matter correlates of reading subskills in children with and without reading disability. Brain Lang. 2023, 241, 105270. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, G.; Song, Z.; Zhang, Z.; Huang, H.; Li, H.; Tang, X. Associations between Brain Microstructure and Phonological Processing Ability in Preschool Children. Children 2022, 9, 782. [Google Scholar] [CrossRef] [PubMed]
- Vandecruys, F.; Vandermosten, M.; De Smedt, B. The role of formal schooling in the development of children’s reading and arithmetic white matter networks. Dev. Sci. 2024, 27, e13557. [Google Scholar] [CrossRef] [PubMed]
- Manning, K.Y.; Reynolds, J.E.; Long, X.; Llera, A.; Dewey, D.; Lebel, C. Multimodal brain features at 3 years of age and their relationship with pre-reading measures 1 year later. Front. Hum. Neurosci. 2022, 16, 965602. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, F.; McCandliss, B.D.; Black, J.M.; Gantman, A.; Zakerani, N.; Hulme, C.; Gabrieli, J.D. Neural systems predicting long-term outcome in dyslexia. Proc. Natl. Acad. Sci. USA 2011, 108, 361–366. [Google Scholar] [CrossRef]
- Kraft, I.; Schreiber, J.; Cafiero, R.; Metere, R.; Schaadt, G.; Brauer, J.; Skeide, M.A. Predicting early signs of dyslexia at a preliterate age by combining behavioral assessment with structural MRI. Neuroimage 2016, 143, 378–386. [Google Scholar] [CrossRef]
- Vanderauwera, J.; De Vos, A.; Forkel, S.J.; Catani, M.; Wouters, J.; Vandermosten, M.; Ghesquière, P. Neural organization of ventral white matter tracts parallels the initial steps of reading development: A DTI tractography study. Brain Lang. 2018, 183, 32–40. [Google Scholar] [CrossRef]
- Huber, E.; Donnelly, P.M.; Rokem, A.; Yeatman, J.D. Rapid and widespread white matter plasticity during an intensive reading intervention. Nat. Commun. 2018, 9, 2260. [Google Scholar] [CrossRef]
- Richards, T.L.; Berninger, V.W.; Yagle, K.; Abbott, R.D.; Peterson, D. Brain’s functional network clustering coefficient changes in response to instruction (RTI) in students with and without reading disabilities: Multi-leveled reading brain’s RTI. Cogent Psychol. 2018, 5, 1424680. [Google Scholar] [CrossRef]
- Bruckert, L.; Borchers, L.R.; Dodson, C.K.; Marchman, V.A.; Travis, K.E.; Ben-Shachar, M.; Feldman, H.M. White matter plasticity in reading-related pathways differs in children born preterm and at term: A longitudinal analysis. Front. Hum. Neurosci. 2019, 13, 139. [Google Scholar] [CrossRef]
- Borchers, L.R.; Bruckert, L.; Dodson, C.K.; Travis, K.E.; Marchman, V.A.; Ben-Shachar, M.; Feldman, H.M. Microstructural properties of white matter pathways in relation to subsequent reading abilities in children: A longitudinal analysis. Brain Struct. Funct. 2019, 224, 891–905. [Google Scholar] [CrossRef]
- Partanen, M.; Kim, D.H.; Rauscher, A.; Siegel, L.S.; Giaschi, D.E. White matter but not grey matter predicts change in reading skills after intervention. Dyslexia 2020, 27, 224–244. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.V.; Sima, D.; Smeets, D.; Ghesquière, P.; Wouters, J.; Vandermosten, M. Structural brain dynamics across reading development: A longitudinal MRI study from kindergarten to grade 5. Hum. Brain Mapp. 2021, 42, 4497–4509. [Google Scholar] [CrossRef] [PubMed]
- Beelen, C.; Blockmans, L.; Wouters, J.; Ghesquiere, P.; Vandermosten, M. Brain-behavior dynamics between the left fusiform and reading. Brain Struct. Funct. 2022, 227, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.E.; Zuk, J.; Mullin, L.J.; Ozernov-Palchik, O.; Norton, E.; Gabrieli, J.D.; Gaab, N. Examining shared reading and white matter organization in kindergarten in relation to subsequent language and reading abilities: A longitudinal investigation. J. Cogn. Neurosci. 2023, 35, 259–275. [Google Scholar] [CrossRef]
- Ghasoub, M.; Perdue, M.; Long, X.; Donnici, C.; Kar, P.; Gibbard, B.; Lebel, C. The brain’s structural connectivity and pre-reading abilities in young children with prenatal alcohol exposure. Dev. Cogn. Neurosci. 2024, 70, 101467. [Google Scholar] [CrossRef]
- Lou, C.; Cross, A.M.; Peters, L.; Ansari, D.; Joanisse, M.F. Patterns of the left thalamus embedding into the connectome associated with reading skills in children with reading disabilities. Netw. Neurosci. 2024, 8, 1507–1528. [Google Scholar] [CrossRef]
- Roy, E.; Richie-Halford, A.; Kruper, J.; Narayan, M.; Bloom, D.; Nedelec, P.; Yeatman, J.D. White matter and literacy: A dynamic system in flux. Dev. Cogn. Neurosci. 2024, 65, 101341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pınar, Y.; Bayat, N.; Yüksel, B.; Özkara, Y. Reading and White Matter Development: A Systematic Review of Neuroplastic Changes in Literacy. Children 2025, 12, 710. https://doi.org/10.3390/children12060710
Pınar Y, Bayat N, Yüksel B, Özkara Y. Reading and White Matter Development: A Systematic Review of Neuroplastic Changes in Literacy. Children. 2025; 12(6):710. https://doi.org/10.3390/children12060710
Chicago/Turabian StylePınar, Yunus, Nihat Bayat, Begümhan Yüksel, and Yasin Özkara. 2025. "Reading and White Matter Development: A Systematic Review of Neuroplastic Changes in Literacy" Children 12, no. 6: 710. https://doi.org/10.3390/children12060710
APA StylePınar, Y., Bayat, N., Yüksel, B., & Özkara, Y. (2025). Reading and White Matter Development: A Systematic Review of Neuroplastic Changes in Literacy. Children, 12(6), 710. https://doi.org/10.3390/children12060710





